Abstract
Careful storage management is required to maintain post-harvest potato tuber quality. The plant growth regulator ethylene has well documented effects on potato tuber respiration rate, fried product color, and sprouting, but data on the amount of ethylene present in ventilated potato storages and how ethylene may affect tubers in commercial storage are not available. To address this need, ethylene concentration in ventilated commercial storage bins located in central Wisconsin was quantified using gas chromatography from shortly after bin filling until unloading. Samples of the storage atmosphere were collected approximately every other week from 17, 18 and 14 storage bins in 2010, 2011 and 2012, respectively. Ethylene was present transiently, and only rarely at concentrations greater than 20 nl l−1. In laboratory-scale experiments, chipping potato tubers responded to ethylene at 20 nl l−1 with an increase in tuber respiration rate, but not with an increase in post-fry chip darkening. These data indicate that the impact of atmospheric ethylene on tuber quality and storage management in ventilated potato storages is likely to be small, except near localized regions of high ethylene production.
Resumen
Se requiere manejo cuidadoso en el almacén para mantener la calidad del tubérculo de papa en postcosecha. El regulador de crecimiento vegetal etileno tiene efectos bien documentados en el nivel de respiración del tubérculo de papa, sobre el color del producto freído y en la brotación, pero no hay datos disponibles sobre la cantidad de etileno presente en almacenamientos ventilados de papa, y de cómo el etileno pudiera afectar a los tubérculos en almacenamiento comercial. Para atender esta necesidad se cuantificó la concentración de etileno en unidades de almacenamiento comercial ventilado ubicados en la parte central de Wisconsin, usando cromatografía de gases, desde justo después del llenado hasta cuando se vaciaron. Se colectaron muestras de la atmosfera del almacén aproximadamente cada dos semanas de 17, 18 y 14 almacenes en 2010, 2011 y 2012, respectivamente. El etileno estuvo presente intermitentemente, y solo raramente en concentraciones mayores a 20 nl–l−1. En experimentos a nivel laboratorio, los tubérculos de freído respondieron al etileno a 20 nl l−1 con incremento en el nivel de respiración del tubérculo, pero no con aumento en oscurecimiento de la hojuela posterior al freído. Estos datos indican que el impacto del etileno atmosférico en la calidad del tubérculo y el manejo en almacenes ventilados de papa, es probable que sea pequeño, excepto cerca de regiones localizadas de alta producción de etileno.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Preserving potato (Solanum tuberosum L.) tuber quality in storage requires careful storage management (Brook et al., 1995; Wustman and Struik, 2007). Temperature, relative humidity, and ventilation rate, for example, must be controlled appropriately to avoid well-documented quality losses. Low temperatures and rapid temperature changes promote tuber sugar accumulation and decrease processing quality (Hopkins, 1924; Sowokinos, 2001; Kumar et al., 2004). Low relative humidity facilitates evaporative water loss from tubers and increases incidence of pressure bruise (Kunkel and Gardner, 1965; Schippers, 1971; Castleberry and Jayanty, 2012). Inadequate ventilation rates contribute to free water formation that promotes pathogen infection and spread. Potato tubers and associated microbial organisms contribute many low molecular weight volatile compounds to the storage atmosphere (Varns and Glynn, 1979; Waterer and Pritchard, 1985; Costello et al., 1999; 2001; Lui et al., 2005). One of these is carbon dioxide (CO2), and CO2 typically accumulates in the storage atmosphere at 2–5 times the concentration found in the intake air. CO2 contents in many storages are monitored and maintained below empirically established thresholds by bringing in ambient air as needed to maintain tuber health and processing quality. Other volatile products produced by potato tubers and tuber-associated microbes are not routinely monitored and are not used for day-to-day management of potato storages. The impact that these compounds have on tuber quality in storage is largely a matter of speculation.
