Abstract
Annual mean salinity, light availability, and sediment depth to bedrock structured the submerged aquatic vegetation (SAV) communities in subtropical mangrove-lined estuaries. Three distinct SAV communities (i.e., Chara group, Halodule group, and Low SAV coverage group) were identified along the Everglades–Florida Bay ecotone and related to water quality using a discriminant function model that predicted the type of plant community at a given site from salinity, light availability, and sediment depth to bedrock. Mean salinity alone was able to correctly classify 78% of the sites and reliably separated the Chara group from the Halodule group. The addition of light availability and sediment depth to bedrock increased model accuracy to 90% and further distinguished the Chara group from the Halodule group. Light availability was uniquely valuable in separating the Chara group from the Low SAV coverage group. Regression analyses identified significant relationships between phosphorus concentration, phytoplankton abundance, and light availability and suggest that a decline in water transparency, associated with increasing salinity, may have also contributed to the historical decline of Chara communities in the region. This investigation applies relationships between environmental variables and SAV distribution and provides a case study into the application of these general principals to ecosystem management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Through the formation of submerged aquatic vegetation (SAV) beds, benthic macrophytes provide the necessary structural habitats and nursery areas for aquatic fauna (see Heck et al. 2003 for review), stabilize sediments (Scoffin 1970; Gacia and Duarte 2001), maintain low water column nutrient concentrations (Kufel and Kufel 2002; Knight et al. 2003), and increase water clarity (Blindlow et al. 2002). Additionally, the high productivity of SAV beds supports complex aquatic food webs (Fry and Parker 1979; Moncrieff et al. 1992) and, in shallow areas, also provides critical seasonal energy sources for migratory waterfowl (Noordhuis et al. 2002; Michot et al. 2008). Many aquatic and estuarine ecosystems, once characterized by SAV abundance and a clear water column, have experienced regime shifts or changes in stable state to ecosystems characterized by phytoplankton dominance and increased turbidity (Orth and Moore 1983; Scheffer et al. 1993). Regime shifts have been attributed to species invasions (MacIsaac et al. 1992), salinity changes (Petersen et al. 2008), and changes in nutrient availability (Scheffer and van Nes 2007), though in many cases, the mechanisms are still unknown. For the conservation of the valuable ecosystem services provided by benthic macrophytes, an increased understanding of the environmental constraints on SAV abundance and species distributions is still needed. Successful restoration of these ecosystems will also require an understanding of the underlying mechanisms of ecosystem regime changes.
A change in light availability is one of the key features of regime shifts in shallow estuaries and lakes because adequate light availability is critical for the establishment and maintenance of SAV communities. Minimum light requirements range from <1% of in-water surface irradiance for most macroalgae to >10% of in-water surface irradiance for sea grasses (Zimmerman 2006). Light penetration through water columns decrease with depth. In shallow environments, light attenuation by resuspended sediments, phytoplankton, and colored dissolved substances may be more important to SAV distribution than water depth (Hemminga and Duarte 2000). Phytoplankton competes with benthic macrophytes for light. Elevated water column nutrients may promote phytoplankton abundance, thereby decreasing light reaching the benthos and therefore decreasing SAV coverage, and once SAV coverage declines, nutrients and sediments in the water column are no longer removed by the benthos (Duarte 1995).
Within estuaries, the relationships between benthic macrophyte species distributions and light availability and nutrients are complex and confounded by interacting relationships with mean salinity and salinity variability. Upstream freshwater SAV communities grade into downstream marine communities as salinity increases. The distributions of individual species along an estuarine gradient often reflect salinity tolerances (McMillan and Moseley 1967). In estuarine areas where freshwater flows are highly seasonal or erratic, benthic macrophyte distribution may be more reflective of salinity variability (Montague and Ley 1993). SAV species composition in estuarine areas of variable salinities may be determined by species’ abilities to tolerate a wide range of salinities. Variable conditions may favor species with high fecundity, dormant seeds or spores that can withstand suboptimal salinity conditions, and large dispersal distances. SAV species composition is also dependent upon substrate type, with most sea grasses requiring sand or mud sediments as opposed to many macroalgal species that require hard substrates for attachment.
Spatially and temporally variable salinities, nutrient concentrations, and underwater light environments are characteristic of estuaries, especially in tropical and subtropical estuaries that have pronounced climate seasonality (Herrera-Silveira 1994; Boyer 2006). Climate change and sea level rise are expected to influence salinity, water depth, and nutrient availability in these transition zones between the marine and freshwater environments (Wanless et al. 1994). In addition, humans alter the delivery of freshwater, sediments, and nutrients to estuaries.
