Abstract
The Endosperm Balance Number (EBN) assigned to each species can foresee success or failure of a given interspecific cross in potatoes, although the underlying molecular basis is poorly studied. We found that the cross of Solanum demissum as female with a breeding line Saikai 35 constantly produced larger seeds (0.94 mg) than those from the reciprocal cross (0.39 mg), suggesting a slightly lower EBN in S. demissum. Crossing behaviors, measured by berry-setting rates, seeds/berry and seed size, in the reciprocal F1 and BC1 progenies suggest at least three genetic factors involved in normal seed development: 1) a cytoplasmic factor, and nuclear genome-encoded factors functioned 2) in female gametophyte and 3) in pollen. Thus, these materials are useful in exploring the molecular mechanism of EBN, because the degree of imbalanced EBN could be measured as quantitative traits.
Resumen
El número de balance endospérmico (EBN) asignado a cada especie puede anticipar éxito o fracaso en una cruza dada interespecífica en papa, aunque la base molecular en la que se respalda es estudiada pobremente. Encontramos que la cruza de Solanum demissum como hembra con una línea de mejoramiento Saikai 35 produjo constantemente semillas más grandes (0.94 mg) que las de la cruza recíproca (0.39 mg), sugiriendo un EBN ligeramente más bajo en S. demissum. Los comportamientos de las cruzas, medidos por los niveles de formación de frutos, semillas/fruto y tamaño de semilla, en las progenies de F1 recíproca y BC1, sugieren por lo menos tres factores genéticos involucrados en el desarrollo normal de semillas: 1) un factor citoplásmico, y funcionamiento de factores citoplásmicos codificados por el genomio nuclear 2) en gametofitos de la hembra y 3) en polen. De aquí que estos materiales son útiles en la exploración del mecanismo molecular de EBN, porque el grado de desbalanceamiento de EBN pudiera medirse como caracteres cuantitativos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the interspecific and inter-ploidy crosses of Arabidopsis, endosperm growth is controlled by a balance between maternally contributed Polycomb repressive complex proteins and paternally contributed AGAMOUS-LIKE Type-1 MADS domain transcription factors in a dosage-dependent manner (Dilkes and Comai 2004; Josefsson et al. 2006; Walia et al. 2009; Köhler et al. 2010). However, little is known on the molecular basis for seed development in interspecific crosses in potato and its relatives (the tuber-bearing Solanum species).
A conceptual explanation, known as the Endosperm Balance Number (EBN) hypothesis (Johnston et al. 1980), has been proposed for endosperm development in interspecific crosses in potato (Ehlendfeldt and Ortiz 1995). According to this hypothesis, a balance of 2:1 maternal to paternal EBN dosage in the endosperm, independent of ploidy, is required for normal endosperm development. EBN values for the various potato species have been determined based on the ease of crossability between standard testers as pollen parents and the species in question, and 2x(1EBN), 2x(2EBN), 4x(2EBN), 4x(4EBN) and 6x(4EBN) species have been identified (Hanneman 1994). The same biological concept, the polar-nuclei activation (PNA) hypothesis has been proposed by Nishiyama and Yabuno (1978) to explain the diverse interspecific crosses in the genus Avena (Katsiotis et al. 1995). The degree of the polar nuclei activation is expressed by the ‘activation index’ (AI), which is the ratio of the ‘activating value’ (AV) of the male gamete to the ‘response value’ (RV) of the female gamete. In a self-pollinated plant AV = RV, and the AI = (AV/2RV) × 100 = 50%, which is the most balanced condition resulting in normal endosperm development. Depending on the AI of the polar nuclei, the kernel type becomes different: AI < 20%—small inviable kernels, 20% < AI < 30%—small viable kernels, 30% < AI < 80%—normal viable kernels, and 80% < AI—large shriveled-empty inviable kernels (Nishiyama and Yabuno 1978). Although for judging the seed viability, plumpness, germinability and/or size are considered (Johnston and Hanneman 1980), seed size itself is not a criterion in determining EBN values. In contrast, kernel size is an important criterion to determine the species’ AV or RV.
