Abstract
Reef fishes, such as gray snapper, support important recreational and commercial fisheries and use a variety of habitats throughout ontogeny. Gray snapper juveniles may be found in estuarine nursery areas, such as seagrass beds, or mangrove shorelines, while adults are most often found in deep channels and farther offshore, associated with hard-bottom habitats. Juvenile and subadult gray snapper were collected from 1996 through 2009 during long-term fishery-independent monitoring of several estuarine systems along the Gulf and Atlantic coasts of Florida. Indices of abundance and habitat suitability were constructed for gray snapper to determine size-specific relationships between abundance, habitat, and environmental conditions. Juvenile and subadult gray snapper were collected year-round only in the southernmost estuaries but were most common from July through December in all estuaries sampled. In addition to timing of estuarine occupancy, abundance varied with latitude; gray snapper were more frequently collected in warmer, southern estuaries. In general, gray snapper were most abundant in euhaline areas with a high percentage of submerged aquatic vegetation (SAV) and, in most cases, where overhanging shoreline vegetation was also present. Annual abundance varied over the sampling period, with some juvenile peaks in abundance translating to subadult peaks in subsequent years. Although strong correspondence between juvenile and subadult populations was not observed in all systems, long-term, broad-scale habitat selection patterns as described in this study are critical to more effectively assess populations of estuarine-dependent species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gray snapper, Lutjanus griseus, are economically important and estuarine-dependent reef fish that are distributed from the mid-Atlantic Bight to Brazil and use a variety of habitat types through ontogeny (Starck and Schroeder 1971; Allman and Grimes 2002). Adult gray snapper spawn during the summer (May–September) in offshore waters around reefs, wrecks, and other structured habitats (Starck and Schroeder 1971; Domeier et al. 1996). Larvae settle out of the plankton in structurally complex estuarine habitats such as seagrass beds (Allman and Grimes 2002; Tzeng et al. 2003; Denit and Sponaugle 2004a). Juvenile gray snapper remain in estuarine nursery areas, such as seagrass beds or mangrove shorelines (Nagelkerken et al. 2000; Cocheret de la Morinière et al. 2002; Whaley et al. 2007; Faunce and Serafy 2008), while subadults move into channels and eventually migrate to offshore structured habitats, where they reach maturity at 175–198 mm SL, or at about 2–3 years of age (Starck and Schroeder 1971; Manooch and Matheson 1981; Domeier et al. 1996). Gray snapper tend to remain in mangrove habitats longer than other reef-associated fishes, and some mature individuals have been found in greater abundance in mangrove habitats than in nearby coral reefs (Nagelkerken et al. 2000; Cocheret de la Morinière et al. 2002; Serafy et al. 2003).
Reef fishes, such as gray snapper, support important recreational and commercial fisheries on the Gulf of Mexico and Atlantic coasts. Recent studies have indicated that many exploited reef fishes (specifically those in the grouper–snapper complex; Ault et al. 2006) along the southeastern United States coast are being overfished due, in part, to increasing pressure from the recreational fishery in recent decades (Ault et al. 1998, 2005a). Gray snapper, as many other exploited reef fishes, are especially susceptible to the effects of overfishing due to unique life history characteristics such as depth and habitat preferences, longevity, and a high probability of discard mortality for undersized individuals (Coleman et al. 1996, 1999; Bartholomew and Bohnsack 2005). The bulk of gray snapper landings in the southeastern United States occur in Florida, especially in the southern portion of the state where they have been reported as being overfished (Ault et al. 2005b). Along both coasts of Florida, the recreational fishery lands more gray snapper per year than do commercial fisheries (FWRI 2010). Gray snapper are also frequently caught during fishing trips targeting other reef fish such as gag (Mycteroperca microlepis), red snapper (Lutjanus campechanus), and red grouper (Epinephelus morio). Despite heavy fishing pressure, a recent status and trend report designates gray snapper stocks as stable (FWRI 2010). Monitoring these trends is important to the maintenance of healthy stocks because overfishing of exploited reef fishes may be manifested in a variety of population-level responses, including declining abundance and reduced size (Coleman et al. 1996; Ault et al. 2005a, b).
Variability in the abundance of young-of-the-year (YOY) gray snapper may be attributed to fluctuations in factors such as fecundity, larval mortality (due to red tide, for example), larval transport, habitat availability, and survival rates in the estuary (Warlen et al. 1998; Epifanio and Garvine 2001). The variability in YOY abundance can sometimes be used to predict annual changes in adult populations. The assessment and management of commercial and recreational fisheries have typically relied heavily on fishery-dependent data, although the importance of incorporating fishery-independent data, when available, has long been recognized (Myers and Cadigan 1993; de Mutsert et al. 2008). Fishery-independent indices of abundance have proved invaluable in the assessment of several stocks in Florida (e.g., common snook and red drum; Muller and Taylor 2006; Murphy and Munyandorero 2009) and have potential applications in forecasting fisheries production from juvenile recruitment. Nevertheless, to be effective, these indices must (1) be part of a statistically robust survey design, (2) incorporate standardized sampling methodologies, and (3) cover a large enough geographical area to adequately capture regional patterns.
The vulnerability of gray snapper to fishing pressure makes it incumbent on fisheries managers to incorporate all aspects of life history, particularly recruitment processes, into assessments. As fishing effort increases, the quantity of biomass available to the fishery is increasingly linked to the strength of recruitment and survival of early life history stages (Smith 1993); accordingly, accurate estimation and prediction of juvenile recruitment is critical to the effective assessment and management of at-risk fisheries (Smith 1993; Koenig and Coleman 1998; Coleman et al. 1999). In addition to developing indices of abundance for gray snapper that reflect yearly cohort strength and long-term trends in abundance, recognizing size-specific habitat preferences through ontogeny can be important when describing the health of a population. Several studies have examined habitat use by gray snapper, but only on a fine temporal or spatial scale (Bartels and Ferguson 2006; Faunce and Serafy 2007, 2008). While these studies provide useful information, they are, in general, not of great utility for large-scale assessments. Accordingly, we conducted a comprehensive series of retrospective analyses of long-term (>10 years) monitoring data collected in four Gulf and two Atlantic coast estuaries in Florida to (1) characterize habitat selection by juvenile and subadult gray snapper and (2) compare interannual recruitment variability to qualitatively characterize concordance of strong year classes among regions.
Methods
Study Area
We investigated patterns of habitat use by gray snapper in four estuarine systems on the Gulf coast (Apalachicola Bay, Cedar Key, Tampa Bay, and Charlotte Harbor; Fig. 1) and two on the Atlantic coast (Northeast Florida and the Indian River Lagoon; Fig. 2) of Florida. The Gulf coast estuaries are proximate to the West Florida Shelf, a broad expanse of ocean bottom containing much of the natural hard-bottom habitat of the Gulf of Mexico (Briggs 1958; McEachran and Fechhelm 1998). Along the Atlantic coast, the Gulf Stream flows northward and is typically defined as the oceanic boundary (Dame et al. 2000); the proximity of this boundary to the estuaries depends on the distance of this current from the shore. The broad geographic expanse of the overall study area encompasses two distinct regions that are reflected in fundamental latitudinal differences in climate and aquatic floral (Sherrod and McMillan 1985) and ichthyofaunal communities (Smith 1976; Gilmore 1995), which are reflected in the differences among estuaries located in the northern and southern ends of the study area.
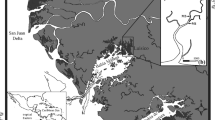
The location of four estuarine systems along the Florida Gulf coast within which monthly stratified random sampling of gray snapper using a 21.3-m and 183-m haul seine was conducted (AB Apalachicola Bay, 1999–2009; CK Cedar Key, 1997–2009; TB Tampa Bay, 1996–2009; CH Charlotte Harbor, 1996–2009). In the upper panel, circles represent locations where samples were collected. In the lower panel, circles represent locations where gray snapper were collected, with the size of the circle representing the total number of gray snapper within each set
The location of estuarine systems along the Florida Atlantic coast within which monthly stratified random sampling of gray snapper using a 21.3-m and 183-m haul seine was conducted (NE Northeast Florida 2002–2009; NIR Northern Indian River Lagoon, 1996–2009; SIR Southern Indian River Lagoon, 1997–2009). In the upper panel, circles represent locations at which samples were collected. In the lower panel, circles represent locations where gray snapper were collected, with the size of the circle representing the total number of gray snapper within each set
Apalachicola Bay, Cedar Key, and Northeast Florida, the three most northerly estuarine systems included in the current study, differ markedly with respect to geomorphological and hydrological characteristics. Apalachicola Bay is a shallow, semienclosed estuary bounded by a barrier island complex with freshwater inputs originating largely from the Apalachicola River and, to a lesser extent, the Carabelle River (Livingston 1983). Cedar Key is the only open estuarine system examined in the current study; freshwater flow is dominated by the Suwannee River and augmented by numerous tidal creeks (Lindberg et al. 1992). The Northeast Florida region is a broad estuarine system encompassing three coastal plain estuaries, defined by their river basins (St. Marys, Nassau, and St. Johns rivers), and interconnected via the Atlantic Intracoastal Waterway. Shoreline vegetation in all three northern estuarine systems consists largely of salt marsh habitat (primarily cordgrass, Spartina spp., and black needlerush, Juncus roemerianus). Both Gulf estuarine systems contain seagrass meadows consisting of mainly shoal grass, Halodule wrightii, turtle grass, Thalassia testudinum, and manatee grass, Syringodium filiforme, whereas in Northeast Florida, bottom vegetation is present only in the oligohaline reaches of the St. Johns River, upriver of downtown Jacksonville (Burns et al. 1997).