The gaseous plant hormone ethylene is required for multiple aspects of plant growth, development and responses to the environment (Schaller, 2012; Vandenbussche et al., 2012). Among these processes are the ripening of fruit, germination of seeds, and senescence of stems, leaves, and flowers. Several responses of potato tubers to endogenous and exogenous ethylene have been described. Endogenous ethylene promotes the establishment of microtuber dormancy (Suttle, 1998). Exogenous ethylene increases tuber respiration rates (Huelin and Barker, 1939; Reid and Pratt, 1972; Chin and Frenkel, 1976), affects tuber dormancy and sprouting (Elmer, 1932; Rylski et al., 1974; Coleman, 1998; Prange et al., 1998; Wills et al., 2004; Daniels-Lake et al., 2005b; Gottschalk, 2011), and can increase tuber reducing sugar concentrations (Huelin and Barker, 1939; Daniels-Lake et al., 2005b; a). An interaction between ethylene and CO2 in the storage atmosphere has been reported, with higher concentrations of both increasing tuber reducing sugar content and darkening fry color (Daniels-Lake et al., 2005a; Daniels-Lake and Prange, 2009). Fry color reflectance values for Russet Burbank exposed to ethylene at 0.25 or 0.5 μl l−1 decreased by approximately 2.5 % as CO2 concentration increased from 0 to 5,000 μl l−1 above that in air alone (Daniels-Lake and Prange, 2009). The effect of long-term exposure to elevated CO2 and ethylene at 0.5 μl l−1 on fry or chip color was found to vary substantially with cultivar (Daniels-Lake, 2012).
Ethylene is produced by plants and microorganisms, and is formed when fossil fuels are burned. Potato tubers produce low amounts of ethylene and are highly sensitive to ethylene in the atmosphere (Creech et al., 1973; Martínez-Romero et al., 2007). Potato tuber respiration rates have been shown to increase with ethylene concentrations as low as 150 nl l−1 for cv. White Rose (Reid and Pratt, 1972) and 20 nl l−1 for cv. Russet Burbank (Rylski et al., 1974). Effects on sprouting have been observed when ethylene was applied at concentrations as low as 2 nl l−1 (Rylski et al., 1974). Reducing sugar accumulation and darkening of French fries have been observed at 250 nl l−1 ethylene for cultivar Russet Burbank (Daniels-Lake and Prange, 2009).
Despite the potential for ethylene to adversely affect the stored potato crop through increased respiration rate and reducing sugar accumulation, data on the amount of ethylene present in the atmosphere of ventilated potato storage bins are not available. In the absence of this data, informed decisions cannot be made about the benefits of installing ethylene monitoring equipment in potato storages and storage management based on the amount of ethylene present. Likewise, there is very little information on the sensitivity to ethylene of chip processing potatoes. Chipping potatoes have been intensively selected for long-term maintenance of low reducing sugar contents and are genotypically distinct from other potato market classes (Hirsch et al., 2013). Decreased ethylene sensitivity could contribute to these breeding goals when ethylene is present in the storage atmosphere by minimizing the adverse effects of ethylene on tuber reducing sugars and respiration rate.
In order to gain quantitative data on the abundance of ethylene in the potato storage atmosphere, measurements of ethylene concentration were made in commercial potato storage bins from shortly after bins were filled until emptied. In order to better assess the potential consequences of ethylene in potato storages, chipping potatoes were treated with concentrations of ethylene similar to those observed in potato storage bins and effects on tuber respiration rate and fried chip color were quantified.