As a case study, the extensive engineering of freshwater flows in the upstream Everglades ecosystem starting around the beginning of the twentieth century, but accelerating in the 1950s and 1960s, has also affected the amount and timing of the delivery of freshwater and nutrients to these transition zones (Light and Dineen, 1994). The mangrove estuaries and brackish “lakes” located along the ecotone between freshwater Florida Everglades and saltwater Florida Bay have changed during the last century. Extensive SAV beds, supporting large populations of wading birds and wintering waterfowl, once characterized these areas (Tabb et al. 1962; Craighead 1971; Ogden 1994; Simmons and Ogden 1998). Present SAV, waterfowl and wading bird abundances are all greatly reduced from historical (1931–1946) levels (Ogden 1994). These reductions are coincident with increasing salinities in this region caused by diminished freshwater inflows due to water management practices (Light and Dineen 1994). With a management goal of restoring historic SAV abundances, increased deliveries of freshwater to these mangrove estuaries are proposed to maintain lower salinities as part of the Comprehensive Everglades Restoration Plan. Increased delivery of freshwater will likely change water quality (i.e., salinities, nutrient concentrations, and the underwater light environments). The ability to predict how SAV communities may change as freshwater flows are restored is built upon understanding the present relationships between water quality and SAV. The focus of this investigation was to describe SAV distribution and abundance and its relationships with salinity climate, sediment depths to bedrock, nutrient availability, and underwater light availability. We were particularly interested in determining potential triggers for regime shift from clear water, SAV-dominated, to turbid, phytoplankton-dominated states. We also investigated the relationship between underwater light availability, water column nutrient concentration, and phytoplankton abundance. Our results contribute toward predicting how the SAV communities will respond to proposed restoration of freshwater flow.
Study Location
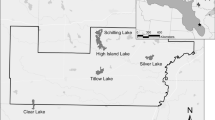
The geographic foci of this investigation are the estuarine mangrove lakes and coastal embayments located along the north shore of Florida Bay (Fig. 1). The 41 study sites are located along two freshwater flow paths that drain portions of the southern Everglades. The West Lake drainage system runs from West Lake through Long Lake (formerly East Lake) to “The Lungs” and finally out into central Florida Bay at Garfield Bight. Long, tenuous, mangrove creeks (Mangrove Creek and Alligator Creek), with small, shallow (<50 cm) ponds within them, effectively separate the lakes and embayments and limit water exchange. In contrast, well-connected lakes running from Seven Palm Lake to Middle Lake to Monroe Lake and out into Florida Bay via Terrapin Bay characterize the Seven Palm drainage system, located to the adjacent east. The watershed that drains into these flow paths lies between the two main conduits for Everglades drainage—Shark Slough to the northwest and Taylor Slough to the northeast. Surface water flows either into or out of these mangrove-lined lakes depending on wind direction, Everglades water levels, and Florida Bay sea level. Deeper areas within the most upstream lakes (West Lake and Seven Palm Lake) had exposed bedrock outcroppings with pockets of mud and sand-sized sediments, while shallow sites and those in the other water bodies had mud sediments.
Materials and Methods
The abundances of benthic macrophytes and water quality parameters were measured at 41 study sites (Fig. 1) from May 2006 through April 2009. These sites were chosen from historic site locations that were previously sampled in short-term SAV monitoring studies (Morrison and Bean 1997; Richards and Fourqurean 2000). Benthic macrophyte abundance at each site was surveyed by snorkeler at approximate 3-month intervals over the study period, a total of 12 times. Mean percent bottom coverage (n = 15 surveys) for each species present was estimated using a 1/4 m2 quadrat. Bottom coverage was estimated to the nearest 5%. During each sampling, the quadrat was arbitrarily placed on the benthos at 15 locations within a 30-m radius of the study site as located using GPS. Sediment depth to bedrock was also measured for each site.
Salinity, water depth (Z), and water transparency (Secchi depth, Z SD) were measured at each site at approximate monthly intervals except when low water levels prevented access by boat. Surface salinity was measured using a combination temperature–conductivity–salinity probe (YSI model 30). Sediment depth to bedrock was also measured at each site. Water and sediment depths to bedrock were measured to the nearest centimeter using a graduated PVC pipe. Water transparency was measured to the nearest 5 cm using a 20-cm diameter Secchi disc. Underwater light availability to benthic macrophytes, expressed as percent light at bottom, was estimated from Secchi depth and water depth measurements using the approximate relations of Holmes (1970) and Kirk (1994), respectively:
where K d is the vertical attenuation coefficient for downwards irradiance of photosynthetically active radiation. Estimates of the percent light at bottom should be considered approximate because the relationship between K d and Secchi depth has been shown to vary (Preisendorfer 1986) especially in more turbid waters where the relation K d = 1.44/Z SD would overestimate K d (Kirk 1994).
Additionally, water column total nitrogen (TN) concentrations, total phosphorus (TP) concentrations, and phytoplankton abundances as water column chlorophyll-a (chl a) were determined from water samples collected at selected sites within each water body (sites 2, 8, 13, 17, 28, 30, 35, and 40; see Fig. 1). Sampling was performed at the same times these sites were sampled for salinity, water depth, and water transparency. TN was measured by high-temperature combustion using an ANTEK 7000N nitrogen analyzer. TP was determined using a dry-ashing, acid hydrolysis technique (Solórzano and Sharp 1980). Chl a concentrations were determined by filtering water samples (25-mm glass fiber GF/F) and extracting the pigment from the filter using 90% acetone. Extracts were analyzed for chl a using a Shimadzu RF-Mini 150 fluorometer equipped with low (10 nm) half-bandwidth filters (excitation = 439, emission = 671) to maximize sensitivity to chl a and minimize interference from phytin a (Welschmeyer 1994). The fluorometer was calibrated with a chl a standard (Sigma-Aldrich) whose concentration was established using a Shimadzu UV Mini 1240 spectrophotometer and the spectrophotometric equations of Jeffrey and Humphrey (1975).
