Abstract
Potato production is being affected by high temperature stresses worldwide due to global warming. The biological basis of carbohydrate metabolism and reactive oxygen species (ROS) activity in potato tubers under high temperature stress is yet to be clearly understood. We evaluated the activities of two of the most important primary ROS members: superoxide (O2.−) and hydrogen peroxide (H2O2) and their scavengers to understand the effects of heat stress on the changes of carbohydrates in growing tubers of five potato varieties including heat-tolerant and heat‐susceptible check varieties. The enzymatic ROS-scavengers were found to be differentially activated in these genotypes. The detoxification mechanism was more efficient in dual-stress (heat and salt) tolerant variety compared to single-stress tolerant variety. The antioxidant activity was increased by several folds in the tolerant variety compared to the susceptible variety. Storage starch accumulation and its composition was affected by O2.− and H2O2 metabolisms in potato tuber. The findings will be helpful in understanding the biological basis of the effect of ROS-detoxification on starch accumulation in growing tubers under heat stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potato (Solanum tuberosum L.), the most important globally cultivated tuber crop, is one of the cheapest sources of starch for both human nutrition and industrial use. Heat stress causes loss in economic yield of potato by increasing internal heat necrosis (Sterrett et al. 1991; Yencho et al. 2008) and reducing carbon accumulation in tuber (Paul et al. 2016; Singh et al. 2019a). Starch, tuber dry matter and reducing sugars are important quality attributes of potato. The structural and functional properties of starch in potato tubers are influenced by both genetic and environmental factors (Noda et al. 2004). High temperature stress significantly affects the yield and quality of storage starch by altering the expressions of starch metabolic genes and the activities of related enzymes (Thalmann and Santelia 2017; Gautam et al. 2023; Hancock et al. 2014). Additionally, it increases the quantity of reducing sugars in tubers, which negatively affect the processing quality of potato tubers (Eldredgc et al. 1996).
Potato plant requires different optimum temperatures for growth and development of different plant parts such as 20–25 °C for shoot biomass and 15–20 °C for root and tuber biomass (Van Dam et al. 1996). High air temperature raises the soil temperature (Watts 1975; Islam et al. 2015). Air temperature stress transmits signals to underground plant parts such as root, which is then transmitted to other developing plant parts of shoot through plasmodesma to begin defense mechanism (Bláha and Středa 2016). Air temperature above 30 °C has been shown to slowdown the tuber growth (Timlin et al. 2006). Soil temperature greater than 22–25 °C increases the risk of heat shock symptom (such as internal browning in tuber), caused by increased activity of oxidising enzymes and tuber respiration (Van Dam et al. 1996; Harper 2004; Rykaczewska 2015).
Under heat stress, abnormal photosynthesis, photorespiration and respiration in cell organelles, such as chloroplasts, mitochondria, peroxisomes and also in plasma membranes and apoplast, increase the level of ROS (Singh et al. 2019b). The ROS readily reacts to other macromolecules such as proteins and lipids and causes oxidative damage to cellular components. This eventually creates imbalances in different cellular metabolic processes, pH and hormone levels, which negatively affect the synthesis and accumulation of starch in storage organs (Yacoubi et al. 2013; Onaga and Wydra 2016).
ROS can be radicals such as superoxide (O2.−), hydroxyl (OH.), perhydroxy (HO2.) and alkoxy (RO.) or non-radicals such as hydrogen peroxide (H2O2), and single oxygen (1O2) (Møller and Sweetlove 2010) etc. O2.− and H2O2 are the two most important initial ROS members that generate other radicals (Quan et al. 2008). The superoxide radical (O2.−) is the first generated member of ROS resulting from the reduction of single electron of atmospheric O2 by photosynthetic and respiratory electron transport system (ETS). In photoautotrophic cells, chloroplasts are the major site of O2.− synthesis. Superoxide dismutase (SOD) causes dismutation of O2.− to generate H2O2 which then converts to OH. by releasing electrons through the Habere Weiss reaction or Fenton’s reaction (Gill and Tuteja 2010). However, complex-I and complex-III of mitochondrial electron transport chain (ETC) are the major sources of these radicals in non-photosynthetic cells (Møller 2001; Quan et al. 2008; Das and Roychoudhury 2014; Janku et al. 2019). H2O2 acts as a signalling molecule under stress. Besides, imbalanced accumulation of H2O2 leads to oxidative stress and can cause injury to the cells. Therefore, imbalanced production of ROS in non-photosynthesizing plant cells can create oxidative stress and toxicity which consequently can cause imbalanced carbon metabolism (Ding et al. 2021).
Plants activate different types of defense mechanisms such as antioxidant enzymes, alternative oxidase, uncoupling protein, and mitochondrial hexokinase activity etc. to reduce ROS level by lowering the rate of ROS generation and by scavenging ROS molecules, depending on the tolerance level of cells (Camacho-Pereira et al. 2009; Baltruschat et al. 2008). Tolerant varieties can maintain tuber yield by increasing the level of both enzymic and non-enzymic antioxidants for upregulating ROS scavenging pathways (Paul et al. 2016). The most important enzymic scavengers of O2.− and H2O2 are SOD, catalase (CAT), ascorbate peroxidase (APX) and guaiacol peroxidase (GPX). SOD scavenges O2.− by generating H2O2 and O2. The H2O2 level is then reduced by CAT, APX and GPX by generating H2O and O2 (Rajput et al. 2021). The levels of synthesis and activities of these antioxidants varies highly among species, cultivars and even among different growth stages of the same cultivar (Saed-Moucheshi et al. 2014). Additionally, soluble sugar metabolism is related to both ROS generation and regulation of defense against various stresses (Ding et al. 2021; Keunen et al. 2013). Besides, the soluble sugars are also the substrate of storage starch synthesis in storage organs such as tubers of potato (Zeeman et al. 2010). Therefore, metabolism of ROS in potato tubers under heat stress may influence the metabolism of storage starch. However, the process is yet to be completely understood.
Plants adaptation mechanisms against heat stress at both physiological and molecular levels depends on types (air and/or soil temperature stress), depths and durations of heat stress as well as genotype and its growth and developmental stages (Bita and Gerats 2013; Singh et al. 2019a). Studies on ROS metabolism under biotic and abiotic stresses mostly focused on leaves of plants (El_Komy et al. 2020). Little is known about effect of heat stress mediated ROS metabolism on development of storage organs. In this study, we investigated the influence of air temperature stress on the level of O2.− and H2O2 and their enzymic and nonenzymic antioxidants along with their effect on the changes of soluble sugars, and quality and quantity of starch in potato tubers at bulking stage. This will enhance our understanding about the ROS mediated response and carbohydrate metabolism in non-photosynthetic plant organs under heat stress.