Materials and Methods
Potato Storage and Sampling of the Storage Atmosphere
Potatoes were grown on commercial farms in the central sands region of Wisconsin and stored in grower storages in 2010, 2011 and 2012. Samples of the storage atmosphere were removed approximately every other week from commercial storage bins at a location above the stored tubers and approximately 2–4 m before the inlet to the return-air fans and ventilation doors. Thus, a mixed sample of air from the entire storage atmosphere was analyzed. In total, 218 samples were collected from 17 bins in 2010, 381 samples from 18 bins in 2011 and 206 samples from 14 bins in 2012. In each year, two of the potato storage bins held approximately 54,000 Mg (120,000 cwt) of potatoes and the remainder contained between 27,000 and 36,000 Mg (60,000–80,000 cwt) of potatoes in bulk storage. Storages were typically filled in late September and some storage bins were emptied as late as May. Storage management followed best management practices at each location. All bins were fogged with an aerosol of chlorpropham (CIPC, Isopropyl-N-(3-chlorophenyl) carbamate) to prevent tuber sprouting. Experiments were conducted blind, with data from the research not shared with the storage manager. Storage management decisions regarding variety, ventilation rate and timing, temperature management, chemical applications, and when to remove product were not shared with the researcher in advance. Potato varieties included Snowden, FL1879, FL2137 and FL2053. A single bin in 2010 and 2011 contained processing russets. Notes were taken on appearance and aroma of each bin, and those were used to develop an assessment of disease pressure. As a general rule, storage bins or partial bins that began to develop active regions of spoilage were unloaded earlier than those that had less evidence of disease.
Ethylene Measurements of Samples From Commercial Bins
Ethylene was measured using gas chromatography (GC). Samples (10 ml) were collected in glass syringes fitted with Teflon seals and integral valves (SGE Analytical Sciences, Melbourne, Australia) and were injected directly into a SRI model 8610C GC outfitted with a purge and trap system and photoionization detector (SRI Instruments, Torrance, CA, USA). The trap contained silica gel and for the 2010 crop year the column was a 2 m by 3.1 mm outside diameter HayeSep D packed column (SRI Instruments). A 2 m by 2 mm inside diameter column packed with 1 % RT-1000 on CarboBlack B was used for the 2011 crop year and later (Restek Corp., Bellefonte, PA, USA). Column temperature was 40°C in 2010 and 60°C in 2011 and later. Changes in GC configuration were made to better resolve compounds other than ethylene that were present in the samples. In both years the carrier gas was helium and the limit of detection for ethylene was 3 nl l−1. Reference samples used to identify and quantify ethylene were prepared by mixing ethylene gas standards (Air Liquide America, Houston, TX USA) with air to a final volume of 10 ml.
Laboratory Measurements of Tuber Respiration Rate and Fried Chip Color in Response to Applied Ethylene
Tubers of cultivars Snowden, Lamoka and Pinnacle (W5015-12) used for respiration rate and fried chip experiments were grown at the University of Wisconsin Hancock Agricultural Research Station and stored in crates, each containing approximately 10 kg tubers, at the Hancock Potato and Vegetable Storage Research Facility until shortly before use. The tubers were preconditioned at 13°C and slowly cooled to a final storage temperature of 9 ± 0.3°C.
Respiration rate measurements were made using a custom built flow-through apparatus in which medical grade air (Airgas Inc., Radnor Township, PA USA) was continuously supplied to custom built glass-walled sample chambers at a rate of 100 ml/min by individual mass flow controllers (model FMA5512, Omega Engineering, Stamford, Connecticut USA). Each chamber held approximately 1,500 g of potato tubers (typically 10–15 tubers) and chambers were held in darkness in a 9°C Percival Model I36NLC8 incubator (Percival Scientific, Perry, IA). Chambers containing tubers were ventilated with medical air alone for two or more days in order to allow baseline tuber respiration rates to stabilize. Ethylene was continuously applied at 0, 20, 50 or 200 nl l−1 for up to 8 days by using a second set