Statistical Analysis
A matrix of Bray–Curtis similarities was constructed from mean SAV species coverages at each site (n = 41) calculated from all sampling events conducted over the study period (n = 12, May 2006–April 2009). To decrease the importance of the rarest species, which are “prone to random and uninterpretable fluctuations” (Clarke and Warwick 2001) and whose occurrence in a particular study plot may be due largely to chance (McCune et al. 2002), the rarest species were removed from the matrix. Only species that had a mean abundance of at least 1%, at any of the 41 sites, were included in the cluster analysis. Hierarchical cluster analysis (McCune et al. 2002) using group average linkage and the calculated similarities was performed to identify natural groups of sites (CLUSTER, Primer-E® version 6.1.5 software). The similarity profile test (Clarke and Gorley 2006) was used to identify significant a posteriori differences in multivariate structure between the identified clusters (SIMPROF, Primer-E® version 6.1.5 software).
Sequential (hierarchical) discriminant analysis (Tabachnick and Fidell 2007) was used to construct discriminant functions that would predict the occurrences of the SAV community groups identified by the cluster analysis as a function of mean salinity, mean sediment depth to bedrock, and median light availability variables calculated from all sampling events (DISCRIMINANT, SPSS version 11.01). Because mean salinity is a targeted performance measure for Florida Bay ecosystem restoration, it was entered first into the discriminant analysis. Mean sediment depth to bedrock and median light availability were entered simultaneously into the discriminant analysis as an additional step. The validity of the additional step in the discriminant analysis was tested using the binomial test for model improvement (Zar 1999). Site classification accuracy was assessed by cross-validation for each of the 41 sites. The discriminant functions were interpreted by examining the correlations between discriminating predictor variables and the discriminant functions. Contrasts of SAV group means were used to assess the contribution of the individual predictor variables to the separation of identified SAV groups after adjustment for the other predictors (covariates; GLM, SPSS version 11.01). Type I error rate was adjusted for the number of contrasts using the Bonferroni adjustment (Tabachnick and Fidell 2007).
Linear and nonlinear regression analyses (Zar 1999) were performed using water column TN, TP, and chl a concentrations to identify possible relationships between Secchi depth, light availability, phytoplankton abundance, and nutrient concentrations (REGRESSION, Sigma-Plot version 9.01). Because it is not possible to infer Secchi depths that exceed water depths, only Secchi depths less than water depths were used in regression analyses.
Results
Four angiosperms (Halodule wrightii Ascherson, Ruppia maritima Linnaeus, Thalassia testudinum Banks ex König, and Rhizophora mangle Linnaeus) and twelve macroalgae (Chara hornemannii Wallman, Batophora oerstedii Agardh, Acetabularia sp., Penicillus capitatus Lamarck, Udotea sp., Halimeda incrassata (Ellis) Lamouroux, Caulerpa prolifera (Forsskål) Lamouroux, Sargassum pteropleuron Grunow, Laurencia intricata Lamouroux, Polysiphonia sp., and Chondria sp.) were observed in sample quadrats during the SAV surveys (May 2006–April 2009). Of the 15 species, only five (H. wrightii, R. maritima, C. hornemannii, B. oerstedii, and Acetabularia sp.) exhibited mean percent bottom coverage (n = 12 sampling times) exceeding 1% at a minimum of one site and were included in the cluster and similarity profile analyses.
The cluster analysis and similarity profile test identified three significant (P < 0.05) benthic communities defined by species composition and percent bottom cover (Fig. 2). The largest group contained 22 sites and was characterized by the dominance of Chara (33% mean bottom cover) within the SAV community (Fig. 2). Also present within the Chara community at mean percent bottom cover >1% was Batophora (2%) which occurred both as an epiphyte and attached to exposed bedrock. The second largest group contained 15 sites and was characterized by the dominance of Halodule within the SAV community (Fig. 2). Batophora was also present in the Halodule community (6% mean bottom cover) where it was attached epiphytically or to hard substrates (e.g., shell fragments). The third group contained four sites and was characterized by very low SAV coverage (<1%, Fig. 2).
A spatial pattern was evident in the distribution of benthic community groups (Fig. 3). The Chara communities were found exclusively within the upstream “lakes” (i.e., West Lake, Long Lake, The Lungs, Seven Palm Lake, Middle Lake, and Monroe Lake), while the Halodule communities were found predominantly in the Florida Bay coastal embayments (Fig. 3). The Low SAV coverage communities were found exclusively in the West Lake drainage in western West Lake and eastern Long Lake (Fig. 3). Ruppia and Acetabularia occurred sporadically and at low mean percent bottom coverages throughout the study area, with Ruppia occurring on mud substrates and Acetabularia attaching to exposed bedrock (Seven Palm Lake) or shell fragments.