Analyses of hybrids between Mexican species of 4x(2EBN) and a South American, colchicine-doubled 4x(2EBN) S. commersonii, or hybrids between a South American 4x(2EBN) S. acaule and a colchicine-doubled 4x(2EBN) S. commersonii, disclosed lack of recombination and segregation for EBN in these hybrids, suggesting that these 4x(2EBN) species carry EBN in a genetically similar way (Bamberg and Hanneman 1990; Bamberg 1994). Ehlendfeldt and Hanneman (1988) obtained exceptional inter-EBN hybrids (1.5 EBN) from the cross between 2x(1EBN) S. commersonii and 2x(2EBN) S. chacoense, and conducted a complete diallele cross including the exceptional hybrids and their parents. They observed that a slight excess of maternal dosage produced viable seeds of reduced size, while a slight excess of paternal dosage produced large seeds or aborted seeds. Based on the observation they proposed a genetic model for EBN, controlled by three unlinked, additive loci in a threshold-like system (Ehlendfeldt and Hanneman 1988). Alternatively, Camadro and Masuelli (1995) proposed a model that the EBN is controlled by two independent loci with two alleles in homozygosity per genome; that is, 4x(2EBN) S. acaule carrying in homozygosity the alleles “0.5” and “0”, 2x(1EBN) S. commersonii carrying the alleles “0.5” and “0” and 2x(2EBN) S. gourlayi carrying the alleles “0.5” and “0.5”. Therefore, although the EBN is practically useful to predict success or failure of a given interspecific cross in potatoes (Ortiz and Ehlenfeldt 1992), genetic understanding of EBN is still controversial. The previous studies were conducted using exceptional hybrids from inter-EBN crosses, so that seemingly, these materials never generated fertile progenies to assess their EBNs. Consequently, studies on genetic and molecular bases of EBN have been greatly limited due to lack of genetic materials.
Solanum demissum Lindl. is a hexaploid Mexican wild species (2n = 6x = 72), and widely used in potato breeding as a source of resistance to the most serious disease, late blight (Phytophthora infestans) (Ross 1986; Plaisted and Hoopes 1989). Both the common potato (S. tuberosum L. 2n = 4x = 48) and S. demissum readily produced plump seeds when crossed with 4x(4EBN) testers, thus having been assigned 4EBN (Johnston and Hanneman 1980; Hanneman 1994). Although S. demissum has an allohexaploid genome structure (AADDDdDd, Matsubayashi 1991), we can easily obtain a pentaploid hybrid from S. demissum × S. tuberosum when S. demissum was used as a female parent. The resultant pentaploid F1 hybrids produce abundant normal-looking pollen grains, but are non-functional as males, and usually produce seeds only if backcrossed with the pollen of S. tuberosum (Dionne 1961).
We successfully obtained interspecific hybrid seeds from S. demissum 5H109-5 by a breeding line Saikai 35 and the reciprocal cross, and noticed that the seed sizes were very different between reciprocal hybrids. For this reason, thousands of crosses between S. demissum and S. tuberosum and among the resultant progenies with various combinations were made. In this article, we report that the crosses between S. demissum 5H109-5 and a breeding line Saikai 35 and the subsequent F1 and BC1 progenies would become useful plant materials to explore the underlying genetic and molecular mechanisms of EBN. We found at least three genetic factors involved in normal seed development: 1) a cytoplasmic factor, and nuclear genome-encoded factors functioned 2) in female gametophyte and 3) in pollen, which are possibly associated with EBN.
Materials and Methods
Plant Materials
Seeds of 25 accessions of S. demissum with different PI numbers (161149, 161155, 161164, 161169, 160208, 160220, 160222, 161365, 161366, 161719, 161729, 175408, 175411, 175423, 186551, 186556, 201850, 201853, 205518, 230488, 230559, 230579, 338619, 365381 and 365382) were obtained from the Potato Introduction Station (NRSP-6), Sturgeon Bay, Wisconsin, USA. A male and female fertile breeding line, Saikai 35, was crossed with S. demissum. Most of S. tuberosum cultivars have chloroplast DNA of T type, as defined by Hosaka (1986), and mitochondrial DNA of β type, as defined by Lössl et al. (1999), (Lössl et al. 2000). The T/β type cytoplasm often causes male sterility by interaction with chromosomal genes (Grun 1979). However, Saikai 35 was maternally descended from S. phureja, having S-type chloroplast DNA and ε-type mitochondrial DNA, which does not cause cytoplasmic male sterility in the progeny.