The southern estuarine systems we investigated (Tampa Bay, Charlotte Harbor, and the Indian River Lagoon) differ by coast with respect to geomorphological characteristics. Tampa Bay and Charlotte Harbor, the two most southerly Gulf coast estuaries sampled during the current study, have historically been viewed as drowned river estuaries (Galperin et al. 1991; Sheng 1998), although recent evidence suggests that Tampa Bay is underlain by a sedimentary basin formed by karst processes (Suthard et al. 2010). Despite this potential geological difference, both Tampa Bay and Charlotte Harbor are bounded by barrier islands and connected to the Gulf of Mexico by several main channels and smaller passes. Freshwater flow into Tampa Bay originates primarily from four rivers (Alafia, Hillsborough, Manatee, and Little Manatee rivers) and numerous smaller tributaries (Schmidt and Luther 2002). Freshwater flow into Charlotte Harbor comes primarily from the Peace, Caloosahatchee, and Myakka rivers. The Indian River Lagoon system is a narrow estuary located along the eastern central coast of Florida and, for this study, was divided into two sampling areas: the sampling area identified as the Northern Indian River Lagoon (NIR) that extends from the Mosquito Lagoon south through the Indian Lagoon proper to Vero Beach and the Southern Indian River Lagoon (SIR) that continues south to Jupiter inlet. The Indian River Lagoon is connected to the Atlantic Ocean by four permanent inlets (Sebastian, Fort Pierce, St. Lucie, and Jupiter) and by one intermittently open conduit via the Canaveral Locks that links the Banana River to the Atlantic Ocean just south of Cape Canaveral. Freshwater inflow comes primarily from the St. Sebastian River in the north, the St. Lucie and Loxahatchee rivers in the south, and numerous creeks located mainly along the western shoreline (Paperno and Brodie 2004; Sime 2005). Unlike the northern estuaries, shoreline vegetation for all three systems consists largely of fringing mangroves (predominantly red mangrove, Rhizophora mangle, and black mangrove, Avicennia germinans) and, to a lesser degree, salt marshes (mostly black needlerush), although the Northern Indian River Lagoon contains a greater proportion of salt marsh plants. Seagrasses (predominantly turtle grass and shoal grass) are widely distributed throughout the shallow regions of all three estuaries.
Field Methods
A stratified random sampling design was employed to allocate monthly sampling efforts within each estuary. The initiation of sampling varied among estuaries and gear types, ranging from 1996 to 2002; sampling continued through 2009 (Table 1). Monthly sampling effort was allocated among spatial zones based on geographic and logistical criteria that defined areas of biological and hydrological homogeneity. Sampling sites were further stratified by habitat type depending on the estuary and gear type used. Monthly sampling effort in each stratum for each estuary was proportional to the total number of potential sampling sites available. All sampling sites were selected randomly without replacement; this procedure was conducted each month.
Juvenile gray snapper were collected with a 21.3-m × 1.8-m center-bag haul seine with 3.2-mm nylon mesh in all systems, except the Southern Indian River Lagoon, where this gear was not used and, therefore, juveniles were not collected. In shallow (≤1.8 m) shoreline and offshore habitats in all estuaries, except Northeast Florida, the 21.3-m seine was pulled a distance of 9.1 m with a width of 15.5 m between the seine poles, sampling an area of approximately 140 m2. Seines were deployed along estuarine shorelines to sample areas with emergent vegetation, mangrove fringes, seawalls, and beaches. Seines were deployed offshore to sample flats at least 5 m from the shoreline; in all estuaries, except Cedar Key, these deployments were stratified by the presence or absence of submerged aquatic vegetation (SAV; presence was defined as ≥25 % SAV cover). Due to the presence of steep shorelines in Northeast Florida, 21.3-m seines were deployed from the stern of a boat in a semielliptical pattern and retrieved onto shore, sampling an area of approximately 68 m2.
Subadult gray snapper were collected using a 183-m × 2.5-m center-bag haul seine with 38-mm stretched nylon mesh netting that was deployed by boat, set in a rectangular shape along the shoreline with a maximum depth of 2.5 m at the bag, and retrieved by hand. The dimensions of the area sampled by the net (approximately 40 m × 103 m = 4,120 m2) were standardized by marking 40 m from each end of the net to designate the corner locations of the rectangular set. In Tampa Bay and Charlotte Harbor, these deployments were stratified based on the presence or absence of overhanging vegetation (presence of overhanging vegetation was defined as ≥10 % cover along the shoreline).
All gray snapper collected in each sample were identified and enumerated, and as many as 40 randomly selected individuals per sample were measured to the nearest millimeter standard length (SL). Location, date, time, and water depth at the bag of the net were recorded at each sampling site. Temperature (degrees Celsius), salinity (practical salinity unit), and dissolved oxygen (milligrams per liter) were recorded at the surface and at 1.0-m depth intervals and were averaged for each sample. Habitat variables (shore type, SAV type, and percentage SAV cover) were recorded at the time of sampling for each net set. (Note: Northeast Florida had very few [<20 % of samples] 21.3-m seine sites with SAV cover present; therefore, this factor was not included in the analyses. Percentage cover of SAV was also not measured consistently at all 183-m seine sites in Northeast Florida or Northern Indian River Lagoon due to frequent high turbidity.) The dominant habitat type (seagrass, marsh, overhang, overSAV, structure, or other) was designated for each sample (Table 2). If the sample was associated with a shoreline (collected within 10 m of shore), a dominant shore type (≥60 % of the shoreline was covered with marsh vegetation, overhanging vegetation, or structure) was included in determining dominant habitat type. Marsh vegetation included emergent plants such as saltmarsh cordgrass and black needlerush; overhanging vegetation included trees and shrubs such as mangroves that provided permanent shade within 1 m of the water surface; and structured shorelines included rip-rap, oyster bars, and seawalls. Seagrass was incorporated into the dominant habitat type of these shoreline-associated sites if SAV cover was at least 25 % (overSAV, seagrass; Table 2). If the sample was collected >10 m from the shoreline, then samples were considered to have a dominant habitat type of seagrass if SAV cover was at least 25 %. If none of the above situations applied, the dominant habitat type was designated as “other” and included primarily shell hash and unconsolidated sediment.
Analytical Methods
Total effort and catch data were summarized for all gray snapper collected in each estuarine system. Locations at which samples were taken, as well as locations at which gray snapper were collected, were plotted for each system in a GIS to delineate spatial patterns of catch and effort. In addition, frequency histograms were constructed by gear type, length, and month to compare variability among the estuarine systems with respect to the timing of juvenile recruitment as well as the duration of estuarine occupancy for juvenile and subadult gray snapper.
Based on previous research, several environmental and habitat metrics (predictor variables) were chosen and used in a generalized linear model to predict gray snapper relative abundance (response variable = number of individuals per seine haul) for each estuary. The relative abundance of gray snapper represents count data, the distribution of which is bounded by zero and, therefore, often highly non-normal. Accordingly, generalized linear models based on the Gaussian distribution with a log transformation, the Poisson distribution, and the negative binomial distribution were fit to the data, and residual diagnostics and goodness-of-fit statistics were examined to determine the most appropriate model. For all estuaries, the model based on the negative binomial distribution was deemed the most appropriate. Based on length–frequency distributions, separate models were developed for juvenile (≤50 mm SL) gray snapper collected in 21.3-m seines and subadult (100–250 mm SL) gray snapper collected in 183-m seines. Our gear did not effectively capture individuals from 50 to 100 mm SL, and a diet shift occurs at around 50 mm SL (Hettler 1989), indicating a possible change in habitat use missed by our sampling regime. A subset of the juvenile data was used to more closely examine the recruitment window (July–December). Although the subadult size range may contain a few mature individuals, these fish are still consistently using estuarine habitats at these sizes and will be referred to as subadults for the remainder of the paper. Year and dominant habitat type were included as categorical variables in the model, while temperature, salinity, dissolved oxygen, water depth, and percentage SAV cover were included as covariates. Variables that did not significantly covary (α = 0.05) with gray snapper abundance were excluded using backward elimination, and the analysis was repeated in a stepwise fashion until only significant variables remained in the model. Results are reported only for statistically significant variables. For each estuarine system, annual least square mean estimates (±SE) of gray snapper relative abundance were plotted in examination of temporal variability. All analyses were fit using the GLIMMIX procedure and SAS software (SAS Institute 2006).