Water quality characteristics were variable at the study sites (Table 1). Mean annual salinities ranged from 14.1 at site 4 in Seven Palm Lake to 35.1 at site 21 in Terrapin Bay. Mean annual salinities increased from the Chara communities in the upstream locations (Seven Palm Lake and West Lake) to the Halodule communities in the downstream coastal embayments (Terrapin Bay and Garfield Bight; Table 1 and Fig. 4a). Median light availability (percent light at bottom) ranged from 1% at sites 27 and 28 in West Lake to >24% (24% is the maximum possible estimate using Secchi disc measurements) at most sites in the Seven Palm Lake drainage system (Table 1). Median light availabilities were lowest at sites within the Low SAV coverage group (Fig. 4b) and were generally lower in West Lake, Long Lake, and The Lungs (sites 22–35, median light availability range = 2–12%) compared to the rest of the study area (sites 1–21, 36–41, median light availability range = 9–>24%; Table 1). Low water transparencies (Secchi depths) during persistent phytoplankton blooms contributed to low light availabilities in West Lake, Long Lake, and The Lungs (Table 1). Light availabilities and Secchi depths declined with increasing phytoplankton abundance (Fig. 5a, b). The censored Secchi depth data did not bias these relationships because there was no significant correlation between phytoplankton abundance (chlorophyll a) and water depths at the sites (Pearson r = 0.026, P = 0.77).
Descriptive statistics of water column salinity (a), light availability (percent light at bottom) (b), and sediment depth to bedrock (c) of the identified SAV community groups. Halodule group: n = 15; Chara group: n = 22; Low SAV coverage group: n = 4. Boxes indicate 25th and 75th percentiles, error bars indicate 10th and 90th percentiles, vertical lines within boxes indicate median values, and dots are outliers
Fitted relationships of Secchi depth to phytoplankton abundance (water column chl a concentration) (a) and light availability (percent of light at bottom) to phytoplankton abundance (water column chl a concentration) (b). Regression equation in Fig. 5a is the inverse relation Z SD = a/(k w + k chl × chlorophyll concentration), where ZSD is Secchi depth, a is the ratio estimate of the light attenuation coefficient (K d) to Secchi depth (Z SD) (Holmes 1970), k w is the light attenuation coefficient for water and other attenuating constituents, and k chl is the light attenuation chlorophyll proportionality constant. Regression equation in Fig. 5b is an unconstrained two-parameter exponential decay equation. Data points are individual sampling events conducted over the study period (May 2006–April 2009) and are coded by drainage system location
Sediment depths to bedrock were spatially variable (Table 1), generally increasing from upstream “lakes” to the coastal embayments, and following the transition from upstream Chara communities to downstream Halodule communities (Fig. 4c). Sediment depths to bedrock ranged from zero in areas of exposed bedrock in Seven Palm Lake to depths exceeding 1 m at most sites in Terrapin Bay and all sites in Garfield Bight (Table 1). Mean water depths ranged from 44 cm at site 38 in Garfield Bight to 157 cm at site 3 in Seven Palm Lake (Table 1). Deeper sites were generally in the middle of the most upstream lakes (Seven Palm Lake and West Lake), while the shallowest sites were located in Garfield Bight (Table 1).
Mean phytoplankton abundances, as estimated by chl a concentrations, ranged from 2.6 μg L−1 in Middle Lake to 31.5 μg L−1 in West Lake, with large differences between the West Lake and Seven Palm Lake drainage systems. Mean chl a concentrations at sites in the West Lake drainage system (range = 11.3–31.5 μg L−1) were approximately an order of magnitude greater than those at sites in the Seven Palm Lake drainage system (range = 2.6–3.8 μg L−1). West Lake exhibited the highest mean chl a concentration (31.5 μg L−1). Linear regression revealed a significant relationship (Pearson r = 0.77, P < 0.001) between log phytoplankton abundance (chl a) and log water column TP (Fig. 6).
The spatial variations in mean water column nutrient concentrations were similar to the spatial variation of chl a concentration, with much higher concentrations in the West Lake drainage system than in the Seven Palm Lake drainage system. Mean TN concentrations in the West Lake system ranged from 81 to 105 μM, while in the Seven Palm Lake system, the range was 51–57 μM. Mean TP concentrations in the West Lake system ranged from 1.82 to 2.86 μM, while in the Seven Palm Lake system, the range was 0.67–0.83 μM. Mean N/P (TN to TP) ratios in the water column, ranging from 65 to 85 in the Seven Palm Lake system and from 37 to 44 in the West Lake system, are much higher than the Redfield ratio (16) and indicate phosphorus (P)-limiting conditions throughout, but also more severe limitation in the Seven Palm Lake system.
The constructed discriminant function models were limited to a maximum of three predictor variables because the total number of predictor variables must be less than the minimum number of cases (sites) in any particular SAV group to avoid overfitting (Tabachnick and Fidell 2007). A discriminant function model with only mean salinity as a predictor variable was able to correctly classify 78% of the sites according to SAV community group, much greater than the 43% by expected by chance based on prior probabilities. The addition of mean sediment depth to bedrock and median percent light at bottom improved the final model (P ≤ 0.10, binomial test; Zar 1999) and increased classification accuracy to 90%. The final model was able to differentiate the SAV community groups along two dimensions (i.e., discriminant functions). The discriminant functions are separate linear combinations of the three predictor variables. The final model was accurate in predicting the occurrence of all three SAV groups (i.e., Halodule—100% accuracy, Chara—86% accuracy, and Low SAV coverage—75% accuracy; Fig. 7a, b). Only four of the 41 sites were misclassified, with two Chara sites misclassified as Low SAV coverage sites (sites 26 and 28), one Low SAV coverage site misclassified as a Chara site (site 30), and one Chara site misclassified as a Halodule site (site 32).