Materials and Methods
Sprouting of Tubers, Plant Growth and Heat Treatment
Two tolerant varieties namely, BARIalu72 (certified as tolerant to heat and salt) and BARIalu73 (certified as tolerant to salt) and three susceptible and high yielding varieties namely, BARIalu7 (Diamant), BARIalu8 (Cardinal) and BARIalu25 (Asterix) were used for this study (for details see Agri-Advisory portal, BARC (2024)). Healthy, disease-free and well sprouted potato tubers were planted in 15 L plastic pot (20 cm x 30 cm x 25 cm) filled with garden soil mix (sandy loam soil and compost at 4:1 ratio). A total of six tubers per genotype (one tuber per pot with a pot-pot distance of 35 cm) were grown under natural field condition in a net house having 22/18 ± 3 °C day/night temperature, 11/13 hr photoperiod (day/night) and natural sunlight using completely randomized design. After 60–65 days after plantation, when the tubers’ diameter became 8–10 mm, three pots from each genotype were transferred to a polyhouse having controlled day/night temperature of 32/25 ± 2 ℃ and a similar photosystem (natural sunlight). All plants were watered when necessary to avoid drought stress. Three weeks after heat stress treatment (80–85 DAP), tubers from all the plants were harvested for data collection with representative samples (3–4 cm in diameter, with 3 replications per genotype of each treatment) being frozen in liquid nitrogen prior to storage at -80 ℃ freezer for further biochemical analyses.
Collection of Morphological Data
Two weeks after heat stress treatment, data on number of stems emerged from a tuber (each stem considered as a plant), plant height, number of compound leaves per 50 cm of the plant (from 10 cm above the ground level), leaf area, chlorophyll content and growth status value were measured. Leaf area was measured from five randomly selected mature compound leaves by LI-3100 C leaf area meter (LI-COR Biosciences, Lincoln, USA). For estimation of chlorophyll content, data was collected from the three randomly selected top most fully mature leaves using a SPAD 502 Plus Chlorophyll meter (Minolta, UK) and calculated to total chlorophyll content by using polynomial model function (Eq. 1) as shown below (Zhang et al. 2022).
Here, Chl is the calculated total chlorophyll content (chl-a + chl-b) of a sample i, SPAD is the SPAD 502 m data (without unit), a (-0.005) and b (1.133) are fitted model parameters and c (-9.119) is model residuals from Zhang et al. (2022).
Growth condition of plants was measured by scoring the growth status of the haulm by following the modified standard evaluation score (SES) ranged from 0 (very healthy) to 9 (completely dead) (Gregorio et al. 1997) for heat stress as shown in Table 1. After harvest, data on number of tubers per plant, diameter of tuber and yield per plant was collected.
Estimation of ROS Activity
Superoxide Radicals (O2.−)
To determine the level of superoxide radicals, the method described by Elstner and Heupel (1976) was used with some modification. A fresh potato tuber sample (0.15 g) was grinded and homogenized with 65 mM potassium-phosphate K-P buffer (pH 7) followed by centrifugation (12,000 rpm, 10 min, 4 ℃). The supernatant, K-P buffer and hydroxylamine hydrochloride (10 mM) were mixed at 12:10:1 ratio and were incubated with sulfanilamide (17 mM) and α–naphthylamine (7 mM) for 20 min. An equal volume of diethylether was added to the mixture and the absorbance was taken at 530 nm using a spectrophotometer (Jasco UV-VIS Spectrophotometer (V-630). The amount of O2.− was calculated using NaNO2 as standard (0 to 1.6 µmol/g).
Hydrogen Peroxide (H2O2)
The H2O2 content was measured by the method described by Jana and Choudhuri (1981). Extraction was done by grinding and homogenizing tuber samples (0.25 g) with 50 mM K-P buffer (pH 6.5) followed by centrifugation at 12,000 rpm for 15 min at 4 °C. The supernatant and reaction assay (0.1% TiCl4 in 20% H2SO4 (v/v) were mixed at 3:1 ratio and stored at room temperature for 10 min followed by centrifugation (12,000 rpm, 15 min, 4 °C). The absorbance was recorded by a spectrophotometer at 410 nm and the concentration of H2O2 was calculated using a standard curve of H2O2 (0.0 to 60 µmol/g).
Extraction of Enzyme and Estimation of ROS Scavenging Activity
Potato tuber samples (1 g) were homogenized in ice cold extraction buffer (50 mM potassium phosphate (pH 7), 100 mM KCl, 1 mM ascorbate, 5 mM β-mercaptoethanol and 10% glycerol) using a cold mortar-pestle. The homogenate was centrifuged (12,000 rpm, 12 min, 4 ℃) and the supernatant was used for measuring the contents of APX, GPX, CAT and SOD by Bradford assay using Bovine Serum Albumin (Sigma Aldrich) as standard (0.0 to 40 mg/g).
APX
The APX activity was estimated as per the method described in Nakano and Asada (1981) which includes oxidizing the ascorbate and measuring the decrease in absorbance. The enzyme extract was added in a cuvette containing APX assay reagent (50 mM K-P buffer (pH 7), 0.5 mM ascorbate, 0.1 mM H2O2, 0.1 mM EDTA) at 1:199 ratio and the data of decreasing absorbance was recorded 2 time points at 30 s interval for 1 min using the extinction coefficient of 2.8 mM− 1cm− 1. The absorbance was calibrated using APX standard curve (0.0 to 2.0 unit/min/mg protein).
SOD
SOD activity was measured by xanthine-xanthine oxidase method described by Sun et al. (1988) based on the inhibition of nitroblue tetrazolium (NBT) reduction by Cu/ZnSOD and MnSOD. The reaction mixture contained K-P buffer (50 mM, pH 7.8), NBT (2.24 mM), xanthine oxidase (0.1 mM), catalase (0.1 mM), 100-xanthine (2.36 mM). The changes in absorbance were recorded at 560 nm as per the method of APX using SOD standard curve (0.0 to 500 unit/min/mg protein).
CAT
The activity of CAT was determined by using the method described in Csiszár et al. (2007). The principle of this method includes decomposition of H2O2 which, in turn, was measured by the decrease in absorbance at 240 nm. The absorbance was calibrated using CAT standard curve (0.0 to 20 µmol/min/mg protein).
GPX
The modified enzyme method (Elia et al. 2003) was used to measure the GPX activity by using H2O2 as substrate. The reaction started at 20 °C with adding substrate in GPX standard assay (100 mM Na3PO4 buffer (pH 7.5), 0.12 mM NADPH, 1 mM EDTA, 1 mM NaN3, 0.6 mM H2O2, 2 mM glutathione, 1 unit glutathione reductase). The absorbance at 340 nm of NADPH oxidation was recorded for 1 min and GPX activity was calculated using an extinction coefficient of 6.62 mM− 1cm− 1 and calibrated using GPX standard curve (0.0 to 1000 nmol/min/mg protein).