Habitat suitability analyses were conducted for variables that were significant across several estuarine systems in order to determine specific patterns of habitat selection by juvenile and subadult gray snapper (Baltz 1990). Suitability curves allow for an examination of resource use that accounts for nonuniform sampling across environmental gradients and provide valuable information regarding patterns of habitat selection. For each univariate habitat suitability analysis, environmental data were subdivided into equal intervals and interval ranges chosen to produce the smoothest habitat suitability curve possible. For each interval or dominant habitat type, habitat suitability values (S) were calculated as:
where P(E | F) represented the proportion of samples in which a species occurred (F, resource use) that fell within a specific environmental interval or dominant habitat type (E), while P(E) represented the proportion of all samples collected, regardless of whether a given species occurred (resource availability), which fell within the same environmental interval (Baltz 1990). Suitability values for each analysis were then standardized by dividing by the greatest observed suitability so that values ranged from 0 (intolerable) to 1 (optimal). Suitability curves were constructed independently for juvenile (July–December) and subadult (year-round) gray snapper collected in each estuarine system.
Canonical discriminant analysis (CDA) was used to test whether environmental conditions differed among estuaries and between sites in which gray snapper were present or absent. A CDA was conducted that included data from samples collected in all estuarine systems to resolve five correlated environmental variables (water depth, salinity, percentage SAV cover, temperature, and dissolved oxygen) into canonical variates (linear combinations of measurement variables) that have the highest possible multiple correlation with the previously defined classes to maximally separate the groups. Observations were weighted by the number of gray snapper present plus one. The CDA was conducted using the CANDISC procedure and SAS software, Wilks' lambda (α = 0.05) was used to discriminate among groups, and the significant canonical correlations were interpreted to describe habitat use (SAS Institute 2006). Variable loadings and canonical variates were calculated independently for each site. Mean environmental conditions sampled were calculated by averaging all canonical variates in each estuarine system by presence or absence of gray snapper. Mean canonical variates (±standard error) were then plotted in two-dimensional space to compare differences between environmental conditions occupied or unoccupied by juvenile (July–December) and subadult (year-round) gray snapper for each respective estuarine system.
Results
From 1996 to 2009, a total of 21,907 21.3-m seine and 16,938 183-m seine samples were collected in six estuarine systems along the Florida coast; of these samples, 5.6 and 12.3 %, respectively, contained gray snapper (Table 1). In total, 3,880 and 9,275 gray snapper were collected during 21.3-m and 183-m seine sampling, respectively. Gray snapper were most frequently collected in Charlotte Harbor, where they occurred in more than 12 % of the 21.3-m seine samples and 25 % of the 183-m seine samples, and in the Southern Indian River Lagoon, where they occurred in 26 % of the 183-m seine samples (Table 1). Gray snapper were rarely collected in Cedar Key and Northeast Florida (Table 1; Figs. 1 and 2). In the semienclosed estuarine systems of Tampa Bay and Charlotte Harbor, more individuals were collected in the polyhaline regions of the estuary near the mouth than in the upper estuary (Fig. 1), and in the Indian River Lagoon, there was a noticeable increase in catches in the southern portion of that estuarine system (Table 1; Fig. 2). Gray snapper collected with the 21.3-m seine ranged from 8 to 315 mm SL (Table 1), although 72 % of individuals were smaller than 50 mm SL (2,785 of 3,880; Fig. 3). Gray snapper collected with the 183-m seine ranged from 28 to 560 mm SL, although the average length was approximately 155 mm SL (Table 1), and most individuals were larger than 100 mm SL in the southern estuaries (Fig. 3). In contrast, the majority of individuals collected with the 183-m seine in the northern estuaries (Apalachicola Bay, Cedar Key, and Northeast Florida) were smaller, on average (Fig. 3). Juvenile gray snapper were most abundant from July through December, although in some cases, they were present year-round, especially in the southernmost estuaries. Subadult gray snapper were collected year-round in Charlotte Harbor and both regions of the Indian River Lagoon, with decreased numbers from January through May (Fig. 4). Gray snapper of all sizes were noticeably absent or reduced in number from collections in the northern estuaries during the same period (Fig. 4).
Results from generalized linear modeling analyses indicated that for most estuarine systems, the relative abundance of juvenile gray snapper strongly covaried with temperature and percentage SAV cover (Table 3). The relative abundance of juvenile gray snapper covaried significantly with salinity in Apalachicola Bay, Tampa Bay, and the Northern Indian River Lagoon, with dissolved oxygen in Charlotte Harbor and Northeast Florida, and with water depth in the east coast estuaries. Significant interannual variability in the relative abundance of juvenile gray snapper was evident for all estuarine systems, although year was not significant in the model for Northeast Florida (Table 3). Observed interannual variability in juvenile recruitment was not universally concordant among estuaries, but relatively strong year classes were evident in 1999, 2001, 2004, and 2006 on the Gulf coast and in 1999 and 2005 on the Atlantic coast (Fig. 5). The relative abundance of juvenile gray snapper for all estuaries was influenced by dominant habitat type (Table 3); in the southern estuaries, juveniles most frequently occurred in habitats with a combination of overhanging and seagrass habitat or just seagrass habitat, and in northern estuaries, juveniles preferred seagrass habitats when it was available. Marsh habitats were also highly suitable in northern estuaries (Fig. 6).
Habitat suitability analyses for juvenile (≤50 mm SL; July–December) and subadult (100–250 mm SL) gray snapper collected within different dominant habitat types during monthly stratified random sampling effort using a 21.3-m haul seine and a 183-m haul seine, respectively, within estuarine systems along the Florida coast. For each estuarine system, the left panel summarizes the relative frequency of the total number of samples collected with 21.3-m seines (black bars), the number of samples collected that contained juvenile gray snapper (white bars), and the results of habitat suitability analyses; the right panel summarizes the same values for samples collected by 183-m seines and those that contained subadult gray snapper. For each plot, the scale on the left Y-axis refers to values for relative frequency and the scale on the right Y-axis refers to values for standardized suitability (gray dots). NS dominant habitat type was not significant in the GLM model
With respect to subadult gray snapper, relative abundance was significantly associated with temperature and, in most cases, percentage SAV cover (Table 4). The relative abundance of subadult gray snapper was significantly associated with water depth in the southern estuaries (Tampa Bay, Charlotte Harbor, and both regions of the Indian River Lagoon), with salinity in Cedar Key and the Indian River Lagoon (NIR and SIR), and with dissolved oxygen in Tampa Bay and the Northern Indian River Lagoon. Significant interannual variability in the relative abundance of subadult gray snapper was also evident for all estuarine systems, and although similar to the juvenile data, year was not significant in the model for Northeast Florida (Table 4). Years of relatively high juvenile recruitment were followed by peaks in subadult abundance in 2002 and 2007 in some Gulf coast estuaries and increases in abundance through 2008 in Northeast Florida (Fig. 5). Other high recruitment years, however, were not followed by peaks in subadult abundance (from 1999 to 2000 on the Gulf coast, for example). The relative abundance of subadult gray snapper in Charlotte Harbor and both regions of the Indian River Lagoon were associated with habitat type; subadults occurred in all habitat types, but the combination of overhanging and seagrass habitat was most suitable in Charlotte Harbor and both regions of the Indian River Lagoon. In the Northern Indian River Lagoon and Tampa Bay, subadults were evenly distributed across most habitat types (Table 4, Fig. 6). Marsh and seagrass habitats were most suitable in northern estuaries, where there was no overhanging habitat.
Habitat suitability analyses of covariates (temperature, salinity, and percentage SAV cover) found to significantly influence the relative abundance of gray snapper identified clear patterns of resource use and selection that were generally consistent across estuarine systems (Figs. 7, 8 and 9). Warm temperatures (26–34 °C) were the most suitable for gray snapper (Fig. 7); no juvenile gray snapper were collected at temperatures less than 14 °C and no subadults at temperatures less than 10 °C (Fig. 7). In general, higher salinity was more suitable for gray snapper than was low salinity, but suitability varied by estuary (Fig. 8). In northern estuaries, juveniles occurred with similar frequency across low and high salinity, but in southern estuaries, juveniles occurred most often in highly saline waters (usually >14 psu). Subadults followed a similar pattern of salinity occurrence, with the exception of Northeast Florida. Very few subadults were collected in Northeast Florida (n = 53), and salinity was not a significant factor in the generalized linear modeling, so the high suitability of oligohaline conditions (2–6 psu) may not hold true with a larger sample size. Generally, gray snapper also occurred in sites with greater than 50 % SAV cover (Fig. 9). This pattern was more variable in the northern estuaries but held true in the southern estuaries.