of mass flow controllers (model 1179A-22614, MKS Instruments, Boulder, CO USA) and a MKS type 247 four-channel mass flow controller power supply to mix ethylene gas (20 μl l−1, Praxair, Danbury, CT USA) with medical air before it entered each chamber. CO2 measurements of the supply air and air exiting the sample chambers were made daily using an infrared gas analyzer (model S154, Qubit Systems Inc., Kingston, ON Canada). Since CO2 concentration in a sample chamber may be affected by variety, tuber mass, respiration rate and CO2 concentration of air supply, tuber mass at the start of the experiment was reduced when needed to maintain CO2 concentrations below 2,000 μl l−1, a range typical of that found in commercial potato storage bins holding chipping potatoes. Four days after the start of the ethylene treatments, a 1–2 mm thick longitudinal slice was removed from the middle of each tuber and fried in vegetable oil at 182°C for 2 min 10 s. Chip color was assessed visually using the 10-point Potato Chip Color Reference Standard rating scale from 1 (light) to 10 (dark) at 0.5 point increments (Potato Chip Institute International, Cleveland, OH USA). Respiration rate and chip color measurements reported here assessed tubers from two or three different crop years and the testing was carried out between January and June of the respective years. Chip color for Snowden and Pinnacle was assessed three times for each variety. Respiration rate measurements at each ethylene concentration were replicated three or more times for each cultivar. To account for developmental changes in tuber respiration rate that occur during storage, peak respiration rates for tubers treated with ethylene are reported relative to baseline respiration rate of tubers from the same experiment without added ethylene (i.e. controls). Statistical significance of ethylene-stimulated respiration rate relative to control respiration rate was assessed with one sample, one-tailed t-tests (GraphPad Prism v6.0d, GraphPad Software, Inc., San Diego, CA).
Results
Low Concentrations of Ethylene Were Observed in Potato Storages
Storage atmosphere samples from commercial potato storages contained low concentrations of ethylene. Observations made over three storage seasons are summarized in Fig. 1. Measured ethylene in the storage atmosphere ranged from less than the limit of detection to as much as 45 nl l−1. Air samples containing less than the detectable amount of ethylene (<3 nl l−1) were the most frequent observation in each of the 3 years. Ethylene was not detectable in over 50 % of the 218 samples in the 2010–2011 storage season (Fig. 1a), and this number increased to over 80 % in the following 2 years (Figs. 1b and c). In most cases when ethylene was observed, it was present at less than 20 nl l−1 (Fig. 1). Ethylene concentrations of greater than 50 nl l−1 were not observed in the commercial storages used for this study.
Histograms of ethylene concentration in the atmosphere of commercial potato storages holding tubers harvested in 2010 (a), 2011 (b) and 2012 (c). Multiple samples as indicated were collected throughout the storage period from 17 (a), 18 (b) or 14 (c) storage bins. Values below individual bars in the histograms indicate mid-point ethylene concentration for the bar, and each bar has a range of 3 nl l−1 ethylene
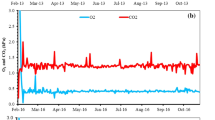
Individual storage bins were sampled multiple times, beginning shortly after bin loading and continuing until unloading. Ethylene was detected in the atmosphere of all storage bins sampled in the 2010 and 2011 crop years. Ethylene was detected in 13 out of 14 storage bins sampled in the 2012 crop year. In storage bins where ethylene was detected, however, it did not persist through the remainder of the storage period. Representative data are presented in Fig. 2, which shows the amount of ethylene in the air of four storage bins sampled from October 2010 through February 2011 Storage bins in which ethylene was observed in the storage atmosphere on a particular date often did not have a detectable quantity of ethylene at a later evaluation date (Fig. 2), and this pattern was often repeated several times within an individual storage bin (Figs. 2 b, c, and d). The only exception to this was when ethylene was first observed immediately prior to bin unloading (data not shown).