Discriminant function site scores generated using sediment depth to bedrock, light availability, and salinity predictor variables to distinguish among the identified SAV community groups. Site scores coded by actual group membership identified using cluster analysis (a). Site scores coded by predicted group membership using the discriminant function model (b)
The three predictor variables were correlated with the discriminant functions (Table 2). Median light availability was positively correlated with both discriminant functions, exhibiting its greatest correlation with discriminant function 2. Mean sediment depth to bedrock was only positively correlated with discriminant function 1. Mean salinity was positively correlated with discriminant function 1 and negatively correlated with discriminant function 2. Interpretation of the discriminant functions and the ordination plots in Fig. 7a, b indicates increasing mean salinities, sediment depths to bedrock, and light availabilities from sites characterized by Low SAV coverage and Chara to sites characterized by Halodule.
Nine contrasts were performed to identify which predictors reliably separated the Low SAV coverage group from the others, the Low SAV coverage group from the Chara group, and the Chara group from the Halodule group after adjustment for the other predictors (Table 3). Mean salinity, median light availability, and mean sediment depth to bedrock were reliable predictors separating the Chara and Halodule groups. Median light availability was also a reliable predictor for separating the Low SAV coverage group from the others and the Low SAV coverage group from the Chara group.
Discussion
The SAV communities of the mangrove-lined estuaries are strongly controlled by salinity climate, sediment depth to bedrock, and light penetration through the water column. Any factor that changes these structuring variables is likely to result in a change in the distribution and abundance of SAV communities. The discriminant function model, in conjunction with the contrast analyses, identified the relative strengths of these structuring variables in differentiating the SAV community groups. Through analogy, the spatial analyses have also provided further insights into understanding the historical regime shift from SAV abundance and clear water columns to phytoplankton abundance and the loss of SAV coverage that has occurred in the mangrove “lakes” of Florida Bay.
Our results provide targets for salinity, water clarity, and water column TP concentration that, if achieved by ecosystem restoration, should see the return of the historical dense Chara communities in the upstream “lakes.” Two SAV community groups were organized along a salinity gradient, with C. hornemannii dominant in the upstream “lakes” at mean annual salinities less than approximately 25 and H. wrightii dominant in the downstream marine embayments at greater mean annual salinities (Fig. 4a). The mean salinities at sites characterized by Halodule dominance (Fig. 4a) were all within the tolerance range (9–65) for H. wrightii isolated in culture (McMahan 1968; Koch et al. 2007). The mean salinities at Chara-dominated sites (Fig. 4a) were also within the salinity range (0–41) reported from field observations of C. hornemannii occurrence (Tabb et al. 1962; Morrison and Bean 1997). Within the “upstream lakes” where Chara occurred were also sites where SAV presence was negligible (Fig. 3). These Low SAV coverage sites were characterized by low light availability caused by a combination of both greater phytoplankton abundance and greater water depth.
As indicated by the 78% accuracy rate for the prediction of community groups using only mean salinity, the discriminant function model justifies the use of salinity as a targeted performance measure for managing the distribution of SAV community types in estuaries. The model also indicated that median light availability and sediment depth to bedrock were valuable predictors of community types whose addition increased model accuracy to 90%. Greater median light availability and greater sediment depth to bedrock characterized Halodule sites and further delimited the Chara and Halodule communities when they were added to the model. Differences in median light availability (Table 3) were uniquely valuable in identifying sites unsuitable for Chara occurrence (i.e., Low SAV coverage sites), even though mean salinities at those sites (i.e., sites 25, 27, 29, and 30 in the West Lake system) were within the approximate favorable range (<25).
The water column throughout the study area was P-limited as indicated by TN/TP ratios exceeding the Redfield ratio and a significant positive relationship between log phytoplankton abundance (chlorophyll a) and log water column TP concentration (Fig. 6). Lower TN/TP ratios in the West Lake system (37–44) relative to those in the Seven Palm Lake system (65–85) indicate less severe P limitation in the western system, with greater phytoplankton concentrations in the West Lake system resulting from greater P availability. The observed P limitation and the east–west gradients in phytoplankton abundance and TN/TP ratios are consistent with larger regional patterns in productivity and P availability within the greater Everglades and Florida Bay ecosystems (Fourqurean et al. 1992; Chen and Twilley, 1999; Noe et al. 2001). The level of productivity and the P limitation gradient reflect differences in proximity to a Gulf of Mexico P source (Fourqurean et al. 1992; Chen and Twilley 1999).
The observed pattern of increasing light availability from the Chara group to the Halodule group is consistent with the pattern of light requirements for algae and sea grasses. Sea grasses generally have higher light requirements than algae because photosynthesis in the sea grass leaves must provide greater energy per unit biomass to support non-photosynthetic roots and rhizomes (Fourqurean and Zieman 1991). Decreasing light availabilities may support only algae that have greater proportions of photosynthetic biomass. Ultimately, light availability decreases past the level to support any SAV.