Proline
The proline content was measured by the method described in Bates et al. (1973). Briefly, potato tubers were homogenized in 3% aqueous sulfosalicylic acid followed by centrifugation at 12,000 rpm for 12 min at 4 ℃. The supernatant, ninhydrin, and glacial acetic acid were mixed at 1:1:1 ratio and incubated at 95 ℃ for 1 h in a water bath. The mixture was then transferred to an ice bath for 15 min to terminate the reaction. After that an equal volume of toluene was added, mixed well and kept at room temperature (25 ℃) for 10 min prior to recording the absorbance at 520 nm. The absorbance readings were calibrated using a proline standard curve (0.0 to 20 µmol/g).
Estimation of Tuber Dry Matter and Carbohydrates
The tuber dry matter was measured by drying a slice (3–4 cm diameter) of marketable tubers (3 slices from each pot) at 65 ℃ in an oven until constant weight. Content of total soluble solids (TSS) was determined by a refractometer (BRIX 0–32%, PSX-BJ-HT113ATC, Perth Scientific PTY Ltd, Malaga WA 6090, Australia). Glucose and fructose content was measured by a digital refractometer (HANNA: HI-96,803 and HI-96,802).
Starch
Fresh potato tubers (2 g) were ground well by mortar and pestle with cold double distilled water (DDW). The sample was transferred into a reaction tube and volumed up to 10 ml with cold DDW. After centrifugation (5500 rpm, 4 ℃, 10 min), the supernatant was discarded and the pellet was washed for several times with cold DDW. The starch pellet was transferred into a 100 ml glass beaker with 20 ml DDW. The solution was heated and the heat was turned off when the temperature raised close to 100 ℃. After the solution was cooled down to 50 ℃, it was filtered, cooled down to room temperature and volumed up to 50 ml with DDW. In a reaction tube the solution was mixed with KI solution (0.26 g I2 + 2.6 g KI) at 1:5 ratio and absorbance was recorded at 600 nm with a spectrophotometer (Photopette). Starch content was calculated from a standard curve of potato starch (Sigma Aldrich) with 0–80% concentration.
Amylose and Amylopectin
Potato dried powder (1 g) was homogenized with 95% (v/v) ethanol and 1 N NaOH (1:9 ratio). After heating (10 min) in hot water bath, the solution was cooled down and was volumed up to 100 ml with deionized water. One milliliter acetic acid (1 N) and 2 ml of iodine solution (0.26 g I2 + 2.6 g KI) were added in a 5 mL aliquot and diluted to 5% (v/v) with deionized water. Spectrophotometric quantification was performed at 620 nm, using the multipoint working curve method with three repetitions and quartz cells of 10 mm path length. The apparent amylose content was calculated using an equation obtained from the standard amylose (Sigma-Aldritch, range from 0 to 30%).
Data Analysis
One-way ANOVA (General Linear Model) was employed to test the significance among the treatments, genotypes and their interactions. Pairwise mean differences were analysed by post hoc (Tukey’s Honest Significant Difference (HSD), with 3 biological replications, p ≤ 0.05) and the principal component (PC) analysis was done to decipher the interrelationship among all the parameters, treatments and genotypes using R-software (R-4.2.3). Additionally, post hoc Tukey’s pairwise comparison was conducted to visualize statistical significance among the mean PC scores of genotype-treatment interactions.
Results
Changes in the Morphological Traits under Heat Stress
Plant height, leaf area, number of leaves, chlorophyll content and growth status values differed significantly among the potato varieties (Table 2). Variety-treatment interaction was significant for growth status value, plant height, chlorophyll content and tuber diameter. At control condition (i.e., at 22/18 oC), the highest plant height, number of leaves and leaf area were observed in the tolerant variety BARIalu72. Chlorophyll content and tuber diameter, on the other hand, were the highest in tolerant varieties BARIalu73, and BARIalu72, respectively. Growth status and yield per plant were the highest in heat susceptible variety Diamant followed by Asterix (Table 2).
After stress imposition (i.e., at 32/25 oC), plant height became the highest in BARIalu73 while the variety maintained the highest chlorophyll content at stressed condition as well (Table 2). BARIalu72 maintained the highest number of leaves and leaf area at stressed condition. In addition, tuber diameter and yield per plant became the highest in this genotype (BARIalu72). The highest growth status value was maintained by Diamant at stress condition.
Plant health condition decreased significantly (higher growth status value indicates higher number of yellow and dead leaves) upon stress imposition; and the maximum decrease were observed in the susceptible genotypes Diamant and Asterix (Table 2). Number of leaves and leaf area did not change significantly whereas chlorophyll content and tuber diameter significantly reduced under stressed condition. Contrastingly, the yield contributing characters such as leaf area (59 cm2), tuber diameter (11.9 cm) and tuber yield per plant (78 g) became the highest in the tolerant variety BARIalu72 under stressed condition followed by Asterix and BARIalu73 (Table 2).
Differential Accumulation of ROS under Heat Stress
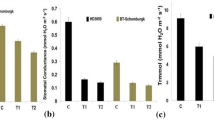
At control condition, the accumulation of superoxide (O2.−) varied significantly among the varieties (Fig. 1). Hydrogen peroxide (H2O2), on the other hand, did not show such significant variation. The O2.− was at the highest level in BARIalu72 followed by Cardinal whereas hydrogen peroxide was the lowest in these two varieties. Upon stress imposition, the O2.− and H2O2 contents increased in all varieties, however the increase was significant in Asterix (from 11.44 µmol/g to 25.7 µmol/g) and Diamant (from 12.24 µmol/g to 19.15 µmol/g). H2O2 content, on the other hand, increased significantly in all varieties except Asterix (Fig. 1).
Changes of superoxide and hydrogen peroxide level and their antioxidant activities (superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), Glutathione peroxidase (GPX) and proline) in potato tuber of five potato cultivars (2 heat resistant (BARIalu73 and BARIalu72) and 3 suspectable check (Cardinal, Asterix and Diamant)) influenced by heat stress (32/25°C: day/night) with 10/14hrs day/night photoperiod. Uppercase same letters indicate no significant difference among the mean values (Tukey HSD; p = 0.05, n = 3)
ROS Scavenging Activity under Heat Stress
Among the scavengers of ROS, the superoxide dismutase (SOD) increased significantly only in Asterix (from 87 to 259 unit) upon stress imposition (Fig. 1). The activity of APX, on the other hand, increased significantly in all varieties, with the highest increase being observed in Asterix (from 0.1 unit to 1.0 unit). The GPX activity also showed significant heat stress mediated increase in all varieties except BARIalu73. Similar to APX activity, GPX activity was also the highest in Asterix (from 112 to 553 unit). The highest significant increase in CAT activity was observed in BARIalu72 (from 3 to 12 unit) followed by Asterix (from 6.7 to 10.2 unit) and Diamant (from 4.5 to 7.1 unit) whereas it decreased in Cardinal (from 7.4 to 3.5 unit) and remained unchanged in BARIalu73 under stress condition. The proline content increased significantly in BARIalu72 (from 1.8 to 5.7 µmol/g) and decreased in BARIalu73 (from 9.3 to 1.2 µmol/g) and cardinal (from 7.9 to 2.6 µmol/g).