Ethylene Application Increased Tuber Respiration Rate
The respiration rate of chipping potato tubers was quantified during short-term exposure to ethylene under laboratory conditions in order to simulate the exposure of tubers to ethylene in storage. Time-dependent changes in observed respiration rate followed kinetics similar to those shown in Fig. 3a. One day after the start of the ethylene treatment, tuber respiration rate began to increase rapidly. Respiration rates were highest at three or four days after ethylene treatment began, and then slowly declined (Fig. 3a). The effect of ethylene on tuber respiration rate is shown in Fig. 3b for three chipping potato varieties. Relative to untreated controls, 20 nl l−1 ethylene increased respiration of Snowden, Lamoka and Pinnacle by 30, 22, and 16 %, respectively. Respiration rates were also significantly elevated at 50 and 200 nl l−1 ethylene. Cultivar differences in ethylene response were most apparent at 200 nl l−1 ethylene, where ethylene-stimulated respiration rates for Pinnacle were almost five times higher than the basal respiration rate of untreated controls (Fig. 3). In contrast, ethylene-stimulated respiration rates in Snowden and Lamoka were 2.5 and 2.1 times, respectively, the respiration rate of the respective untreated controls (Fig. 3b).
Respiration rate (a) of Pinnacle and Lamoka tubers exposed to 200 nl l−1 ethylene for up to 8 days. Note that the highest respiration rate for both varieties was observed three days after ethylene addition to the atmosphere. Relative respiration rate (b) of Snowden, Lamoka and Pinnacle tubers treated with 20, 50 and 200 nl l−1 ethylene. Respiration rates are relative to that of tubers treated with air alone (controls), i.e. 2.05 ± 0.16, 2.44 ± 0.19 and 2.49 ± 0.14 mg CO2 kg−1 h−1 (mean ± standard error) for Snowden, Lamoka and Pinnacle, respectively, averaged across all measurement dates. Values in (b) that are statistically greater than 1.0 at p = 0.05 and p = 0.01 are indicated by * and **, respectively
Chip Darkening Resulting from Short-Term Exposure to Ethylene
Short-term exposure to low concentrations of ethylene similar to those observed in commercial storage bins did not result in chip darkening. Typical examples of chips made from Pinnacle and Snowden tubers treated with 0, 20, 50 or 200 nl l−1 ethylene for four days are shown in Fig. 4. In this figure, each chip represents a different tuber. Mean chip color scores for Pinnacle (Fig. 4a) were 3.1, 3.1, 3.4 and 4.9 at 0, 20, 50 and 200 nl l−1 ethylene respectively. Mean chip color scores for Snowden (Fig. 4b) were 2.7, 2.6, 2.6 and 2.9 at 0, 20 50 and 200 nl l−1 ethylene respectively. For both varieties, chips exposed to 200 nl l−1 ethylene were darker than chips that were not exposed to ethylene and the difference in chip color score was statistically significant at p = 0.05 in 5 out of 6 experiments. Chips treated with 20 nl l−1 ethylene, a concentration of ethylene comparable to that observed in commercial potato storages, were not darker than controls in three independent experiments for each variety. The effect of ethylene at 50 nl l−1 on chip color darkening was less consistent than at 20 nl l−1, with a statistically significant, modest increase in chip color observed in some experiments (for example Fig. 4a) but not in others (Fig. 4b).
Discussion
Potato tubers are highly sensitive to atmospheric ethylene concentrations. How this might impact the quality of potato tubers in ventilated commercial storages depends on when the ethylene is present and at what concentration. In this study, ethylene concentration was quantified in 805 individual air samples removed from commercial potato storages during a 3-year period. Ethylene was not detectable in most samples in each year (Fig. 1), and the measured ethylene concentration in any sample did not exceed 50 nl l−1. Measurements were made immediately before the return air fans. In the case where fresh air is not being introduced into the storage bin, measured ethylene concentrations approximate closely the ethylene concentration in the air delivered to the tubers though the ventilation supply. At many other times, however, fresh air is mixed with the return air and the amount of ethylene in the cooling air forced though the pile would be, on average, less than that measured in this study. These findings parallel closely observations made on ethylene in commercial sugarbeet storages (Fugate et al., 2010). In that case, measured ethylene concentrations in sugarbeet storage piles were less than 2 nl l-l during the first 21 days after piling and less than 60 nl l−1 ethylene at later times (Fugate et al., 2010).