Habitat suitability analyses along a temperature gradient for juvenile (≤50 mm SL; July–December) and subadult (100–250 mm SL) gray snapper collected in monthly stratified random sampling effort using a 21.3-m haul seine and a 183-m haul seine, respectively, within estuarine systems along the Florida coast. For each estuarine system, the left panel summarizes the relative frequency of the total number of samples collected with 21.3-m seines (black bars), the number of samples collected that contained juvenile gray snapper (white bars), and the results of habitat suitability analyses; the right panel summarizes the same values for samples collected by 183-m seines and those that contained subadult gray snapper. For each plot, the scale on the left Y-axis refers to values for relative frequency and the scale on the right Y-axis refers to values for standardized suitability (black line). NS temperature was not significant in the GLM model
Habitat suitability analyses along a salinity gradient for juvenile (≤50 mm SL; July–December) and subadult (100–250 mm SL) gray snapper collected in monthly stratified random sampling effort using a 21.3-m haul seine and a 183-m haul seine, respectively, within estuarine systems along the Florida coast. The description of these plots is the same as described in the caption for Fig. 7. NS salinity was not significant in the GLM mode
Habitat suitability analyses along a gradient of estimated percent cover for submerged aquatic vegetation (SAV) for juvenile (≤50 mm SL; July–December) and subadult (100–250 mm SL) gray snapper collected within monthly stratified random sampling effort using a 21.3-m haul seine and a 183-m haul seine, respectively, within estuarine systems along the Florida coast. The description of these plots is the same as described in the caption for Fig. 7. NS SAV cover was not significant in the GLM model, N/A not included in the GLM model due to large numbers of missing values
Mean environmental conditions differed significantly between sites based on the presence and absence of gray snapper as well as among estuaries for both juvenile and subadult gray snapper (Wilks' lambda, F = 113.7, p < 0.0001 and F = 193.2, p < 0.0001, respectively). With respect to juvenile gray snapper, the CDA of combined environmental data from all estuaries identified five significant canonical variates (p < 0.0001), although the first two variates together explained 90.9 % of total variability. The first canonical variate was generally a positive measure of temperature (0.400) and percentage SAV cover (0.950; Fig. 10a). The second variate had positive loadings for salinity (0.592) and water depth (0.426) and negative loadings for dissolved oxygen (−0.692; Fig. 10a). The CDA for subadult sampling also identified five significant canonical variates (p < 0.0001), and the first two variates together explained 85.6 % of total variability. The first canonical variate was generally a positive measure of temperature (0.667), percentage SAV cover (0.860), and salinity (0.505; Fig. 10b). The second variate had positive loadings for water depth (0.910) and negative loadings for dissolved oxygen (−0.390; Fig. 10b).
Mean canonical variates (±standard error) for sites in each estuarine system occupied (gray snapper present; open circles) and not occupied (gray snapper absent; filled circles) by juvenile (a ≤50 mm SL, July through December) and subadult (b 100–250 mm SL) gray snapper in two-dimensional canonical space. Note differences in scale
Mean estuary-specific canonical variates for samples collected with and without gray snapper identified clear differences in environmental conditions sampled in all estuarine systems (Fig. 10). Regardless of whether gray snapper were present, northern estuaries (Apalachicola Bay, Cedar Key, and Northeast Florida) were typically cooler than southern estuaries (Tampa Bay, Charlotte Harbor, and the Indian River Lagoon), reflecting latitudinal differences in solar input. Sites sampled in the northern estuaries also had lower salinity and less SAV cover than did the southern estuaries, and sampling sites in Cedar Key and Northeast Florida were generally deeper than those sampled in the remaining estuaries. Mean estuary-specific canonical variates for samples that contained gray snapper indicated that mean environmental conditions of habitat occupied by gray snapper differed significantly from mean conditions sampled in unoccupied habitat for all estuarine systems. Juvenile gray snapper occupied warmer and deeper sites with higher salinity and higher SAV cover than were measured in unoccupied habitats (Fig. 10a). Similarly, subadult gray snapper occupied warmer and deeper sites with higher salinity and higher SAV cover than in unoccupied habitats, although these trends were not as pronounced in Cedar Key and Northeast Florida (Fig. 10b).
Discussion
Long-term, broad-scale habitat selection patterns of gray snapper over a wide geographic region were described in this study; this type of detailed information is critical for effective population assessments of estuarine-dependent species. Earlier studies describing the habitat use of gray snapper have generally been of limited scope, either focusing on particular habitats (Thayer et al. 1987; Valentine-Rose et al. 2007; Faunce and Serafy 2008) and specific size classes (Rutherford et al. 1989b; Chester and Thayer 1990), or encompassing limited temporal (2006–2007; Patterson et al. 2009) or spatial coverage (Charlotte Harbor: Whaley et al. 2007; southeastern Florida: Serafy et al. 1997; Faunce et al. 2002; Faunce and Serafy 2007; Serafy et al. 2007; Florida Keys: Eggleston et al. 2004; Bartels and Ferguson 2006; Campeche Bank: Carrió et al. 2004; various Caribbean islands: Nagelkerken et al. 2000; Cocheret de la Morinière et al. 2002; Rypel and Layman 2008). The results of this study provide a comprehensive view of estuarine habitat use by gray snapper across an expanded spatial and temporal range.
Although gray snapper were collected in all Florida estuarine habitats sampled during this study, the timing and duration of estuarine occupancy varied latitudinally, with an expanded recruitment window and continuous subadult occupancy in more southerly estuaries. Gray snapper occurred most often in the warmer temperatures (i.e., generally >18 °C) common in the southern estuaries (Charlotte Harbor and the Southern Indian River Lagoon). Juvenile gray snapper recruited from July through December, although they were present year-round in some southern estuaries. The observed differences in timing of settlement may indicate regional differences in the timing of spawning; spawning by gray snapper in Florida is generally most frequent during warm summer months (Starck and Schroeder 1971; Domeier et al. 1996), although Allman and Grimes (2002), using back-calculated fertilization dates, found that some juvenile gray snapper from southern estuaries originated from winter spawning. Subadult gray snapper were noticeably absent from or reduced in number in collections from the northern estuaries from January through May; although gray snapper were collected year-round in Tampa Bay, Charlotte Harbor, and throughout the Indian River Lagoon, abundance declined markedly during the same time period. It is difficult to ascertain whether these patterns reflect a suitable temperature range or factors (e.g., timing of spawning, occurrence of seagrass, and mangrove habitat) that correlate strongly with temperature.
Gray snapper also exhibited differences in size among estuaries of varying latitude. The mean size of juvenile gray snapper increased with decreasing latitude, and similarly, several studies have revealed a positive correlation between juvenile growth and temperature (Denit and Sponaugle 2004c; Wuenschel et al. 2004). In the estuaries sampled, the mean size of subadult gray snapper did not exhibit the same trend, although subadults were noticeably absent from the northern estuaries. The recruitment pulse happens earlier in northern estuaries and is markedly different from the protracted recruitment typical of the southern estuaries. Larger individuals of a cohort are more successful in surviving extended periods of cold temperatures than smaller individuals (Sogard 1997), so the early recruitment and lack of subadults in the northern estuaries may be evidence of overwintering mortality, emigration from the estuary, or rapid growth rates during the reduced time in which high temperatures occur (Conover and Present 1990). Adult gray snapper collected from offshore waters exhibit growth rates that differ latitudinally along both coasts of Florida (Burton 2001; Allman and Goetz 2009). In these studies, individuals from northern Florida were found to reach a greater maximum size and age than those from southern Florida. In the southern regions, the size-selective mortality that results from increased fishing pressure may also account for the lack of larger, older fish and for the selection of more slowly growing individuals. Increased fishing can decrease intraspecific competition for prey, resulting in increased growth rates, but it also results in selective removal of the largest fish influencing observed growth patterns (Kritzer 2002). A combination of differential recruitment, survival, growth, mortality, and fishing pressure likely influences the latitudinal differences in the size of gray snapper in these estuaries.