Heat Stress Mediated Alterations in Carbohydrate Contents in Potato Tuber
All the starch biosynthesis related traits such as contents of total soluble solid (TSS), reducing sugars, starch, amylose and amylopectin varied significantly among the varieties and treatments (Table 2). All traits but tuber dry matter varied significantly for variety-treatment interaction. The starch content decreased in all the varieties except BARIalu73 under heat stress. However, the decrease was significant only in Asterix and Diamant. The highest level of starch content was observed in BARIalu72 under both control and stressed temperatures. In Asterix and Diamant, soluble solids increased significantly whereas tuber dry matter and starch content decreased at stressed condition. Although the amylose content of BARIalu73 and BARIalu72 decreased under stress condition, it increased in Asterix and Diamant. Furthermore, the opposite was observed for amylopectin (data not shown). In addition, the ratio of amylopectin-amylose content increased in BARIalu72 and BARIalu73, and decreased (by 2 folds) in Asterix and Diamant. The soluble and insoluble carbohydrates of Cardinal remained unaffected by heat stress.
Principal Component Analysis (PCA)
PCA of all the parameters under stressed and controlled conditions extracted major contrast in the activities of ROS and their scavengers in the five potato cultivars. The first three principal components (PCs) explained 75% of the total variation in the dataset (Table 3). PC1 accounted for 48% of the total variation which had large positive associations with the contents of proline, chlorophyll, starch, amylopectin, tuber dry matter, tuber diameter and yield etc. (as indicated by higher positive coefficients for these traits) versus the lower negative co-efficient for growth status, activities of APX, GPX, SOD, superoxide and H2O2, contents of fructose, glucose, and amylose etc. (Fig. 2). So, it is apparent that the first principal component separated the cultivars primarily based on their tuber yield performances against the activities of ROS and their respective scavengers and sugar compounds. PC2, on the other hand, accounted for 16% of the total variation which was mainly manifested by higher positive coefficients for the number of leaves, leaf area, and contents of total soluble sugars, proline, amylose and activity of superoxide etc. and lower negative coefficients for number and weight of tuber, ratio of amylose and amylopectin, yield per plant, contents of amylopectin, starch and glucose etc.
Biplot of morphological, activity of ROS and their scavengers, and starch contents of five stressed (32/25°C: day/night) and control (22/18°C: day/night) cultivars of potato as determined by the principal component analysis (PCA). Cultivars indicated by upward triangle and bold red fonts represents stressed condition; and that of by squared and blue fonts represent control condition. H2O2: hydrogen peroxide, SUP: superoxide, APX: ascorbate peroxidase, GPX: glutathione peroxidase, CAT: catalase, SOD: superoxide dismutase, PRL: proline, PH: plant height, LA: Leaf area, Chl: chlorophyll content, NOL: number of leaf, TN: Tuber number plant− 1, TD: tuber diameter, PPY: yield plant− 1, GS: growth status value, PRT: protein content, TSS: total soluble solid, GLU: glucose content, FRU: fructose content, ITW: individual tuber wt. DRM: tuber dry matter, STAR: starch content, AMYL: amylose, AMYP: amylopectin, AMYLP_R: ratio of amylose and amylopectin. The number 1 to 10 is defined as genotype number
Discussion
Heat Stress Modulates Source-Sink Relations during Tuber Bulking Stage
Plant growth depends on the efficiency of biosynthesis and utilization of carbon assimilates. After tuber initiation, potato plants utilize their photosynthates in the underground tuber growth instead of aboveground vegetative growth (Golovko and Tabalenkova 2019; Hastilestari et al. 2018). In this study, we observed that the heat stress during tuber bulking stage (stress applied after tubers became 8–10 mm in diameter) did not significantly affect plant height, leaf area and leaf number. Instead, the major effects of heat stress were observed on chlorophyll content and plant health conditions (e.g., the higher growth status value, the higher number of dead and yellow leaves).
The economic yield related traits such as tuber diameter, tuber number and yield were not significantly affected by stress imposition in all the studied varieties, except the susceptible variety Diamant. This is consistent with previous findings as potato plants, grown at moderately elevated temperatures, did not exhibit classic symptoms of abiotic stress (Hancock et al. 2014). However, increment of every 5 °C temperature (above the optimum requirement) negatively affected yield and quality of tubers through inhibition of the rate of photosynthesis, growth, development and carbon translocation to potato tuber (Timlin et al. 2006; Dahal et al. 2019). Delayed high temperature stress, on the other hand, was found to exert little negative effect on the yield of tuber (Rykaczewska 2013, 2015).
Additionally, in some commercial cultivars the gross photosynthesis rates were found to be maximum at 20 to 30 oC temperature, which then started to decline beyond 35 oC onwards (Dwelle et al. 1981). Gross photosynthesis is also related to plant leaf area. In our study, although the chlorophyll content decreased significantly at 32 °C temperature, the leaf area and leaf number were not significantly affected in the studied genotypes (except a significant increase of leaf number in BARIalu72). Application of heat stress during tuber bulking stage thus did not affect tuber number, diameter and yield in the both tolerant and two susceptible (Cardinal and Asterix) varieties, although plant health in most of the varieties declined significantly (with exception in BARIalu73 and BARIalu72 (Pic. 1).
During tuber bulking, plants utilize their photosynthates mostly for starch accumulation in the tubers. The reduced chlorophyll contents probably were still enough to supply the assimilates to the tubers instead of haulm growth at tuber bulking stage in tolerant varieties. Additionally, under stressed condition (when the photosynthetic potentiality is decreased), the non-structural carbohydrates, stored in potato stem, are found to act as buffer to maintain tuber bulking by promoting the remobilization of storage reserve from source to sink (Zheng et al. 2009; Duque and Setter 2013). Hence, the degradation and remobilization of reserves, from haulm to storage organ (tuber), might have contributed to an increase in the growth status value (higher yellow/dead leaves) which, in turn, might have helped in reducing the yield losses in Cardinal and Asterix under stress condition.
Significant variations in growth status value and tuber diameter due to variety-treatment interaction indicated that under heat stress, the tuber size of potato depends on the tolerance level of the plant. At unstressed condition, three growth indexes (plant height, leaf number and leaf area) in the dual stress tolerant variety BARIalu72 and two growth indexes (plant height and chlorophyll content) in the single stress tolerant variety BARIalu73 were the highest during vegetative growth. These indexes were less affected by heat stress during tuber bulking stage in these tolerant varieties. This vigorous growth at vegetative stage (without stress) might also contribute in supplying more photo assimilates to tubers upon stress imposition during bulking stage in a way to reduce yield loss. This might have resulted in the highest yield of BARIalu72 followed by BARIalu73, under heat stress.