Ethylene was observed more frequently and at higher concentrations in 2010 than in 2011 or 2012 (Fig. 1). Disease pressure during the 2010 storage year was higher than that during the subsequent 2 years. Wet spots resulting from condensation above diseased areas developed on the top of the potato pile early in multiple storages in 2010, and several bins we emptied at mid-season (Figs. 2a, b and d). In contrast, 2011 and especially 2012 had low disease pressure in almost all of the storage bins used for this study. Ethylene production from tubers infected with pathogens has been shown to be greater than that from uninfected tubers (Creech et al., 1973), and many microbes can produce ethylene (Arshad and Frankenberger, 1992). It may be hypothesized that observed year-to-year differences in the frequency at which ethylene was observed in storage, how long ethylene persisted and the amount of ethylene observed may be related to differences in pathogen activity. In this regard, it is worth noting that tuber infections are dynamic processes in which infected tubers may be secondarily infected by other pathogens, such as the soft rot bacteria, Pectobacterium spp, which are facultative anaerobes. Ethylene production is likely to decrease whenever localized conditions become anaerobic, since ethylene production requires oxygen (Lin et al., 2009).
Ethylene was present transiently in potato storage bins (Fig. 2), but in almost all cases the concentration was less than 20 nl l−1 (Fig. 1). Short-term exposure to this concentration of ethylene under laboratory conditions did not result in darkening of Snowden or Pinnacle chips (Fig. 4). Thus, ethylene in the storage atmosphere may not have a significant effect on fried chip color for most tubers in bulk storage. However, ethylene in the atmosphere of potato storages may have a measureable effect on tuber respiration rates. Tuber respiration rates increased with ethylene concentration in the range of 20–200 nl l−1. Rates of respiration are proportional to rates of heat and carbon dioxide production. Thus even small amounts of ethylene can increase cooling requirements and the need for fresh air. These needs relate directly to electricity usage by fan motors and the cooling system. It has also been suggested that higher rates of respiration promote more rapid rates of physiological aging (Blauer et al., 2013). Thus low amounts of ethylene could potentially decrease the duration of storage prior to the onset of senescent sweetening.
Although ethylene concentrations were very low in the combined storage atmosphere of the whole bin, the data presented here do not preclude the possibility that localized, higher concentrations of ethylene exist in parts of the bulk pile where air exchange is poor. Ethylene production from pathogen-infected tubers and restricted air-flow resulting from tuber breakdown may result in localized regions within the potato pile that have ethylene concentrations higher than those reported here. Elevated tuber and microbial respiration rates in these locations might contribute to increased rates of heat production and the creation of “hot spots”. Condensation on the top of the potato pile may thus occur when warm moist air from such regions cools near the top of the pile.
In lab-scale experiments, potato tubers responded to applied ethylene in a consistent manner that included a delay of about one day, an increase in respiration rate that occurred over several days, and a slow decline (Fig. 3a). The delay in the initial response is consistent with the slow binding kinetics of ethylene to its receptor (Morgan, 2011). In previous experiments with applied ethylene, comparable changes in respiration rate with time were observed (Huelin and Barker, 1939; Rylski et al., 1974; Day et al., 1978). In those studies, respiration rates declined to near baseline rates when ethylene was applied continuously. If, on the other hand, ethylene was removed for a period of time and then reapplied, tubers again responded with an increase in respiration (Huelin and Barker, 1939). In the current study, ethylene was not found to persist in individual storage bins, but to appear episodically (Fig. 2). A consequence of this may be that tubers are likely to respond to ethylene each time it appears in the storage, rather than becoming desensitized to it through constant exposure. As a consequence, multiple, discrete periods of ethylene exposure may have a greater effect on average tuber respiration rate than ethylene at a similar concentration that is present during the entire storage period.
References
Arshad, M., and W.T. Frankenberger. 1992. Microbial biosynthesis of ethylene and its influence on plant growth. In Advances in Microbial Ecology, ed. K.C. Marshall. Boston: Springer US.