F1 and BC1 progenies analyzed in this study were all derived from 5H109-5 (S. demissum PI 186551) and Saikai 35. Since S. demissum is highly self-fertile and homogeneous as evidenced by random amplified polymorphic DNA analysis (unpublished data), we assumed 5H109-5 and the selfed plants were all genetically identical and collectively referred to as D. Saikai 35 was referred to as T hereinafter. The cross between D as female and T as male generated DT family (6H37) and the reciprocal cross TD family (6H38). Four plants of DT family (6H37-2, -6, -13 and -23) and four plants of TD family (6H38-7, -8, -19 and -23) were crossed as female with T, deriving BC1 families (DT)T and (TD)T, respectively. One plant of DT family (6H37-23) and four plants of TD family (6H38-43, -58, -84 and -73) were crossed as female with D, deriving BC1 families (DT)D and (TD)D, respectively.
Crossing
All crosses were made in an ordinary manner. Berries were collected 1 month after pollination and seeds were extracted after another 1 month. When seeds were extracted, matured berries were squeezed in water and the debris was flushed out to collect only plump seeds. The seeds were dried naturally and then in a desiccator, followed by counting number of seeds and measuring the weight.
Hybridity Test
Somatic chromosome numbers in root-tip cells were counted by the method of Kurata and Omura (1978) with a slightly modified enzyme solution (2% Cellulase Onozuka RS, 1.5% Macerozyme R-10, 0.3% Pectolyase, 1 mM EDTA, pH adjusted to 4.2). A median from four or five cell counts was used as the somatic chromosome number of each plant.
Total DNA was extracted from fresh leaves by the method of Hosaka and Hanneman (1998). Amplified fragment length polymorphism (AFLP) analysis (Vos et al. 1995) was performed to compare parents and the reciprocal hybrids. Total DNA was double-digested by MspI and EcoRI, ligated to adapters, pre-amplified and selectively amplified by the method essentially described in Vos et al. (1995). Adapter and primer sequences were described in Xiong et al. (1999). For pre- and selective amplification, PCR was set-up in volumes of 10 μl consisting of 0.3 μM primer, 5 μl of Ampdirect® Plus (Shimadzu Co., Japan) and 0.25 units Taq DNA polymerase (Nova Taq™ Hot Start DNA polymerase, Novagen®, USA). A total of 126 primer combinations with seven EcoRI primers and 18 MspI primers were used for selective amplification. The amplification products were electrophoresed on 4% denaturing polyacrylamide gels, and silver-stained (Bassam et al. 1991).
Observation of Pollen Tube Growth
Flowers were emasculated a day before flowering and pollinated. Styles and ovaries were collected 48 h later after pollination, fixed in FAA (1 : 3 = glacial acetic acid : ethanol) at 4°C for 24 h, and after rinsing in water for 30 min, stored in 70% ethanol at 4°C. The pollen tube growth was observed using the aniline blue method modified from Sitch and Snape (1987) as follows . The samples were washed and rehydrated in distilled water for 30 min, and then, softened in 70% lactic acid in a boiling water bath for 10 min. After cooling to room temperature, samples were washed in distilled water for 1 h and left in 0.1 M K3PO4 buffer at 4°C overnight. They were then stained in decolorized aniline blue solution (0.2% w/v in 0.1 M K2HPO4 buffer, pH 11.0) for 3 h. The stained samples were cut with a pair of tweezers, mounted under a cover slip and examined using a fluorescence microscope (Nikon HB-10101AF). The yellow florescence emitted by the stained callose plugs and the linings of pollen tubes were visualized under ultra-violet light.