Taken together, it is apparent that an increase in air temperature during a later part of potato growing season (i.e., tuber bulking state), tuber yield may not be affected due to the remobilization of storage reserves from haulm to growing tubers. However, the tuber quality such as soluble sugar content, starch and its composition may be affected.
ROS Detoxification Occur Differentially among Varieties
Previous research has demonstrated that the rate of ROS production is increased under biotic and abiotic stress conditions and that the antioxidant mediated detoxification level depends on the tolerance level of the plant. Potato cultivars show varied responses to heat stress in terms of ROS biosynthesis and defense mechanism. Heat tolerance of potato is a polygenic trait and thus, is expected to be substantially influenced by genotype-environment interactions (Tang et al. 2018). Consistence with this, our results also indicate that the ROS synthesis and their detoxification metabolism in potato tuber under heat stress depends on the genotypes.
Superoxide Radical (O2.−)
The dismutation of O2.− into H2O2 and O2 is the first line of defense against ROS in protecting the cell and in that conversion, SOD is one of the key enzymes (Uchida et al. 2002). The H2O2 acts as signal molecule for triggering tolerance to various abiotic and biotic stresses (Fukao and Bailey-Serres 2004; Quan et al. 2008). In our study, the levels of both O2.− and SOD increased significantly in the variety Asterix. A sharp increase of O2.− in Asterix (2.2 times higher than control) may lead to the significant level of increase in SOD (3 times higher than control). No significant difference of SOD and O2.− contents were observed between the control and stress conditions in BARIalu73, BARIalu72 and Cardinal. However, in Diamant, although the O2.− increased significantly under stress, the level of SOD did not increase. This result indicates that under heat stress, O2.− dismutation depends on the genotypes. Previous researches reported that fatty acid β-oxidation, photorespiratory glycolate oxidase reaction, the enzymatic reaction of flavin oxidases and disproportionation of O2 are the most important metabolic processes for the generation of H2O2 in peroxisomes (Del Río et al. 2002; Río et al. 2006). SOD catalyses disproportionation of O2.− to give one molecule of H2O2 from two molecules of O2.−. In our result, under stress, the amount of H2O2 was half of the amount of O2.− in Asterix (Sheng et al. 2014). From this we can assume that H2O2 synthesis in tuber of Asterix was due to disproportionation of O2.− by SOD.
Hydrogen Peroxide (H2O2)
H2O2 acts as a signal molecule to induce antioxidation mechanisms under abiotic stress. But at excess level, it becomes toxic to plants. It was reported that exogenous application of H2O2 can effectively protect plant from heat and salt stress by increasing the synthesis of several antioxidant enzymes such as SOD, CAT, APX and GPX (Uchida et al. 2002; Furtana et al. 2019). The amount of H2O2 increased in all of our varieties under heat stress. Significant increase of H2O2 was found in BARIalu73, Cardinal and Diamant. However, the increase of H2O2 in Asterix and BARIalu72 were not significant. This may be due to the increase of most of the H2O2 scavengers in BARIalu72 (CAT, APX, GPX and proline) and in Asterix (CAT, APX, GPX). CAT and APX are two most powerful enzymes for dismutation of H2O2 (Gill and Tuteja 2010). The levels of their activities were reported to be increased in tolerant genotypes due to the generation of H2O2 by overexpressed SOD (Tanaka et al. 1999). The O2.− radical is a moderately reactive species with approximately 2–4 µs of half-life (Gill and Tuteja 2010). Due to such short life, O2.− radical immediately converts to O2 and H2O2 by SOD. H2O2, on the other hand, have comparatively longer half-life and is broken down into O2 and water by CAT (Møller 2001). In BARIalu72, the level of CAT was 4 times higher under heat stress compared to the control condition. Additionally, the level of proline increased 3 times under stress condition. Proline contributes to the detoxification of H2O2 by enhancing the APX activity (Hossain et al. 2010; Patade et al. 2011). Therefore, increased activities of APX and CAT are needed to scavenge H2O2 which was generated by the dismutation of O2.− (Patade et al. 2011). Otherwise, there remains the risk of generation of highly toxic radical (HO.) via Habere Weiss reaction. Thus, the coordinated activities of CAT and APX are essential for effective scavenging of ROS (Chagas et al. 2008).
The accumulation of H2O2 in the dual stress (heat and salt) tolerant variety BARIalu72 was insignificant. This may be due to the fact that the H2O2 was scavenged immediately by increased activities of CAT, APX and proline. In the variety BARIalu73 (a salt tolerant variety), the level of H2O2 was significantly increased because the levels of enzymatic and non-enzymatic scavengers (except APX) were not increased. It indicates that although BARIalu73 was tolerant under salt stress, it probably could not avoid the effect of oxidative stress under heat stress. The varieties Diamant, Asterix and Cardinal, which are widely grown for high yield, showed significantly higher levels of H2O2 and O2.−, although, the levels of CAT, APX and GPX were also increased by several folds. This may be due to the fact that the levels of scavengers were not high enough to reduce the toxicity of these ROS members in these varieties. Previous studies also suggested that the activities of SOD and APX should be high enough to efficiently reduce the oxidative damage (Asada 2006; Chagas et al. 2008). Therefore, considering the levels of H2O2 and O2.−, both enzymatic and non-enzymatic scavengers, it can be suggested that BARIalu72 is the most efficient variety in detoxifying ROS in tuber.
Carbon Metabolism in Tuber is Affected under Heat Stress
The dry matter accumulation in storage organs depends on the mobilization and conversion efficiency of sugar to storage starch (Mohabir and John 1988). Elevated temperature significantly hampers starch biosynthesis due to decrease in precursors and alteration in the activities of the enzymes related to starch metabolic pathway (Geigenberger et al. 1998); Lafta and Lorenzen 1995; Gautam et al. 2023). Tuber dry matter was reported to be drastically reduced when grown under higher temperature (Gautam et al. 2023). In our study, the lower contents of dry matter corresponded to the lower contents of starch and higher contents of soluble solids (TSS) and reducing sugars (glucose and fructose) (Table 2). Due to sensitivity to the heat stress, maximum reductions in the contents of starch and dry matter were observed in Diamant and Asterix which corresponded to the maximum levels of TSS, glucose and fructose. Therefore, the increased level of TSS and hexose sugars can be the outcome of reduction in starch content and conversion of dry matter from soluble sugar. This result is supported by the previous finding that the high temperature limits the biosynthesis efficiency of starch from sucrose (Krauss and Marschner 1984). The increase of the soluble sugars under moderate heat stress may be due to the negative effect of heat stress on the activities of starch biosynthetic enzymes (Lafta and Lorenzen 1995; Xalxo et al. 2020). With exception of glucose in BARIalu72, the changes of soluble and insoluble carbohydrates under heat stress were insignificant in tolerant varieties BARIalu73 and BARIalu72.