Blauer, J.M., L.O. Knowles, and N.R. Knowles. 2013. Evidence that tuber respiration is the pacemaker of physiological aging in seed potatoes (Solanum tuberosum L.). Journal of Plant Growth Regulation 32: 708–720.
Brook, R.C., R.J. Fick, and T.D. Forbush. 1995. Potato storage design and management. American Journal of Potato Research 72: 463–480.
Castleberry, H.C., and S.S. Jayanty. 2012. An experimental study of pressure flattening during long-term storage in four russet potato cultivars with differences in at-harvest tuber moisture loss. American Journal of Potato Research 89: 269–276.
Chin, C., and C. Frenkel. 1976. Influence of ethylene and oxygen on respiration and peroxide formation in potato tubers. Nature 264: 60–60.
Coleman, W.K. 1998. Carbon dioxide, oxygen and ethylene effects on potato tuber dormancy release and sprout growth. Annals of Botany-London 82: 21–27.
Costello, B., P. Evans, R. Ewen, H. Gunson, N. Ratcliffe, and P. Spencer-Phillips. 1999. Identification of volatiles generated by potato tubers (Solanum tuberosum CV-Maris Piper) infected by Erwinia carotovora, Bacillus polymyxa and Arthrobacter sp. Plant Pathology 48: 345–351.
Costello, B., P. Evans, R. Ewen, H. Gunson, P. Jones, N. Ratcliffe, and P. Spencer-Phillips. 2001. Gas chromatography–mass spectrometry analyses of volatile organic compounds from potato tubers inoculated with Phytophthora infestans or Fusarium coeruleum. Plant Pathology 50: 489–496.
Creech, D., M. Workman, and M. Harrison. 1973. Influence of storage factors on endogenous ethylene production by potato-tubers. American Potato Journal 50: 145–150.
Daniels-Lake, B.J. 2012. Effects of elevated CO2 and trace ethylene present throughout the storage season on the processing colour of stored potatoes. European Potato Journal 55: 157–173.
Daniels-Lake, B.J., and R.K. Prange. 2009. The interaction effect of carbon dioxide and ethylene in the storage atmosphere on potato fry color is dose-related. HortScience 44: 1641–1644.
Daniels-Lake, B., R. Prange, and J. Walsh. 2005a. Carbon dioxide and ethylene: a combined influence on potato fry color. HortScience 40: 1824–1828.
Daniels-Lake, B., R. Prange, J. Nowak, S. Asiedu, and J. Walsh. 2005b. Sprout development and processing quality changes in potato tubers stored under ethylene: 1 Effects of ethylene concentration. American Journal of Potato Research 82: 389–397.
Day, D.A., G.P. Arron, R.E. Christoffersen, and G.G. Laties. 1978. Effect of ethylene and carbon dioxide on potato metabolism: stimulation of tuber and mitochondrial respiration, and inducement of the alternative path. Plant Physiology 62: 820–825.
Elmer, O. 1932. Growth inhibition of potato sprouts by the volatile products of apples. Science 75: 193.
Fugate, K.K., J.C. Suttle, and L.G. Campbell. 2010. Ethylene production and ethylene effects on respiration rate of postharvest sugarbeet roots. Postharvest Biology and Technology 56: 71–76.
Gottschalk, K. 2011. Recent developments in potato storage in Europe. Potato Journal 38: 85–99.
Hirsch, C.N., C.D. Hirsch, K. Felcher, J. Coombs, D. Zarka, A. van Deynze, W. De Jong, R.E. Veilleux, S. Jansky, P. Bethke, D.S. Douches, and C.R. Buell. 2013. Retrospective view of North American potato (Solanum tuberosum L.) breeding in the 20th and 21st centuries. G3 3: 1003–1013.
Hopkins, E.F. 1924. Relation of low temperatures to respiration and carbohydrate changes in potato tubers. Botanical Gazette 78: 311–325.
Huelin, F., and J. Barker. 1939. The effect of ethylene on the respiration and carbohydrate metabolism of potatoes. New Phytologist 38: 85–104.