Results
Reciprocal Crosses between S. tuberosum and S. demissum
One hundred and twenty-two plants raised from 25 S. demissum accessions with different PI numbers (3–10 seedlings per accession) were selfed. The mean berry-setting rate among 122 plants was very high (92.8%) compared with that of Saikai 35 self (68.1%) (Table 1). The number of seeds per berry (166.8) and the mean seed weight (0.41 mg) were not much different from those of Saikai 35 self.
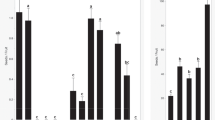
All 110 S. demissum plants (2–5 seedlings per accession) set berries with the pollen from Saikai 35. The mean berry-setting rate was 81.2%. The reciprocal cross was also successful, although the mean berry-setting rate was low (18.7%). The cross of S. demissum × Saikai 35 produced 34.0 seeds per berry and the mean seed weight of 0.94 mg, which were significantly lower and heavier (P < 0.001) than the reciprocal cross (113.2 and 0.39 mg, respectively) (Fig. 1a). The seed size obtained in a berry was considerably uniform in these crosses. When the mean seed size was over 0.80 mg, it is defined as large, because seed sizes in inter-varietal and interspecific hybrids ranged normally from 0.40 to 0.70 mg (unpublished data). In the following experiments, only two parental genotypes were extensively used: 5H109-5 (S. demissum PI 186551) and the selfed progeny (collectively referred to as D) and Saikai 35 (referred to as T).
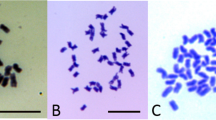
Characterization of reciprocal interspecific hybrids between D and T. a Seed size in TD (left) and DT (right). b AFLP banding patterns with E-AGC and M-TGC primers (1 T, 2 D, 3 TD bulk, 4 DT bulk). c Somatic chromosomes in the root tip cells of TD (left, 6H38-19, 2n = 60) and DT (right, 6H37-6, 2n = 60). d Meiosis at metaphase I in TD (left, 6H38-19, 1IV + 5III + 17II + 7I) and DT (right, 6H37-6, 1IV + 5III + 16II + 9I)
Hybridity Test of Reciprocal Interspecific Hybrids
DNA samples from 6 DT hybrids and those from 7 TD hybrids were bulked, separately. Using 126 AFLP primer pairs, over 12,500 DNA fragments were compared between the two bulked DNA samples, which were identical and the sum of the parental AFLP banding patterns (Fig. 1b), except for a few bands reported elsewhere (Sanetomo and Hosaka 2011). These results supported their hybrid status.
Cytological Analysis of Reciprocal Hybrids and Backcross Progenies
Three DT (6H37-5, -6 and -15) and three TD (6H38-2, -8 and -19) hybrids were counted for somatic chromosome numbers, all of which had 60 chromosomes (Fig. 1c). Chromosome pairing configurations at metaphase-I were observed for one DT hybrid (6H37-6) and one TD hybrid (6H38-19), which showed 0.50IV + 4.75III + 17.08II + 9.67I (n = 12) and 0.33IV + 5.25III + 17.92II + 7.08I (n = 12), respectively (Fig. 1d). These configurations were similar to those reported by Irikura (1976) and Matsubayashi (1991), confirming these were pentaploid interspecific hybrids.
Somatic chromosome numbers were determined for 105 plants of (DT)T (derived from 6H37-6 × T) and 100 plants of (TD)T (derived from 6H38-19 × T). Both BC1 families showed normal distributions for the chromosome number; (DT)T ranging from 49 to 59 with an average of 53.5 (SD = 1.98) and (TD)T ranging from 49 to 60 with an average of 53.8 (SD = 1.93). No significant difference was found between the two families (t = 1.15, P > 0.25).
Crossing Behavior of DT and TD Reciprocal Hybrids
Selfing and sib-crossing of DT hybrids as male were all unsuccessful (Table 2), whereas 4 of 17 TD hybrids set 1–3 berries by selfing and 6 of 53 sib-mating combinations using TD hybrids as male set 1–3 berries, resulting in relatively low mean berry-setting rates (1.7–5.6%) and small numbers of seeds/berry (3.7–10.1).