The ratio of amylopectin-amylose determines the physicochemical properties of starch granule. The content of amylopectin is usually 3 times higher than the amylose because, in a growing tuber, the amount of amylopectin increases during maturation (Jansky and Fajardo 2016). However, in storage starch, this ratio differs among cultivars under different growing temperatures (Feng et al. 2019; Zhao et al. 2023). At the final stage of starch granule formation, the activity of several key enzymes such as GBSS, BE and DBE are reduced by high temperature which results in the reduction of the ratio of amylopectin and amylose and the content of total starch (Ahmed et al. 2015; Feng et al. 2019). In consistent with this, the total starch content and the ratio of amylopectin-amylose were significantly changed in our varieties under stress condition. The ratio was reduced in temperature sensitive varieties (Asterix and Diamant) which may negatively affected the starch content and tuber yield.
Tuber Yield and Starch Quality are Influenced by ROS Detoxification
The tuber yield, the growth status value of aboveground parts and storage starch accumulation in our contrastingly tolerant varieties indicates that metabolism of the two ROS members namely, O2.− and H2O2 may have an influence on tuber yield and its quality. Tuber yield, starch content and the ratio of amylopectin-amylose in the tolerant variety BARIalu72 was significantly higher under heat stress. This may be due to the increase in the activities of antioxidant enzymes by several folds that efficiently reduced the toxicity levels of O2.− and H2O2 in this genotype. Paul et al. (2016) reported that tuber yield has strong correlation with the levels of antioxidant activities. These antioxidants may protect the starch metabolic enzymes and the tuber cell for starch accumulation. The accumulation was comparatively higher in BARIalu72 and BARIalu73 compared to susceptible varieties Asterix, Cardinal and Diamant. However, the structural properties may differ among the varieties due to difference in the ratio of amylopectin-amylose.
Soluble sugar has a dual function in ROS metabolism. Accumulation of sugars can (i) promote ROS generation by supplying NADH for mitochondrial respiratory chain (Ding et al. 2021; Ravera et al. 2015) and (ii) regulate defence mechanisms against ROS-generated stress (Couée et al. 2006; Keunen et al. 2013). It was reported in cotton that, the varieties with high sugar content promote ROS accumulation in a way to stimulate cellulose synthesis while enhanced ROS synthesis arrested cellulose elongation which resulted in high fibre yield with low quality (Ding et al. 2021). In our study, accumulation of soluble sugar and ROS members increased significantly in the susceptible varieties Asterix and Diamant under stress condition without any visual external or internal necrosis. However, the tuber dry matter and starch content decreased. These differences may have two possible explanations: (i) the soluble sugars mobilized from the aboveground plant part were accumulated in the tuber for utilization of ROS synthesis and the accumulated ROS interfered with the starch metabolic pathways or (ii) under stress, accumulation of O2.− interfered in the starch metabolism which resulted in the increased level of sugar molecules and these sugar molecules further stimulated the synthesis of both O2.− and H2O2. However, further research is necessary to elucidate the interference of ROS on starch metabolism on potato tuber under heat stress.
Conclusion
ROS detoxification capacity of potato tuber has an influence on carbon assimilation and structural properties of starch in potato tubers under heat stress in a variety dependent manner. One of the reasons of the tolerance in BARIalu72 could be the increase of antioxidant levels by several folds under heat stress. Therefore, it will be interesting to see the influence of ROS on starch metabolism in developing potato tuber. Additionally, this study only considered the effects of air temperature. Effects of both above ground and underground temperatures on starch metabolism can also be studied. The findings of this study provide a direction for future research on the influence of ROS metabolism on starch accumulation in non-photoautotrophic storage organs of tuber crop.
Data Availability
Not applicable.
Change history
13 June 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12230-024-09956-4
References
Agri-Advisory Portal, Bangladesh Agricultural Research Council (BARC), Ministry of Agriculture. 2024. http://cropzoning.gov.bd:82/CropInfo/CropVarieties?c=36
Ahmed, N., I. J. Tetlow, S. Nawaz, A. Iqbal, M. Mubin, M. S. N. U. Rehman, A. Butt, D. A. Lightfoot, and M. Maekawa. 2015. Effect of high temperature on grain filling period, yield, amylose content and activity of starch biosynthesis enzymes in endosperm of basmati rice. Journal Science Food Agriculture 95(12): 2159–2354.
Asada, K. 2006. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology 141(2): 391–396.
Baltruschat, H., J. Fodor, B. D. Harrach, E. Niemczyk, B. Barna, G. Gullner, A. Janeczko, K.-H. Kogel, P. Schäfer, I. Schwarczinger, A. Zuccaro, and A. Skoczowski. 2008. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytologist 180(2): 501–510.
Bates, L. S., R. P. Waldren, and I. D. Teare. 1973. Rapid determination of free proline for water-stress studies. Plant and Soil 39: 205–207.
Bita, C. E., and T. Gerats. 2013. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Frontiers in Plant Science 4: 1–18.
Bláha, L., and T. Středa. 2016. Plant integrity-the important factor of adaptability to stress conditions. Abiotic and Biotic Stress in Plants-Recent Advances and Future Perspectives InTech: https://doi.org/10.5772/60477.
Camacho-Pereira, J., L. E. Meyer, L. B. Machado, M. F. Oliveira, and A. Galina. 2009. Reactive oxygen species production by potato tuber mitochondria is modulated by mitochondrially bound hexokinase activity. Plant Physiology 149(2): 1099–1110.
Chagas, R. M., J. A. G. Silveira, R. V. Ribeiro, V. A. Vitorello, and H. Carrer. 2008. Photochemical damage and comparative performance of superoxide dismutase and ascorbate peroxidase in sugarcane leaves exposed to paraquat-induced oxidative stress. Pesticide Biochemistry and Physiology 90: 181–188.
Couée, I., C. Sulmon, G. Gouesbet, and A. Amrani. 2006. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. Journal of Experimental Botany 57(3): 449–459.
Csiszár, J, E., I. Lantos, E. Tari, B. Madoşă, Á. Wodala, F. Vashegyi, A. Horváth, M. Pécsváradi, B. Szabó, Á. Bartha, A. Gallé, G. Lazăr, M. Coradini, S. Staicu, S. Postelnicu, G. Mihacea, Nedelea, and L. Erdei. 2007. Antioxidant enzyme activities in Allium species and their cultivars under water stress. Plant Soil and Environment 53(12): 517–523.
Dahal, K., X-Q. Li, H. Tai, A. Creelman, and B. Bizimungu. 2019. Improving potato stress tolerance and tuber yield under a climate change scenario - a current overview. Frontiers in Plant Science 10: 1–16.