Our findings indicate that both juvenile and subadult gray snapper use estuarine areas with a high percentage of SAV cover. This trend was evident across most estuarine systems and holds true despite latitudinal differences in seagrass production (Phillips 1960; Dawes et al. 2004; Handley et al. 2007). These results were supported by a study that utilized similar fishery-independent data collected in several Gulf estuaries, where for over a 2-year period, it was found that presence of seagrass habitat was the single most important factor in predicting the presence of age 0 gray snapper (Patterson et al. 2009). Seagrass beds are known to serve as refuge and feeding habitat for various species of nekton. In addition, seagrass bed architecture, species composition, location, and water quality can all affect the value of seagrass as habitat (Bell et al. 1987, 1988; Robbins and Bell 1994; Raposa and Oviatt 2000; Jelbart et al. 2007; Flaherty et al. 2010). Increasing seagrass canopy cover can indicate greater protection for fish from predators in these environments and has been positively correlated with juvenile gray snapper abundance in Tampa Bay (Orth et al. 1984; Flaherty and Matheson 2008). Seagrass leaf density varies seasonally, however, so other characteristics may be important in determining juvenile fish density. For example, in some estuaries, juvenile abundance may be more related to the location of the seagrass bed in relation to sources of larvae than with the architecture of the bed (Bell et al. 1988). Seagrass species composition has also been shown to influence habitat selectivity by juvenile gray snapper (Chester and Thayer 1990; Bartels and Ferguson 2006). Flaherty and Matheson (2008) did not find a relationship between seagrass type and juvenile gray snapper abundance in Tampa Bay, even though gray snapper juveniles were more abundant in polyhaline portions of the bay characterized with higher proportions of turtle grass. By examining data collected in multiple estuarine systems at sites with differing habitat types and SAV cover, this study builds upon previous work by characterizing overall patterns of habitat selection (Baltz 1990).
In addition to SAV, results from our analyses indicated that the structure provided by overhanging vegetation was important for gray snapper. Nekton are generally thought to select structured habitats (e.g., SAV, mangrove, and hard bottom) because they provide food and refuge, although unraveling the relative importance of various structured habitats is often difficult. The use of seagrass habitat by juvenile gray snapper has been well documented, although prior studies were generally conducted exclusively in polyhaline seagrass beds and did not explicitly examine the relative importance of different habitat types (Chester and Thayer 1990; Bartels and Ferguson 2006). In areas in which other structured habitats were readily available (mangrove and structured shorelines in the southern estuaries and marsh shorelines in the northern estuaries), gray snapper appeared to exhibit an ontogenetic shift in the relative importance of various habitat types. In our study, juvenile gray snapper were markedly more abundant in habitats containing seagrass (seagrass, overSAV) over those containing solely structured shorelines, whereas subadult gray snapper were somewhat indiscriminate across available structured habitats. Similarly, Faunce and Serafy (2007) demonstrated that juvenile gray snapper occupied seagrass, while subadults occupied mangrove shorelines year-round and inland mangroves during the dry season and mangrove shorelines closest to the reef tract were used at much greater levels than their availability would suggest (Faunce and Serafy 2008). Documented ontogenetic shifts may reflect a decreasing suitability of seagrass habitats as refuge for larger fish or, possibly, an increase in mobility with growth. It is not clear at what point gray snapper's habitat use patterns change; in our study, we captured relatively few gray snapper from 50 to 100 mm SL, although recent sampling of deeper seagrass beds using otter trawls suggests that these sizes of gray snapper still utilize SAV habitat (Switzer et al. 2011). In an extensive review, Heck et al. (2003) concluded that, compared with other structured habitats, seagrass meadows were not correlated with greater abundance, growth, or survival of nekton. Other studies, however, have found that nekton habitat use was greater in seagrass habitats than in marsh edges and mangrove shorelines (Bloomfield and Gillanders 2005; Rozas et al. 2012), but these responses differ depending on season, prey availability, and life history stage (Yáñez-Arancibia et al. 1993; Verweij et al. 2006; Faunce and Serafy 2007). In an experimental study of habitat use (Verweij et al. 2006), herbivorous and diurnally active zoobenthivorous reef fish were attracted to mangroves and seagrass beds by food availability, but nocturnal zoobenthivores were attracted to structure (i.e., shade) for predator avoidance. Nagelkerken et al. (2000) found that gray snapper of all sizes used mangrove shorelines in contrast to other reef-associated species that migrated permanently to deeper reefs when they reach a specific size. Nevertheless, within the current study, identifying the relative importance of shoreline versus benthic habitat proved problematic because both juvenile and subadult gray snapper selected sites in which both overhanging shorelines and seagrass were present. Further study on the complexity of habitat use by gray snapper is needed to document the effects of habitat fragmentation, proximity of alternate habitats, and edge effects.
Juvenile and subadult gray snapper generally occupied euhaline and, to a lesser extent, polyhaline environments. This trend was most evident in southern estuaries in which there were saltier environments with a greater degree of SAV cover, while in the northern estuaries, habitat use was more variable. In this study, juveniles were collected in a broader range of salinity than subadults. Juvenile gray snapper have a wide tolerance for salinity and usually occur in intermediate salinity (Wuenschel et al. 2004; Serrano 2008), but growth efficiency has been positively correlated with salinity (Wuenschel et al. 2004). In some cases, juvenile preference for high-salinity habitats may simply be related to where larvae settle out in close proximity to inlets or by movement due to estuarine currents, but patterns of subadult distribution are likely driven more by active habitat selection. In addition to occupying high-salinity habitats, subadults have also been collected in tidally influenced rivers not sampled in this study and in portions of upper Tampa Bay during hook-and-line studies (FWRI, unpublished data). Physiologically, subadult gray snapper can withstand a broad salinity range (Serrano 2008), so greater catches of gray snapper observed in the lower polyhaline regions of some estuaries (Tampa Bay and Charlotte Harbor) may be more strongly associated with the increasing SAV cover, deeper channels, and close proximity to offshore spawning sites common to those areas (Luo et al. 2009). A combination of increasing SAV, optimal temperature and salinity ranges, and close proximity to inlets or channels leading to offshore habitats all likely contribute to the observed distribution of gray snapper.
Observed differences in depths occupied by gray snapper likely reflect an ontogenetic shift in habitat preference. Juvenile gray snapper were found more often in slightly deeper areas, in contrast to previous studies, which indicate that juveniles are present in shallow areas with a higher proportion of seagrass habitat (Nagelkerken et al. 2000; Cocheret de la Morinière et al. 2002; Whaley et al. 2007; Faunce and Serafy 2008). This result may not be a strong indication of habitat selectivity, since sites sampled by the 21.3-m seine do not vary significantly in water depth (range: 0.3–1.5 m), and depth was only marginally associated with the second canonical variate (0.426) that explained a small portion of the variability (8.1 %). Subadult gray snapper showed a stronger affinity for deepwater habitat, especially in the southern estuaries, possibly demonstrating migration from seagrass and mangrove estuarine habitats to deeper structure as the fish grow. Another possibility is a diel migration of subadults that our restricted daytime sampling did not detect; Luo et al. (2009) demonstrated that gray snapper move from mangrove shoreline and deepwater areas during the day to more productive seagrass beds at night for feeding. The smaller numbers of subadults collected in northern estuaries may be related to movement into deeper water or into undersampled structured habitats; the gear types used in this study were constrained by depth (21.3-m seines ≤ 1.8 m, 183-m seines ≤ 2.5 m) and could not be used in areas with obstructions (e.g., artificial reefs and natural hard bottom). In the case of the northern estuaries, juvenile survival may be reduced, or subadult gray snapper may leave the estuary, resulting in decreased abundance throughout the year. Movement into deeper water as gray snapper mature would reinforce previous findings on their life history and juvenile migration patterns (Nagelkerken et al. 2000; Cocheret de la Morinière et al. 2002; Denit and Sponaugle 2004b), since adult gray snapper are most often found in deeper channels and farther offshore, associated with hard bottom (Starck and Schroeder 1971). Gray snapper adults staging for migrations offshore during the spawning season have also been found to use estuarine passes close to the ocean (Luo et al. 2009).
Although several years of relatively high recruitment were evident among multiple estuarine systems, we did not detect strong spatial concordance in the relative strength of juvenile recruitment. While the Florida Gulf and Atlantic stocks differ genetically, they do not differ significantly between areas along each coast (Gold et al. 2009), so concordant coast-specific abundance patterns would be expected in years of similar environmental conditions. In the present study, there were a few cases in which a high recruitment year translated into peaks in subadult abundance, but a clear pattern was not evident across systems. Gray snapper have not been observed in large site-specific spawning aggregations, although it has been well documented that gray snapper occur in dense schools year-round and that large numbers of adults migrate offshore during the spawning season (Domeier et al. 1996; Domeier and Colin 1997). Because of this diffuse spawning pattern, concordance in recruitment strength across several estuaries would not be due to one particular spawning site but to favorable postsettlement conditions in that year. For example, several estuaries showed increased recruitment in 1999, possibly in response to decreased precipitation as a result of La Niña conditions (Abtew and Trimble 2010), which expanded the area exhibiting euhaline and polyhaline conditions suitable for gray snapper recruitment. In 2001, Florida was undergoing a major drought (Abtew et al. 2002), which also may have enhanced the survival of juveniles on the Gulf Coast. Similarly, Rutherford et al. (1989a) demonstrated an inverse relationship between gray snapper recruitment and water levels (indicative of rainfall) in Florida Bay. In 2005, the impacts of a major red tide event on fish populations in Tampa Bay and Sarasota Bay (Gannon et al. 2009; Flaherty and Landsberg 2011) may help explain the low recruitment in southwest Florida that year. The influence of red tide has been found to be species-specific (Flaherty and Landsberg 2011), however, so a more detailed investigation into the response of gray snapper to red tides is needed. The concordant juvenile peaks in abundance in 2005 (Atlantic) and 2006 (Gulf) did not relate directly to the large-scale environmental trends mentioned above, but a combination of other factors such as wind- and buoyancy-driven flow or changes in prevailing currents feeding individual estuaries may have contributed to the survival of a large recruitment class during these years (Epifanio and Garvine 2001). Continuing to collect these monitoring data over a large area is essential to the tracking of population trends over time and to increase our understanding of the correlation and interaction of various habitat and environmental factors with gray snapper recruitment and development within the estuary.