Increasing sediment thickness (depth to bedrock) from the upstream “lakes” to the downstream coastal embayments reflect the progression of sea level rise and associated marine carbonate sedimentation that created Florida Bay over the past 5,000 years (Enos and Perkins 1979). Sediment depths to bedrock reliably separated the upstream Chara communities from the downstream Halodule communities when used as a predictor variable in the discriminant function model (Table 3). The greater sediment depths to bedrock, which have the ability to store more nutrients, may favor the establishment of Halodule that can take up nutrients through its roots over Chara that predominantly takes up nutrients from the water column (Kufel and Kufel 2002).
Pre-drainage SAV data for the mangrove estuaries along the ecotone between Florida Bay and the freshwater Everglades are scarce, but anecdotal reports indicate that historical SAV abundances were much higher than those observed recently. Based on qualitative observations, Tabb et al. (1962) first described the disappearance of the Chara–Ruppia–Batophora community and associated waterfowl in Whitewater and Coot Bays (located to the adjacent west from present study area), following the construction of the Buttonwood Canal in 1956–57 that permitted the entry of saline water from Florida Bay. They also estimated that these SAV communities were in chronic decline starting in 1920 when Everglades drainage operations began south of Lake Okeechobee. During the time of their study, they also reported that the Chara–Ruppia–Batophora community in West Lake was less affected than areas further west and likely represented pre-drainage conditions. Shortly after their study, the conditions in West Lake deteriorated when a channel was cut between West and Long Lakes that permitted more saline water from Long Lake to enter West Lake, causing another acute loss of the Chara communities (Craighead 1971). The decline of these communities since pre-drainage times is also associated with an estimated 20–30 increase in mean salinities along the north shore of Florida Bay (McIvor et al. 1994).
Though earlier investigators attributed historical Chara loss to increasing salinities (Tabb et al. 1962; Craighead 1971; Simmons and Ogden 1998), the unique ability of light availability to distinguish the Low SAV coverage group from the Chara and Halodule groups (Fig. 4b and Table 3), suggests an additional mechanism for the loss of Chara communities. Decreased freshwater deliveries permit the intrusion of surface seawater from Florida Bay (Davis et al. 2003) and increase the discharge of brackish groundwater where fresh and saline groundwater meet (Price et al. 2006). Both Florida Bay seawater and brackish groundwater are enriched with P relative to upstream freshwater sources (Rudnick et al. 1999; Davis et al. 2003; Price et al. 2006); therefore, increased saltwater intrusion likely increased P loading to these estuaries. The clear relationships between P availability, phytoplankton abundance, and light availability indicate that historic SAV communities will not be restored in the freshwater end of these estuarine systems unless water column TP concentrations are reduced, especially in the West Lake system. Though increased freshwater flow will decrease salinity and may also decrease P loading, the reestablishment of flourishing Chara communities in the estuaries of the southern Everglades may be inhibited by the long-term retention of P in P-limited carbonate ecosystems (Herbert and Fourqurean 2008). Because 75% of the sites characterized by Chara dominance exhibited median light at bottom exceeding 5% of surface light and all sites characterized with Low SAV coverage were below this level of light availability (Fig. 4b), 5% light at bottom is a reasonable estimate for the maintenance of Chara communities. Using a light availability target of 5% of surface light at bottom and the regression equations relating light availability to chl a, and chl a to TP, we estimate that annual mean water column TP concentrations should be maintained at or below 2.2 ± 1.1 μM (mean ± 95% confidence interval) to limit phytoplankton abundance and allow for the shift back to the Chara-dominated regime in the upstream “lakes.” However, if the Chara-dominated and the phytoplankton-dominated regimes are distinct stable states characterized by hysteresis (Scheffer et al. 1993), a much lower TP concentration may be required.
This study has demonstrated how SAV distribution in the mangrove lakes and estuaries relates to salinity, light availability, and sediment depth to bedrock and has also provided water quality restoration targets based upon these relationships. The study also suggests that in addition to salinity intrusion, decreased light availability facilitated by phytoplankton blooms that are fueled by increased nutrient availability may have also contributed to a historical regime shift from SAV abundance to phytoplankton domination.
References
Blindlow, I., A. Hargeby, and G. Andersson. 2002. Seasonal changes of mechanisms maintaining clear water in a shallow lake with abundant Chara vegetation. Aquatic Botany 72: 315–334.
Boyer, J.N. 2006. Shifting N and P limitation along a north–south gradient of mangrove estuaries in South Florida. Hydrobiologia 569: 167–177.
Chen, R., and R.R. Twilley. 1999. Patterns of mangrove forest structure and soil nutrient dynamics along the Shark River estuary, Florida. Estuaries 22: 955–970.
Clarke, K.R., and R.N. Gorley. 2006. Primer v6: User manual/tutorial. Plymouth: PRIMER-E Ltd.
Clarke, K.R., and R.M. Warwick. 2001. Change in marine communities: An approach to statistical analysis and interpretation, 2nd ed. Plymouth: PRIMER-E.
Craighead Sr., F.C. 1971. The trees of South Florida, vol. I. The natural environments and their succession. Coral Gables: University of Miami Press.