DT × T showed similar crossing behavior to TD × T. 66.7% of DT and 73.2% of TD hybrids set berries with the mean berry-setting rates of 24.5 and 30.8% and the mean seeds/berry of 32.3 and 33.2, respectively. DT (grown from large seeds) × T produced large seeds of 0.93 mg, and interestingly, TD (grown from small seeds) × T produced also large seeds of 0.97 mg. When DT and TD were used as male onto T, no berry set.
Likewise, DT × D showed similar crossing behavior to TD × D: 40.0% of DT and 50.0% of TD hybrids set berries with the mean berry-setting rates of 21.7 and 25.3%, respectively. The mean seeds/berry was 62.2–84.3 and the mean seed weight of 0.53–0.59 mg. In the reciprocal crosses, although 7 of 9 DT and all 16 TD hybrids set berries onto D, DT and TD hybrids showed significantly different performances. TD hybrids set berries with the significantly higher berry-setting rate (64.9%) and larger number of seeds/berry (46.2) than DT hybrids (24.2% and 30.0, respectively) (P < 0.001 and P = 0.023, respectively). Their mean seed weights were similar (0.62–0.63 mg). In these reciprocal crosses with D, however, it was noticed that berries contained many large empty shriveled, or aborted seeds (uncounted), and even the size of plump seeds remarkably varied in a berry, although the standard deviation among mean seed weights for each cross combination was not high (Table 2).
Crossing Behavior of BC1 Plants
In any cross combinations involving BC1 plants (DT)T, (TD)T, (DT)D and (TD)D, successfulness of crossing largely differed between individual plants, or likely segregated. Moreover, successful crosses produced berries containing many large empty shriveled, aborted seeds, and a wide range of seed size variation was observed (Fig. 2). In a few cross combinations, seed size within a berry was relatively uniform.
Ten of 83 (DT)T and 43 of 112 (TD)T plants set berries by selfing. Thus, (TD)T was more successful (the berry-setting rate of 25.1%) and produced a higher number of mean seeds/berry (55.9) than (DT)T (8.4% and 19.3, respectively) (Table 3). In the sib-crosses, again, (TD)T plants were more successful as males: the pollen from (TD)T plants set more berries (13.7–14.8%) and produced the higher number of seeds/berry (35.3–48.7) than that from (DT)T plants (3.3–11.0% and 14.8–27.2 seeds/berry, respectively). In the crosses with T, (DT)T × T and (TD)T × T showed higher berry-setting rates (28.6–49.2%) and heavier mean seed weight (0.80–0.84 mg) than their reciprocal crosses (1.5–14.7% and 0.57–0.67 mg, respectively). In addition, (TD)T × T showed higher berry-setting rates than (DT)T × T (49.2% vs. 28.6%), and T × (TD)T higher than T × (DT)T (14.7% vs. 1.5%). In the reciprocal crosses with D, the berry-setting rates were relatively high except for the cross D × (DT)T (8.4%). (DT)T × D and (TD)T × D produced the larger number of mean seeds/berry (66.3–74.3) and smaller seed weight (0.45–0.48 mg) than their reciprocal crosses (14.4–31.5 and 0.80–0.85 mg, respectively).
Selfing of (DT)D and (TD)D plants was unsuccessful. One (DT)D plant set one seedless berry, and another one set two selfed berries, of which only one contained one seed weighing 0.50 mg. Thus, the mean seeds/berry for (DT)D family became 0.50. Likewise, five of 111 (TD)D plants set 14 berries by selfing, but only six berries from one plant contained a total of 11 seeds. (DT)D × T and (TD)D × T set berries (the mean berry-setting rates of 14.5% and 22.7%, respectively), but the number of seeds/berry was low (5.2 and 5.3, respectively). T × (DT)D and T × (TD)D set no berry, although the number of pollinations might not be sufficient. In the reciprocal crosses of (DT)D and (TD)D with D, only (TD)D × D showed the moderate berry-setting rate (13.4%). In the cross D × (TD)D, only one of seven plants set one berry containing the relatively high number of seeds (85) with uniformly small size (the mean seed weight of 0.30 mg, ranging from 0.2 to 0.4 mg). (DT)D × D and the reciprocal crosses failed to set berry.