Although the relationship between juvenile and subadult abundance is variable, this does not necessarily rule out the use of juvenile peaks in forecasting recruitment into the offshore adult population. The inconsistency of correlations between juvenile and subadult abundance may be partially due to the limitations inherent in using traditional nets to sample structured habitats (e.g., bridges, limestone rock outcrops, and natural and artificial reefs). These structured habitats in the estuary and nearshore coastal areas have been under-represented in fishery-independent monitoring; thus, there is little long-term information on the relative importance of these habitats to reef species. In future studies, these habitats should be targeted for sampling to further elucidate the ontogeny of gray snapper. Information on the estuarine and nearshore occupancy of larger subadults and adults (legal-size fish) that are associated with these habitats will fill this gap in knowledge, improve indices of abundance, and add to our overall understanding of habitat use in gray snapper. The accurate estimation and prediction of juvenile recruitment into the offshore population is critical for effective assessment and management (Smith 1993; Koenig and Coleman 1998; Coleman et al. 1999).
In this study, we have determined where and when gray snapper recruit to several Florida estuarine systems and have identified specific patterns of habitat selection by estuary and size class. The timing of recruitment reported in this study complements findings of studies on larval ingress (Tzeng et al. 2003), reproductive activity (Domeier et al. 1996; Allman and Grimes 2002), and juvenile habitat use (Nagelkerken et al. 2000; Cocheret de la Morinière et al. 2002; Faunce and Serafy 2007). In future work, better understanding of the complexity of habitat use and the function of nursery habitats should be explored. Additional dietary studies will build on previous work (Franks and VanderKooy 2000; Cocheret de la Morinière et al. 2003), help identify why each habitat is important and, possibly, add insight into differential growth rates. Otolith microchemistry has been used to link juvenile gray snapper to particular nursery habitats (Allman and Grimes 2002; Lara et al. 2008; Patterson et al. 2009) and can be used to determine the source of adults that survive to join the offshore population. These additional methods, in conjunction with the findings in this study, will aid in determining which estuarine habitats or regions are essential to the long-term health of the population.
References
Abtew, W., and P. Trimble. 2010. El Niño–southern oscillation link to South Florida hydrology and water management applications. Water Resources Management 24: 4255–4271.
Abtew, W., R.S. Huebner, and S. Sunderland. 2002. Part I. Hydrological analysis of the 2000–2001 drought in South Florida. Technical Report EMA-405. West Palm Beach, FL: South Florida Water Management District.
Allman, R.J., and L.A. Goetz. 2009. Regional variation in the population structure of gray snapper, Lutjanus griseus, along the West Florida Shelf. Bulletin of Marine Science 84: 315–330.
Allman, R.J., and C. Grimes. 2002. Temporal and spatial dynamics of spawning, settlement, and growth of gray snapper (Lutjanus griseus) from the West Florida Shelf as determined from otolith microstructures. Fishery Bulletin 100: 391–403.
Ault, J.S., J.A. Bohnsack, and G.A. Meester. 1998. A retrospective (1979–1996) multispecies assessment of coral reef fish stocks in the Florida Keys. Fishery Bulletin 96: 395–414.
Ault, J.S., J.A. Bohnsack, S.G. Smith, and J. Luo. 2005a. Towards sustainable multispecies fisheries in the Florida, USA, coral reef ecosystem. Bulletin of Marine Science 76: 595–622.
Ault, J.S., S.G. Smith, and J.A. Bohnsack. 2005b. Evaluation of average length as an estimator of exploitation status for the Florida coral-reef fish community. ICES Journal of Marine Science 62: 417–423.
Ault, J.S., S.G. Smith, J.A. Bohnsack, J. Luo, D.E. Harper, and D.B. McClellan. 2006. Building sustainable fisheries in Florida's coral reef ecosystem: positive signs in the Dry Tortugas. Bulletin of Marine Science 78: 633–654.
Baltz, D.M. 1990. Autecology. In Methods for fish biology, ed. C.B. Schreck and P.B. Moyle, 585–607. Bethesda, Maryland: American Fisheries Society.
Bartels, C.T., and K.L. Ferguson. 2006. Preliminary observations of abundance and distribution of settlement-stage snappers in shallow, nearshore seagrass beds in the Middle Florida Keys. Proceedings of the Gulf and Caribbean Fisheries Institute 57: 235–248.
Bartholomew, A., and J.A. Bohnsack. 2005. A review of catch-and-release angling mortality with implications for no-take reserves. Reviews in Fish Biology and Fisheries 15: 129–154.
Bell, J.D., M. Westoby, and A.S. Steffe. 1987. Fish larvae settling in seagrass: Do they discriminate between beds of different leaf density? Journal of Marine Biology and Ecology 111: 133–144.
Bell, J.D., A.S. Steffe, and M. Westoby. 1988. Location of seagrass beds in estuaries: Effects on associated fish and decapods. Journal of Marine Biology and Ecology 122: 127–146.
Bloomfield, A., and B. Gillanders. 2005. Fish and invertebrate assemblages in seagrass, mangrove, saltmarsh, and nonvegetated habitats. Estuaries and Coasts 28(1): 63–77.
Briggs, J.C. 1958. A list of Florida fishes and their distribution. Bulletin of the Florida State Museum, Biological Sciences 2(8): 223–318.
Burns, J.W., A.D. Chapman, E. Messer, and J. Konwinski. 1997. Submerged aquatic vegetation of the lower St. Johns River. Palatka, FL: St. Johns River Water Management District Report.
Burton, M.L. 2001. Age, growth, and mortality of gray snapper, Lutjanus griseus, from the east coast of Florida. Fishery Bulletin 99: 254–265.
Carrió, E.G., C.R. Vazquez, and A.J. Sanchez. 2004. Trophic association in dominant fish species from Laguna de Terminos, Campeche. Proceedings of the Gulf and Caribbean Fisheries Institute 55: 1022.
Chester, A.J., and G.W. Thayer. 1990. Distribution of spotted seatrout (Cynoscion nebulosus) and gray snapper (Lutjanus griseus) juveniles in seagrass habitats of western Florida Bay. Bulletin of Marine Science 46: 345–357.
Cocheret de la Morinière, E., B.J. Pollux, I. Nagelkerken, and G. Van der Velde. 2002. Post-settlement life cycle migration patterns and habitat preference of coral reef fish that use seagrass and mangrove habitats as nurseries. Estuarine, Coastal and Shelf Science 55: 309–321.
Cocheret de la Morinière, E., B.J.A. Pollux, I. Nagelkerken, M.A. Hemminga, A.H.L. Huiskes, and G. Van der Velde. 2003. Ontogenetic dietary changes of coral reef fishes in the mangrove-seagrass-reef continuum: Stable isotopes and gut-content analysis. Marine Ecology Progress Series 246: 279–289.
Coleman, F.C., C.C. Koenig, and L.A. Collins. 1996. Reproductive styles of shallow-water groupers (Pisces: Serranidae) in the eastern Gulf of Mexico and the consequences of fishing spawning aggregations. Environmental Biology of Fishes 47: 129–141.
Coleman, F.C., C.C. Koenig, A.M. Eklund, and C.B. Grimes. 1999. Management and conservation of temperate reef fishes in the grouper–snapper complex of the southeastern United States. American Fisheries Society Symposium 23: 233–242.
Conover, D.O., and T.M.C. Present. 1990. Countergradient variation in growth rate: Compensation for length of the growing season among Atlantic silversides from different latitudes. Oecologia 83(3): 316–324.
Dame, R., M. Alber, D. Allen, M. Mallin, C. Montague, A. Lewitus, A. Chalmers, R. Gardner, C. Gilman, B. Kjerfve, J. Pinckney, and N. Smith. 2000. Estuaries of the South Atlantic Coast of North America: Their geographical signatures. Estuaries 23(6): 793–819.
Dawes, C.J., R.C. Phillips, and G. Morrison. 2004. Seagrass communities of the Gulf coast of Florida: Status and ecology. St. Petersburg, FL: Florida Fish and Wildlife Conservation Commission Fish and Wildlife Research Institute and the Tampa Bay Estuary Program.
de Mutsert, K., J.H. Cowan, T.E. Essington, and R. Hilborn. 2008. Reanalyses of Gulf of Mexico fisheries data: Landings can be misleading in assessments of fisheries and fisheries ecosystems. Proceedings of the National Academy of Sciences 105(7): 2740–2744.