Kumar, D., B. Singh, and P. Kumar. 2004. An overview of the factors affecting sugar content of potatoes. Annals of Applied Biology 145: 247–256.
Kunkel, R., and W.H. Gardner. 1965. Potato tuber hydration and its effect on blackspot of russet burbank potatoes in the Columbia basin of Washington. American Potato Journal 42: 109–124.
Lin, Z., S. Zhong, and D. Grierson. 2009. Recent advances in ethylene research. Journal of Experimental Botany 60: 3311–3336.
Lui, L., A. Vikram, Y. Abu-Nada, A. Kushalappa, G. Raghavan, and K. Al-Mughrabi. 2005. Volatile metabolic profiling for discrimination of potato tubers inoculated with dry and soft rot pathogens. American Journal of Potato Research 82: 1–8.
Martínez-Romero, D., G. Bailén, M. Serrano, F. Guillén, J.M. Valverde, P. Zapata, S. Castillo, and D. Valero. 2007. Tools to maintain postharvest fruit and vegetable quality through the inhibition of ethylene action: a review. Critical Review of Food Science and Nutrition 47: 543–560.
Morgan, P.W. 2011. Another look at interpreting research to manage the effects of ethylene in ambient air. Crop Science 51: 903–913.
Prange, R.K., W. Kalt, B.J. Daniels-Lake, C.L. Liew, R.T. Page, J.R. Walsh, P. Dean, and R. Coffin. 1998. Using ethylene as a sprout control agent in stored “russet burbank” potatoes. Journal of the American Society of Horticultural Science 123: 463–469.
Reid, M.S., and H.K. Pratt. 1972. Effects of ethylene on potato tuber respiration. Plant Physiology 49: 252–255.
Rylski, I., L. Rappaport, and H.K. Pratt. 1974. Dual effects of ethylene on potato dormancy and sprout growth. Plant Physiology 53: 658–662.
Schaller, G. 2012. Ethylene and the regulation of plant development. BMC Biology 10: 9.
Schippers, P.A. 1971. The relation between storage conditions and changes in weight and specific gravity of potatoes. American Potato Journal 48: 313–319.
Sowokinos, J.R. 2001. Biochemical and molecular control of cold-induced sweetening in potatoes. American Journal of Potato Research 78: 221–236.
Suttle, J. 1998. Involvement of ethylene in potato microtuber dormancy. Plant Physiology 118: 843–848.
Vandenbussche, F., I. Vaseva, K. Vissenberg, and D. Van Der Straeten. 2012. Ethylene in vegetative development: a tale with a riddle. New Phytologist 194: 895–909.
Varns, J., and M.T. Glynn. 1979. Detection of disease in stored potatoes by volatile monitoring. American Potato Journal 56: 185–197.
Waterer, D., and M. Pritchard. 1985. Production of volatile metabolites in potatoes infected by Erwinia carotovora var carotovora and Erwinia carotovora var atroseptica. Canadian Journal of Plant Pathology 7: 47–51.
Wills, R.B.H., M.A. Warton, and J.K. Kim. 2004. Effect of low levels of ethylene on sprouting of potatoes in storage. HortScience 39: 136–137.
Wustman, R., and P.C. Struik. 2007. The canon of potato science: 35. Seed and ware potato storage. Potato Research 50: 351–355.
Acknowledgments
The author thanks Heartland Farms, Hancock, WI and Mortenson Brothers Farms, Plainfield, WI for access to their potato storages. Financial support from the Wisconsin Potato and Vegetable Growers Association Chip Committee and United States Potato Board is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bethke, P.C. Ethylene in the Atmosphere of Commercial Potato (Solanum Tuberosum) Storage Bins and Potential Effects on Tuber Respiration Rate and Fried Chip Color. Am. J. Potato Res. 91, 688–695 (2014). https://doi.org/10.1007/s12230-014-9400-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-014-9400-1