Das, K., and A. Roychoudhury. 2014. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science 2: 1–13.
Del Río, L. A., F. J. Corpas, L. M. Sandalio, J. M. Palma, M. Gómez, and J. B. Barroso. 2002. Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes. Journal of Experimental Botany 53(372): 1255–1272.
Del Río, L. A., L. M. Sandalio, F. J. Corpas, J. M. Palma, and J. B. Barroso. 2006. Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging and role in cell signalling. Plant Physiology 141: 330–335.
Ding, X., X. Li, L. Wang, J. Zeng, L. Huang, L. Xiong, S. Song, J. Zhao, L. Hou, F. Wang, and Y. Pei. 2021. Sucrose enhanced reactive oxygen species generation promotes cotton fibre initiation and secondary cell wall deposition. Plant Biotechnology Journal 19: 1092–1094.
Duque, L. O., and T. L. Setter. 2013. Cassava response to water deficit in deep pots: root and shoot growth, ABA, and carbohydrate reserves in stems, leaves and storage roots. Tropical Plant Biology 6: 199–209.
Dwelle, R. B., G. E. Kleinkopf, and J. J. Pavek. 1981. Stomatal conductance and gross photosynthesis of potato (Solanum tuberosum L.) as influenced by irradiance, temperature, and growth stage. Potato Research 24: 49–59.
Watts, W. R. 1975. Air and soil temperature differences in controlled environments, as a consequence of high radiant flux densities and of day/night temperature changes. Plant and Soil 42: 299–303.
El_Komy, M. H., A. A. Saleh, Y. E. Ibrahim, and Y. Y. Molan. 2020. Early production of reactive oxygen species coupled with an efficient antioxidant system play a role in potato resistance to late blight. Tropical Plant 45: 44–55.
Eldredgc, E. P., Z. A. Holmes, A. R. Mosley, C. C. Shock, and T. D. Stieber. 1996. Effect of transitory water stress on potato tuber stem-end reducing sugar and fry colour. American Potato Journal 73: 517–530.
Elia, A. C., R. Galarini, M. I. Taticchi, A. J. M. Dörr, and L. Mantilacci. 2003. Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicology and Environmental Safety 55: 162–167.
Elstner, E. F., and A. Heupel. 1976. Inhibition of nitrite formation from hydroxylammonium chloride: a simple assay for superoxide dismutase. Analytical Biochemistry 70: 616–620.
Feng, P., Y. Wang, Y. Mu, J. Un, J. Kang, N. Wu, and X. Lu. 2019. Effect of high temperature on potato starch content, amylase activity and yield. Southwest China Journal of Agricultural Sciences 32(6): 1253–1258.
Fukao, T., and J. Bailey-Serres. 2004. Plant responses to hypoxia - is survival a balancing act? Trends in Plant Science 9(9): 449–456.
Furtana, G. B., K. Sönmez, Ş.Ş. Ellialtıoğlu, and R. Tıpırdamaz. 2019. The effects of hydrogen peroxide and nitric oxide on ion contents and lipid peroxidation levels of pepper callus tissues under salt stress. Research Journal of Chemistry and Environment 23(1): 123–130.
Gautam, S., R. Morey, N. Rau, D. C. Scheuring, D. Kurouski, and M. I. Vales. 2023. Raman spectroscopy detects chemical differences between potato tubers produced under normal and heat stress growing conditions. Front Plant Science 14: 1–15.
Geigenberger, P., M. Geiger, and M. Stitt. 1998. High-temperature perturbation of starch synthesis is attributable to inhibition of ADP-Glucose pyrophosphorylase by decreased levels of glycerate-3-phosphate in growing potato tubers. 117: 1307–1316.
Gill, S. S., and N. Tuteja. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry. Plant Physiology 117: 1307–1316.
Golovko, T. K., and G. N. Tabalenkova. 2019. Source-sink relationships in potato plants. Russian Journal of Plant Physiology 66(4): 313–320.
Gregorio, G. B., D. Senadhira, and R. D. Mendoza. 1997. Screening rice for salinity tolerance. IRRl Discussion Paper Series 22:1–30.
Hancock, R. D., W. L. Morris, L. J. M. Ducreux, J. A. Morris, M. Usman, S. R. Verrall, J. Fuller, C. G. Simpson, R. Zhang, P. E. Hedley, and M. A. Taylor. 2014. Physiological, biochemical and molecular responses of the potato (Solanum tuberosum L.) plant to moderately elevated temperature. Plant, Cell and Environment. 37: 439–450.
Harper, S. 2004. Potato tuber quality management in relation to environmental and nutritional stress. Horticultural Australia Ltd. Sydney.
Hastilestari, B. R., J. Lorenz, S. Reid, J. Hofmann, D. Pscheidt, U. Sonnewald, and S. Sonnewald. 2018. Deciphering source and sink responses of potato plants (Solanum tuberosum L.) to elevated temperatures. Plant Cell & Environment 41: 2600–2616.
Hossain, M. A., M. Hasanuzzaman, and M. Fujita. 2010. Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiology and Molecular Biology of Plants 16(3): 259–272.
Islam, K. I., A. Khan, and T. Islam. 2015. Correlation between atmospheric temperature and soil temperature: a case study for Dhaka, Bangladesh. Atmospheric and Climate Sciences 5: 200–208.
Jana, S., and M. A. Choudhuri. 1981. Glycolate metabolism of three submersed aquatic angiosperms: effect of heavy metals. Aquatic Botany 11: 67–77.
Janku, M., L. Luhová, and M. Petrivalský. 2019. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 8: 1–15.
Jansky, S., and D. Fajardo. 2016. Amylose content decreases during tuber development in potato. Journal of Science Food Agriculture 96(13): 4560–4564.
Keunen, E., D. Peshev, J. Vangronsveld, W. V. D. Ende, and A. Cuypers. 2013. Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant Cell and Environment 36: 1242–1255.
Krauss, A., and A. Marschner. 1984. Growth rate and carbohydrate metabolism of potato tubers exposed to high temperatures. Potato Research 27: 297303.
Lafta, A. M., and J. H. Lorenzen. 1995. Effect of high temperature on plant growth and carbohydrate metabolism in potato. Plant Physiology 109(2): 637–643.
Mohabir, G., P, John. 1988. Effect of temperature on starch synthesis in potato tuber tissue and in amyloplasts. Plant Physiology 88: 1222–1228.
Møller, I. M. 2001. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annual Review of Plant Biology 52: 561–591.
Møller, I. M., and L. J. Sweetlove. 2010. ROS signalling – specificity is required. Trends in Plant Science 15: 370–374.
Nakano, Y., and K. Asada. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology 22(5): 867–880.