Denit, K., and S. Sponaugle. 2004a. Growth of early stage gray snapper, Lutjanus griseus, across a latitudinal gradient. Proceedings of the Gulf and Caribbean Fisheries Institute 55: 839–843.
Denit, K., and S. Sponaugle. 2004b. Growth variation, settlement, and spawning of gray snapper across a latitudinal gradient. Transactions of the American Fisheries Society 133: 1339–1355.
Denit, K., and S. Sponaugle. 2004c. Local and latitudinal differences in growth during the early life history of gray snapper, Lutjanus griseus. Proceedings of the Gulf and Caribbean Fisheries Institute 55: 1019.
Domeier, M.L., and P.L. Colin. 1997. Tropical reef fish spawning aggregations: Defined and reviewed. Bulletin of Marine Science 60: 698–726.
Domeier, M. L., C. Koenig, and F. Coleman. 1996. Reproductive biology of the gray snapper (Lutjanus griseus), with notes on spawning for other western Atlantic snappers (Lutjanidae). In Biology and culture of tropical groupers and snappers, eds. F. Arreguín-Sánchez, J.L. Munro, M.C. Blagos, and D. Pauly, 189–201. ICLARM Conference Proceedings 48, Manila.
Eggleston, D.B., C.P. Dahlgren, and E.G. Johnson. 2004. Fish density, diversity, and size-structure within multiple back reef habitats of Key West National Wildlife Refuge. Bulletin of Marine Science 75: 175–204.
Epifanio, C.E., and R.W. Garvine. 2001. Larval transport on the Atlantic continental shelf of North America: A review. Estuarine, Coastal and Shelf Science 52: 51–77.
Faunce, C.H., and J. Serafy. 2007. Nearshore habitat use by gray snapper (Lutjanus griseus) and bluestriped grunt (Haemulon sciurus): Environmental gradients and ontogenetic shifts. Bulletin of Marine Science 80: 473–495.
Faunce, C.H., and J.E. Serafy. 2008. Selective use of mangrove shorelines by snappers, grunts, and great barracuda. Marine Ecology Progress Series 356: 153–162.
Faunce, C.H., J.J. Lorenz, J.A. Ley, and J.E. Serafy. 2002. Size structure of gray snapper (Lutjanus griseus) within a mangrove “no-take” sanctuary. Bulletin of Marine Science 70: 211–216.
Fish and Wildlife Research Institute (FWRI). 2010. Status and trends of Florida nearshore and inshore fishes—species account, gray snapper. http://myfwc.com/media/195570/gray_snapper.pdf.
Flaherty, K.E., and J.H. Landsberg. 2011. Effects of a persistent red tide (Karenia brevis) bloom on community structure and species-specific relative abundance of nekton in a Gulf of Mexico estuary. Estuaries and Coasts 34: 417–439.
Flaherty, K.E., and R.E. Matheson Jr. 2008. Fish communities associated with seagrass beds in Tampa Bay of differing architecture, species composition and varying degrees of freshwater influence. State Wildlife Grant 6317, Final Report.
Flaherty, K.E., R.E. Matheson Jr., F.X. Courtney, and R.F. Jones. 2010. Nekton communities associated with seagrass in Tampa Bay: the effects of seagrass bed architecture, seagrass species composition, and varying degrees of freshwater influence. In Proceedings, Tampa Bay Area Scientific Information Symposium, BASIS 5, 20–23October 2009, St. Petersburg, FL, ed. S.T. Cooper, 275–298.
Franks, J.S., and K.E. VanderKooy. 2000. Feeding habits of juvenile lane snapper Lutjanus synagris from Mississippi coastal waters, with comments on the diet of gray snapper Lutjanus griseus. Gulf and Caribbean Research 12: 11–17.
Galperin, B., A.F. Blumberg, and R.H. Weisberg. 1991. A time-dependent three-dimensional model of circulation in Tampa Bay. In Tampa Bay Area Scientific Information Symposium, ed. S.F. Treat and P.A. Clark, 77–97. Tampa, FL: Tampa Bay Regional Planning Council.
Gannon, D.P., E.J. Berens-McCabe, S.A. Camilleri, J.G. Gannon, M.K. Brueggen, A.A. Barleycorn, V.I. Palubok, G.J. Kirkpatrick, and D.R.S. Wells. 2009. Effects of Karenia brevis harmful algal blooms on nearshore fish communities in Southwest Florida. Marine Ecology Progress Series 378: 171–186.
Gilmore, G.R. 1995. Environmental and biogeographic factors influencing ichthyofaunal diversity: Indian River Lagoon. Bulletin of Marine Science 57: 153–170.
Gold, J.R., E. Saillant, N.D. Ebelt, and S. Lem. 2009. Conservation genetics of gray snapper (Lutjanus griseus) in US waters of the northern Gulf of Mexico and western Atlantic Ocean. Copeia 2009(2): 277–286.
Handley, L., D. Altsman, and R. DeMay. 2007. Seagrass status and trends in the northern Gulf of Mexico: 1940–2002. US Geological Survey Scientific Investigations Report 2006–5287 and US Environmental Protection Agency 855-R-04-003.
Heck Jr., K.L., G. Hays, and R.J. Orth. 2003. Critical evaluation of the nursery role hypothesis for seagrass meadows. Marine Ecology Progress Series 253: 123–136.
Hettler, W.F. 1989. Food habits of juveniles of spotted seatrout and gray snapper in western Florida Bay. Bulletin of Marine Science 44(1): 155–162.
Institute Inc, S.A.S. 2006. Base SAS® 9.1.3 procedures guide, 2nd ed., Volumes 1–4. Cary: SAS Institute Inc.
Jelbart, J.E., P.M. Ross, and R.M. Connolly. 2007. Patterns of small fish distributions in seagrass beds in a temperate Australian estuary. Journal of the Marine Biological Association of the United Kingdom 87: 1297–1307.
Koenig, C.C., and F.C. Coleman. 1998. Absolute abundance and survival of juvenile gags in seagrass beds of the northeastern Gulf of Mexico. Transactions of the American Fisheries Society 127: 44–55.
Kritzer, J.P. 2002. Variation in the population biology of stripey bass Lutjanus carponotatus within and between two island groups on the Great Barrier Reef. Marine Ecology Progress Series 243: 191–207.
Lara, M.R., D.L. Jones, Z. Chen, J.T. Lamkin, and C.M. Jones. 2008. Spatial variation of otolith elemental signatures among juvenile gray snapper (Lutjanus griseus) inhabiting southern Florida waters. Marine Biology 153(3): 235–248.
Lindberg, W.J., T.M. Bert, and G.P. Genoni. 1992. Alternative hypotheses for low landings in the Cedar Key stone crab (genus Menippe) fishery, 1984–85. Florida Marine Research Publications 50: 50–57.
Livingston, R.J. 1983. Resource atlas of the Apalachicola estuary. Gainesville, FL: Florida Sea Grant College Program Report 55.
Luo, J., J.E. Serafy, S. Sponaugle, P.B. Teare, and D. Kieckbusch. 2009. Movement of gray snapper Lutjanus griseus among subtropical seagrass, mangrove, and coral reef habitats. Marine Ecology Progress Series 380: 255–269.
Manooch III, C.S., and R.H. Matheson III. 1981. Age, growth and mortality of gray snapper collected from Florida waters. Proceedings of the Southeastern Association of Fish and Wildlife Agencies 35: 331–344.
McEachran, J.D., and J.D. Fechhelm. 1998. Fishes of the Gulf of Mexico. Volume 1: Myxiniformes to Gasterosteiformes. Austin: University of Texas Press.
Muller, R.G., and R.G. Taylor. 2006. The 2005 stock assessment update of common snook, Centropomus undecimalis—final. IHR 2006–003. St. Petersburg, FL: Fish and Wildlife Research Institute.
Murphy, M.D., and J. Munyandorero. 2009. An assessment of the status of red drum in Florida waters through 2007. St. Petersburg: Fish and Wildlife Research Institute.
Myers, R.A., and N.G. Cadigan. 1993. Is juvenile mortality in marine demersal fish variable? Canadian Journal of Fisheries and Aquatic Sciences 50: 1591–1598.
Nagelkerken, I., G. van der Velde, M.W. Gorissen, G.J. Meijer, T. van't Hof, and C. den Hartog. 2000. Importance of mangroves, seagrass beds and the shallow coral reef as a nursery for important coral reef fishes: Using a visual census technique. Estuarine, Coastal and Shelf Science 51: 31–44.
Orth, R.J., K.L. Heck Jr., and J. van Montfrans. 1984. Faunal communities in seagrass beds: A review of the influence of plant structure and prey characteristics on predator–prey relationships. Estuaries 7: 339–350.