Observation of Pollen Tube Growth
Pollen tubes in reciprocal crosses between T and D penetrated styles normally, reached the base of styles (Fig. 3b) and entered gaps between ovules (Fig. 3c), which strongly indicated that normal fertilization occurred. Even in unsuccessful crosses such as selfing DT and TD, or T × DT and T × TD, pollen tubes were penetrating through styles towards ovaries (Fig. 3a) and reached ovaries (Fig. 3d). In the crosses D × DT and D × TD, a differential crossing ability was found as described above. Yet, pollen tubes of both DT (less successful parent, Fig. 3e) and TD (successful parent, Fig. 3f) apparently reached ovaries of T.
Parental and Cytoplasmic Effects
Mean values of the berry-setting rate, seeds/berry and seed weight were extracted from Tables 1, 2 and 3 and arranged to display parental (as female or male) and cytoplasmic (D or T cytoplasm) differences in Table 4.
There was a tendency that the percentage berries/flower was positively correlated with mean seeds/berry (r = 0.503), but the mean seeds/berry was negatively correlated with seed weight (r = −0.460). Thus, the berry-setting rate, seeds/berry and seed weight were likely associated among them, indicating that if a certain cross easily sets berries, more number of seeds with small sizes were expected.
In addition to this general association, we found three tendencies. First, the cytoplasmic difference was prominent. Irrespective of being crossed as male or female, F1 and BC1 progenies with T cytoplasm always showed higher berry-setting rates (the average of 2.04 times) than those with the D cytoplasm (t = 3.852, P < 0.001, by the paired t test). Second, irrespective of cytoplasm or male parent, when F1 and BC1 progenies were crossed as female, the berry-setting rates decreased with increasing D-derived germplasm in the female; that is, (DT)D < DT < (DT)T or (TD)D < TD < (TD)T (theoretically consisted of the average of 82%, 60% and 33% D germplasm, respectively). The third finding was a little complicated. When the F1 and BC1 progenies were used as males, berry-setting rates were optimized under a certain balance: onto T, only (DT)T or (TD)T hybrids with 33% D germplasm set berries, whereas onto D, DT or TD hybrids with 60% D germplasm showed the highest berry-setting rates. In the latter case, (DT)T or (TD)T plants with less D germplasm (33%) in pollen produced heavier seeds, while (DT)D or (TD)D plants with larger D germplasm (82%) in pollen produced smaller seeds.
Discussion
It is generally known that S. demissum easily sets berries with the pollen of S. tuberosum, while the reciprocal cross is unsuccessful. The resultant pentaploid F1 hybrids are non-functional as males, and produce seeds only if backcrossed with the pollen of S. tuberosum (Dionne 1961). The present study reconfirmed this unilateral incompatibility (UI) in the cross between a large number of S. demissum accessions and T. However, the degree of UI was not complete, but rather quantitative as observed in the berry-setting rates; 81.2% in S. demissum × T vs. 18.7% in T × S. demissum. Dionne (1961) suggested that this male sterility was attributed to the interaction of a cytoplasmic factor or factors in S. demissum with nuclear factors contributed by the male parents. However, the pollen from TD hybrids that had the T cytoplasm was also non-functional on T, and in fact, the pollen from both DT and TD hybrids were functional on D (Table 2). These facts make it implausible to attribute the non-functional pollen on S. tuberosum to male sterility caused by interaction with the S. demissum cytoplasm.