Davis III, S.E., D.L. Childers, J.W. Day Jr., D.T. Rudnick, and F.H. Sklar. 2003. Factors affecting the concentration and flux of materials in two southern Everglades mangrove wetlands. Marine Ecology Progress Series 253: 85–96.
Duarte, C.M. 1995. Submerged aquatic vegetation in relation to different nutrient regimes. Ophelia 41: 87–112.
Enos, P., and R.D. Perkins. 1979. Evolution of Florida Bay from island stratigraphy. Geological Society of America Bulletin 90: 59–83.
Fourqurean, J.W., and J.C. Zieman. 1991. Photosynthesis, respiration and whole plant carbon budget of the seagrass Thalassia testudinum. Marine Ecology Progress Series 69: 161–170.
Fourqurean, J.W., J.C. Zieman, and G.V.N. Powell. 1992. Phosphorus limitation of primary production in Florida Bay: Evidence from C:N:P ratios of the dominant seagrass Thalassia testudinum. Limnology and Oceanography 37: 162–171.
Fry, B., and P.L. Parker. 1979. Animal diet in Texas seagrass meadows: 13C evidence for the importance of benthic plants. Estuarine and Coastal Marine Science 8: 499–509.
Gacia, E., and C.M. Duarte. 2001. Sediment retention by a Mediterranean seagrass Posidinia oceanica meadow: The balance between deposition and resuspension. Estuarine and Coastal Shelf Science 52: 505–514.
Heck Jr., K.L., G. Hays, and R.J. Orth. 2003. Critical evaluation of the nursery role hypothesis for seagrass meadows. Marine Ecology Progress Series 253: 123–136.
Hemminga, M.A., and C.M. Duarte. 2000. Seagrass ecology. New York: Cambridge University Press.
Herbert, D.A., and J.W. Fourqurean. 2008. Ecosystem structure and function still altered two decades after short-term fertilization of a seagrass meadow. Ecosystems 11: 688–700.
Herrera-Silveira, J.A. 1994. Phytoplankton productivity and submerged macrophyte biomass variation in a tropical coastal lagoon with groundwater discharge. Vie Milieu 44: 257–266.
Holmes, R.W. 1970. The Secchi disk in turbid coastal zones. Limnology and Oceanography 15: 688–694.
Jeffrey, S.W., and G.F. Humphrey. 1975. New spectrophotometric equations for determining chlorophylls a, b, c1, and c2 in higher plants, algae and natural phytoplankton. Biochemie Physiologie Pflanzen 167: 191–194.
Kirk, J.T.O. 1994. Light and photosynthesis in aquatic ecosystems, 2nd ed. New York: Cambridge University Press.
Knight, R.L., B. Gu, R.A. Clarke, and J.M. Newman. 2003. Long-term phosphorus removal in Florida aquatic systems dominated by submerged aquatic vegetation. Ecological Engineering 20: 45–63.
Koch, M.S., S.A. Schopmeyer, C. Kyhn-Hansen, C.J. Madden, and J.S. Peters. 2007. Tropical seagrass species tolerance to hypersalinity stress. Aquatic Botany 86: 14–24.
Kufel, L., and I. Kufel. 2002. Chara beds acting as nutrient sinks in shallow lakes—A review. Aquatic Botany 72: 249–260.
Light, S.S., and J.W. Dineen. 1994. Water control in the Everglades: A historical perspective. In Everglades: the ecosystem and its restoration, ed. S.M. Davis and J.C. Ogden, 47–84. Boca Raton: St. Lucie Press.
MacIsaac, H.J., G. Sprules, O.E. Johannson, and J.H. Leach. 1992. Filtering impacts of larval and sessile zebra mussels (Dreissena polymorpha) in western Lake Erie. Oecologia 92: 30–39.
McCune, B., J.B. Grace, and D.L. Urban. 2002. Analysis of ecological communities. Gleneden Beach: MJM Software Design.
McIvor, C.C., J.A. Ley, and R.D. Bjork. 1994. Changes in freshwater inflow from the Everglades to Florida Bay including effects on biota and biotic processes: A review. In Everglades: the ecosystem and its restoration, ed. S.M. Davis and J.C. Ogden, 117–148. Boca Raton: St. Lucie Press.
McMahan, C.A. 1968. Biomass and salinity tolerance of Shoalgrass and Manateegrass in Lower Laguna Madre, Texas. The Journal of Wildlife Management 32: 501–506.
McMillan, C., and F.N. Moseley. 1967. Salinity tolerances of five marine spermatophytes of Redfish Bay, Texas. Ecology 48: 503–506.
Michot, T.C., M.C. Wooden, and A.J. Nault. 2008. Food habits of redheads (Athya americana) wintering in seagrass beds of coastal Louisiana and Texas, USA. Acta Zoologica Academiae Scientiarum Hungaricae 54: 239–250.
Moncrieff, C.A., M.J. Sullivan, and A.E. Daehnick. 1992. Primary production dynamics in seagrass beds of Mississippi Sound: The contributions of seagrass, epiphytic algae, sand microflora, and phytoplankton. Marine Ecology Progress Series 87: 161–171.