Paperno, R., and R.B. Brodie. 2004. Effects of environmental variables upon the spatial and temporal structure of a fish community in a small, freshwater tributary of the Indian River Lagoon, Florida. Estuarine, Coastal and Shelf Science 61(2): 229–241.
Patterson, III, W.F., C.A. Lounder, R.J. Allman, and R.S. McBride. 2009. Recruitment dynamics and population connectivity of gray snapper, Lutjanus griseus, among West Florida estuarine systems. Final Report. Gainesville, FL: Florida Sea Grant College Program R/LR-B-59.
Phillips, R.C. 1960. Observations on the ecology and distribution of the Florida seagrasses. St. Petersburg: Florida State Board of Conservation Marine Laboratory.
Raposa, K.B., and C.A. Oviatt. 2000. The influence of contiguous shoreline type, distance from shore, and vegetation biomass on nekton community structure in eelgrass beds. Estuaries 23: 46–55.
Robbins, B.D., and S.S. Bell. 1994. Seagrass landscapes: A terrestrial approach to the marine subtidal environment. Trends in Ecology & Evolution 9(8): 301–304.
Rozas, L., T. Minello, and D. Dantin. 2012. Use of shallow lagoon habitats by nekton of the northeastern Gulf of Mexico. Estuaries and Coasts 35(2): 572–586.
Rutherford, E.S., T.W. Schmidt, E.B. Thue, and J.T. Tilmant. 1989a. Fishery harvest and population dynamics of gray snapper, Lutjanus griseus, in Florida Bay and adjacent waters. Bulletin of Marine Science 44: 139–154.
Rutherford, E.S., T.W. Schmidt, and J.T. Tilmant. 1989b. Early life history of spotted seatrout (Cynoscion nebulosus) and gray snapper (Lutjanus griseus) in Florida Bay, Everglades National Park, Florida. Bulletin of Marine Science 44: 49–64.
Rypel, A.L., and C.A. Layman. 2008. Degree of aquatic ecosystem fragmentation predicts population characteristics of gray snapper (Lutjanus griseus) in Caribbean tidal creeks. Canadian Journal of Fisheries and Aquatic Sciences 65: 335–339.
Schmidt, N., and M.E. Luther. 2002. ENSO impacts on salinity in Tampa Bay, Florida. Estuaries 25: 976–984.
Serafy, J.E., K.C. Lindeman, T.E. Hopkins, and J.S. Ault. 1997. Effects of freshwater canal discharge on fish assemblages in a subtropical bay: Field and laboratory observations. Marine Ecology Progress Series 160: 161–172.
Serafy, J.E., C.H. Faunce, and J.J. Lorenz. 2003. Mangrove shoreline fishes of Biscayne Bay, Florida. Bulletin of Marine Science 72: 161–180.
Serafy, J.E., M. Valle, C.H. Faunce, and J. Luo. 2007. Species-specific patterns of fish abundance and size along a subtropical mangrove shoreline: An application of the delta approach. Bulletin of Marine Science 80: 609–624.
Serrano, X.M. 2008. Ecophysiology of the gray snapper Lutjanus griseus: salinity effects on abundance, physiology and behavior. M.S. Thesis, University of Miami, Miami.
Sheng, P.S. 1998. Circulation in the Charlotte Harbor estuarine system. In Proceedings of the Charlotte Harbor Public Conference and Technical Symposium, ed. S.F. Treat, 99–110. South Florida Water Management District and Charlotte Harbor National Estuary Program, West Palm Beach and North Fort Myers, Florida.
Sherrod, C.L., and C. McMillan. 1985. The distributional history and ecology of mangrove vegetation along the northern Gulf of Mexico coastal region. Contributions in Marine Science 28: 129–140.
Sime, P. 2005. St. Lucie Estuary and Indian River Lagoon conceptual ecological model. Wetlands 25(4): 898–907.
Smith, G.B. 1976. Ecology and distribution of eastern Gulf of Mexico reef fishes. Florida Marine Research Publications 19: 1–78.
Smith, P.E. 1993. Balancing sampling precision and fisheries management objectives: Minimal methods. Bulletin of Marine Science 53(2): 930–935.
Sogard, S.M. 1997. Size-selective mortality in the juvenile stage of teleost fishes: A review. Bulletin of Marine Science 60(3): 1129–1157.
Starck II, W.A., and R.E. Schroeder (eds.). 1971. Investigations on the gray snapper, Lutjanus griseus. Studies in Tropical Oceanography No. 10. Coral Gables, FL: University of Miami Press.
Suthard, B.C., A.C. Hine, S.D. Locker, D.S. Duncan, and R.A. Morton. 2010. A siliciclastic-filled sedimentary basin in a mid-carbonate platform setting, Tampa Bay, Florida. In Proceedings, Tampa Bay Area Scientific Information Symposium, BASIS 5, 20–23 October 2009. St. Petersburg, FL, ed. S.T. Cooper, 35.
Switzer, T.S., B. Mahmoudi, B.L. Winner, K.E. Flaherty, and S.F. Keenan. 2011. Improved indices of juvenile and pre-fishery abundance for gray snapper, gag, and other estuarine-dependent reef fishes along the Gulf coast of Florida. NOAA/NMFS Marine Fisheries Initiative (MARFIN) Final Report (Grant # NA09NMF4330152), submitted October 29, 2011.
Thayer, G.W., D.R. Colby, and W.F. Hettler Jr. 1987. Utilization of the red mangrove prop root habitat by fishes in South Florida. Marine Ecology Progress Series 35: 25–38.
Tzeng, M.W., J.A. Hare, and D.G. Lindquist. 2003. Ingress of transformation stage gray snapper, Lutjanus griseus (Pisces: Lutjanidae) through Beaufort Inlet, North Carolina. Bulletin of Marine Science 72: 891–908.
Valentine-Rose, L., C.A. Layman, D.A. Arrington, and A.L. Rypel. 2007. Habitat fragmentation decreases fish secondary production in Bahamian tidal creeks. Bulletin of Marine Science 80: 863–877.
Verweij, M.C., I. Nagelkerken, D. de Graaff, M. Peeters, E.J. Bakker, and G. van der Velde. 2006. Structure, food and shade attract juvenile coral reef fish to mangrove and seagrass habitats: A field experiment. Marine Ecology Progress Series 306: 257–268.
Warlen, S.M., P.A. Tester, and D.R. Colby. 1998. Recruitment of larval fishes into a North Carolina estuary during a bloom of the red tide dinoflagellate, Gymnodinium breve. Bulletin of Marine Science 63: 83–95.
Whaley, S.D., J.J. Burd Jr., and B.A. Robertson. 2007. Using estuarine landscape structure to model distribution patterns in nekton communities and in juveniles of fishery species. Marine Ecology Progress Series 330: 83–99.
Wuenschel, M.J., A.R. Jugovich, and J.A. Hare. 2004. Effect of temperature and salinity on the energetics of juvenile gray snapper (Lutjanus griseus): Implications for nursery habitat value. Journal of Experimental Marine Biology and Ecology 312: 333–347.
Yáñez-Arancibia, A., A. Lara-Domínguez, and J. Day. 1993. Interactions between mangrove and seagrass habitats mediated by estuarine nekton assemblages: Coupling of primary and secondary production. Hydrobiologia 264(1): 1–12.
Acknowledgments
First and foremost, we acknowledge the countless FWC personnel and volunteers who have assisted with the collection and processing of data since the inception of this survey. Special thanks to G. McLaughlin, A. Tyler-Jedlund, and J. Davis for logistical support and leadership in the field portion of the Marine Fisheries Initiative (MARFIN) grant that helped support this research. Thanks to K. Fischer, D. Leffler, S. Parks, R. Paperno, J. Ley, B. Crowder, and two anonymous reviewers for providing feedback and useful editorial comments that greatly improved the quality of this manuscript, and to R. McMichael Jr., for his support and assistance. This project was supported, in part, by proceeds from State of Florida saltwater recreational fishing licenses, by funding from the Department of the Interior, US Fish and Wildlife Service, Federal Aid for Sportfish Restoration Project Number F-43, and from the Department of Commerce, National Oceanographic and Atmospheric Administration, National Marine Fisheries Service, MARFIN grant (# NA09NMF4330152). The statements, findings, views, conclusions, and recommendations contained in this document are those of the authors and do not necessarily reflect the views of the US Department of Interior or Commerce and should not be interpreted as representing the opinions or policies of the US government. Mention of trade names or commercial products does not constitute their endorsement by the US government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flaherty, K.E., Switzer, T.S., Winner, B.L. et al. Regional Correspondence in Habitat Occupancy by Gray Snapper (Lutjanus griseus) in Estuaries of the Southeastern United States. Estuaries and Coasts 37, 206–228 (2014). https://doi.org/10.1007/s12237-013-9652-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-013-9652-x