Noda, T., S. Tsuda, M. Mori, S. Takigawa, C. Matsuura-Endo, N. Hashimoto, and H. Yamauchiet. 2004. Properties of starches from potato varieties grown in Hokkaido. Journal of Applied Glycoscience 51: 241–246.
Onaga, G., and K. Wydra. 2016. Advances in plant tolerance to biotic stresses. In: Plant Genomics. Ed. Ibrokhim Y. Abdurakhmonov. InTech. 229–272.
Patade, V. Y., S. Bhargava, and P. Suprasanna. 2011. Salt and drought tolerance of sugarcane under iso-osmotic salt and water stress: growth, osmolytes accumulation and antioxidant defense. Journal of Plant Interactions 6(4): 275–282.
Paul, S., M. Farooq, and N. Gogoi. 2016. Influence of high temperature on carbon assimilation, enzymatic antioxidants and tuber yield of different potato cultivars. Russian Journal of Plant Physiology 63(3): 319–325.
Quan, L. J., B. Zhang, W. W. Shi, and H. Y. Li. 2008. Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. Journal of Integrative Plant Biology 50(1): 2–18.
Rajput, V. D., R. K. Harish, K. K. Singh, L. Verma, F. R. Sharma, M. Quiroz-Figueroa, V. S. Meena, T. Gour, Minkina, and S. Sushkova. 2021. Recent developments in enzymatic antioxidant defense mechanism in plants with special reference to abiotic stress. Biology 10: 1–28.
Ravera, S., M. Bartolucci, P. Cuccarolo, E. Litamè, M. Illarcio, D. Calzia, P. Degan, A. Morelli, and I. Panfoli. 2015. Oxidative stress in myelin sheath: the other face of the extramitochondrial oxidative phosphorylation ability. Free Radical Research 49(9): 1156–1164.
Rykaczewska, K. 2013. The impact of high temperature during growing season on potato cultivars with different response to environmental stresses. American Journal of Plant Sciences 4: 2386–2393.
Rykaczewska, K. 2015. The effect of high temperature occurring in subsequent stages of plant development on potato yield and tuber physiological defects. American Journal of Potato Research 92: 339–349.
Saed-Moucheshi, A., A. Shekoofa, and M. Pessarakli. 2014. Reactive oxygen species (ROS) generation and detoxifying in plants. Journal of Plant Nutrition 37: 1573–1585.
Sheng, Y., I. A. Abreu, D. E. Cabelli, M. J. Maroney, A. F. Miller, and M. Teixeira. 2014. and J.S. Valentine. Superoxide dismutases and superoxide reductases. Chemical Reviews 114: 3854–3918.
Singh, B., S. Kukreja, and U. Goutam. 2019a. Impact of heat stress on potato (Solanum tuberosum L.): present scenario and future opportunities. Journal of Horticultural Science and Biotechnology 95(4): 407–424.
Singh, A., A. Kumar, S. Yadav, and I. K. Singh. 2019b. Reactive oxygen species-mediated signalling during abiotic stress. Plant Gene 18:1–7.
Sterrett, S. B., M. R. Henningre, and G. S. Lee. 1991. Relationship of internal heat necrosis of potato to time and temperature after planting. Journal of the American Society for Horticultural Science 116(4): 697–700.
Sun, Y., L. W. Oberley, and Y. Li. 1988. A simple method for clinical assay of superoxide dismutase. Clinical Chemistry 34(3): 497–500.
Tanaka, Y., T. Hibino, Y. Hayashi, A. Tanaka, S. Kishitani, T. Takabe, S. Yokota, and T. Takabe. 1999. Salt tolerance of transgenic rice overexpressing yeast mitochondrial Mn-SOD in chloroplasts. Plant Science 148: 131–138.
Tang, R., S. Niu, G. Zhang, G. Chen, M. Haroon, Q. Yang, O. P. Rajora, and X. Q. Li. 2018. Physiological and growth responses of potato cultivars to heat stress. Botany 96: 897–912.
Thalmann, M., and D. Santelia. 2017. Starch as a determinant of plant fitness under abiotic stress. New Phytologist 214: 943–951.
Timlin, D., L. S. M. Rahman, J. Baker, V. R. Reddy, D. Fleisher, and B. Quebedeaux. 2006. Whole plant photosynthesis, development, and carbon partitioning in potato as a function of temperature. Agronomy Journal 98: 1195–1203.
Uchida, A., A. T. Jagendorf, T. Hibino, T. Takabe, and T. Takabe. 2002. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Science 163: 515–523.
Van Dam, J., P. L. Kooman, and P. C. Struik. 1996. Effects of temperature and photoperiod on early growth and final number of tubers in potato (Solanum tuberosum L). Potato Research 39: 51–62.
Xalxo, R., B. Yadu, J. Chandra, V. Chandrakar, and S. Keshavkant. 2020. Alteration in carbohydrate metabolism modulates thermotolerance of plant under heat stress. Heat Stress Tolerance in Plants: Physiological, Molecular and Genetic Perspectives (First Edition), John Wiley & Sons Ltd: 77–115.
Yacoubi, R., C. Job, M. Belghazi, W. Chaibi, and D. Job. 2013. Proteomic analysis of the enhancement of seed vigour in osmoprimed alfalfa seeds germinated under salinity stress. Seed Science Research 23(2): 99–110.
Yencho, G. C., P. H. McCord, K. G. Haynes, and S. B. R. Sterrett. 2008. Internal heat necrosis of potato - A review. American Journal of Potato Research 85: 69–76.
Zeeman, S. C., J. Kossmann, and A. M. Smith. 2010. Starch: its metabolism, evolution, and biotechnological modification in plants. Annual Review of Plant Biology 61: 209–234.
Zhang, R., P. Yang, S. Liu, C. Wang, and J. Liu. 2022. Evaluation of the methods for estimating leaf chlorophyll content with SPAD chlorophyll meters. Remote Sensing 14: 1–17.
Zhao, X., P. Hofvander, M. Andersson, and R. Andersson. 2023. Internal structure and thermal properties of potato starches varying widely in amylose content. Food Hydrocoll 135: 1–8.
Zheng, X., Y. Jitsuyama, T. Terauchi, and K. Iwama. 2009. Effects of drought and shading on non-structural carbohydrate stored in the stem of potato (Solanum tuberosum L). Plant Production Science 12(4): 449–452.
Acknowledgements
This work was funded by Bangladesh Agricultural University Research System (BAURES). The analytical work was supported by laboratory of Bangladesh Agricultural Research Institute. The experiments comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimers: All co-authors read and approved the manuscript. There is nothing to disclaim.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naher, J., Sabuj, Z.H., Sumona, S.I. et al. Heat Stress Modulates Superoxide and Hydrogen Peroxide Dismutation and Starch Synthesis during Tuber Development in Potato. Am. J. Potato Res. 101, 275–289 (2024). https://doi.org/10.1007/s12230-024-09950-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-024-09950-w