Montague, C.L., and J.A. Ley. 1993. A possible effect of salinity fluctuation on abundance of benthic vegetation and associated fauna in northeastern Florida Bay. Estuaries 16: 703–717.
Morrison, D., and D.L. Bean. 1997. Benthic macrophyte and invertebrate distribution and seasonality in the Everglades–Florida Bay ecotone. Final project report submitted to Everglades National Park South Florida Natural Resources Center, Homestead, Florida.
Noe, G.B., D.L. Childers, and R.D. Jones. 2001. Phosphorus biogeochemistry and the impact of phosphorus enrichment: Why is the Everglades so unique? Ecosystems 4: 603–624.
Noordhuis, R., D.T. van der Molen, and M.S. van den Berg. 2002. Response of herbivorous water-birds to the return of Chara in Lake Veluwemeer, The Netherlands. Aquatic Botany 72: 349–367.
Ogden, J.C. 1994. A comparison of wading bird nesting colony dynamics (1931–1946 and 1974–1989) as an indication of ecosystem conditions in the southern Everglades. In Everglades: The ecosystem and its restoration, ed. S.M. Davis and J.C. Ogden, 533–570. Boca Raton: St. Lucie Press.
Orth, R.J., and K.A. Moore. 1983. Chesapeake Bay: An unprecedented decline in submerged aquatic vegetation. Science 222: 51–53.
Petersen, J.K., J.W. Hansen, M.B. Laursen, P. Clause, J. Carstensen, and D.J. Conley. 2008. Regime shift in a coastal marine ecosystem. Ecological Applications 18: 497–510.
Preisendorfer, R.W. 1986. Secchi disk science: Visual optics of natural waters. Limnology and Oceanography 31: l909–l1926.
Price, R.M., P.K. Swart, and J.W. Fourqurean. 2006. Coastal groundwater discharge—An additional source of phosphorus for the oligotrophic wetlands of the Everglades. Hydrobiologia 569: 23–36.
Richards, J.H., and J.W. Fourqurean, 2000. Benthic macrophyte distribution and seasonality in the Everglades–Florida Bay ecotone: Pattern and processes. Final report submitted to Everglades National Park South Florida Natural Resources Center, Homestead, Florida.
Rudnick, D.T., Z. Chen, D.L. Childers, J.N. Boyer, and T.D. Fontaine III. 1999. Phosphorus and nitrogen inputs to Florida Bay: The importance of the Everglades watershed. Estuaries and Coasts 22: 398–416.
Scheffer, M., and E.H. van Nes. 2007. Shallow lakes theory revisited: Various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 584: 455–466.
Scheffer, M., S.H. Hosper, M.-L. Meijer, B. Moss, and E. Jeppesen. 1993. Alternative equilibria in shallow lakes. Trends in Ecology and Evolution 8: 275–279.
Scoffin, T.P. 1970. The trapping and binding of subtidal carbonate sediments by marine vegetation in Bimini Lagoon, Bahamas. Journal of Sedimentary Petrology 40: 249–273.
Simmons, G., and L. Ogden. 1998. Gladesmen. Miami: University Press of Florida.
Solórzano, L., and J.H. Sharp. 1980. Determination of total dissolved P and particulate P in natural waters. Limnology and Oceanography 25: 754–758.
Tabachnick, B.G., and L.S. Fidell. 2007. Using multivariate statistics, 5th ed. New York: Pearson Education Inc.
Tabb, D.C., D.L. Dubrow, and R.B. Manning. 1962. The ecology of northern Florida Bay and adjacent estuaries. State of Florida Board of Conservation Technical Series No. 39. Miami, Florida.
Wanless, H.R.R., R.W. Parkinson, and L.P. Tedesco. 1994. Sea level control on stability of Everglades wetlands. In Everglades: the ecosystem and its restoration, ed. S.M. Davis and J.C. Ogden, 199–224. Boca Raton: St. Lucie Press.
Welschmeyer, N.A. 1994. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnology and Oceanography 39: 1985–1992.
Zar, J.H. 1999. Biostatistical analysis, 4th ed. Upper Saddle River: Prentice-Hall, Inc.
Zimmerman, R.C. 2006. Light and photosynthesis in seagrass meadows. In Seagrasses: Biology, ecology and conservation, ed. A.W.D. Larkum, R.J. Orth, and C.M. Duarte, 303–321. Dordrecht: Springer.
Acknowledgments
This research was funded by a grant from Everglades National Park under cooperative agreement number H5297-05-0041. We thank P.J. Walker for permit issuance and Dave Hallac for use of ENP facilities. Additional support was provided by the Florida Coastal Everglades Long-Term Ecological Research program funded by the National Science Foundation. We also thank Kevin Cunniff, Steven Huddleston, Elizabeth Kozma, and Dave Ward for field assistance. We thank two anonymous reviewers and Associate Editor Jacob Carstensen for their constructive comments and recommendations that greatly improved the manuscript. This is contribution number 469 from the Southeast Environmental Research Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frankovich, T.A., Morrison, D. & Fourqurean, J.W. Benthic Macrophyte Distribution and Abundance in Estuarine Mangrove Lakes and Estuaries: Relationships to Environmental Variables. Estuaries and Coasts 34, 20–31 (2011). https://doi.org/10.1007/s12237-010-9279-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-010-9279-0