Although UI is usually explained by differential pollen tube growth between reciprocal crosses, no apparent inhibition of pollen tube growth was observed in this study in any cross combinations that failed seed formation. Thus, the UI between S. tuberosum and S. demissum and the subsequent incompatibilities occurred in backcrossing were likely caused by a post-zygotic failure of seed formation. We observed apparent size difference between DT and TD seeds, the former being significantly larger than the latter. Both EBN and PNA hypotheses predict that a slight excess of maternal dosage will produce small seeds, while a slight excess of paternal dosage will produce large seeds (Nishiyama and Yabuno 1978; Ehlendfeldt and Hanneman 1988; Ehlendfeldt and Ortiz 1995). Thus, we propose that there was an imbalance of EBN between D and T, D having a slightly lower EBN than T. In this context, we can explain our results as follows; the cross D × T produced large seeds with much higher aborted seeds that were actually flushed out during seed extraction, resulting in a higher berry-setting rate, smaller number of seeds/berry and large seeds. In contrast, the cross T × D produced small or too small inviable seeds not sufficient to retain berries, resulting in a low berry-setting rate, larger number of seeds/berry and small seeds.
We found three genetic factors involved in normal seed development: 1) a cytoplasmic factor, and nuclear genome-encoded factors functioned 2) in female gametophyte and 3) in pollen. Reducing D germplasm in female gametophyte increased or recovered berry-setting rates (Table 4), indicating nuclear genome-encoded factor(s) of D functioned negatively as a suppressor for endosperm development. In pollen, the proportion of D germplasm in optimizing the berry-setting rates depended upon whether the female was D or T (Table 4), implying that the EBN-like balance system functioned. Three additive loci in a threshold-like system (Ehlendfeldt and Hanneman 1988) or two independent loci with two alleles in homozygosity (Camadro and Masuelli 1995) have been proposed for EBN-controlling genes, and the EBN-controlling genes would segregate and are randomly distributed into gametes (Camadro and Masuelli 1995). Apparently, these nuclear genome-encoded factor(s) of D were segregating in the BC1 populations due to aneuploidy and recombination. BC1 plants as aneuploids showed wide variation in berry-setting rates due to segregation to berry-setting/non-berry-setting plants and the degree of berry-setting rate in each of berry-setting plants (Table 3). In addition, the number of seeds/berry and seed weight also varied between plants (Table 3, Fig. 2). Thus, the BC1 populations are useful as mapping populations to genetically localize the nuclear genome-encoded factor(s). We have constructed a molecular marker-based genetic map of D using one of BC1 populations (unpublished), and characterization of each BC1 plant for crossability is under going.
Since the postulated EBN-controlling genes have additive effect (Ehlendfeldt and Hanneman 1988), both DT and TD hybrids would have the same EBN value consisted of a half of D and a half of T. Nevertheless, DT and TD hybrids were apparently different in the crossing behaviors, particularly in crosses onto D (Table 2). We compared pollen DNA of DT and TD, which revealed parental differences of chloroplast and mitochondrial DNA as expected, and in addition, a few DNA and DNA methylation level differences (Sanetomo and Hosaka 2011). Thus, other factors than EBN were likely involved. We found a cytoplasmic, or maternally inherited factor of D, which reduced berry-setting rates in all cross combinations (Table 4). This maternally inherited factor suppressed berry-setting in the female germ line, and interestingly in pollen or through pollen, too. As generally recognized, paternal organelle DNA is not delivered with sperm cells into egg cell nor central cell. Sperm nuclei in pollen, however, are modified differentially by DNA methylation or histone modification from vegetative nucleus during pollen maturation and have a distinct and diverse transcriptional profile, which may deliver specific mRNA or small RNA by fertilization to the central cell (Gutiérrez-Marcos et al. 2006; Okada et al. 2006; Borges et al. 2008; Singh et al. 2008; Ribeiro et al. 2009; Slotkin et al. 2009). Thus, the D cytoplasm in pollen might affect central cell and repress endosperm development, which consequently becomes equivalent to non-functional pollen as described by Dionne (1961).
In conclusion, the cross between S. demissum 5H109-5 and a breeding line Saikai 35, and the derived F1 and BC1 progenies would become very useful plant materials to explore underlying genetic and molecular mechanisms of EBN, because the degree of imbalanced EBN could be seen visibly as variable seed sizes and measured quantitatively. We found at least three contributory factors to the development of interspecific hybrid seeds in these materials. Fortunately, these materials do not encounter cytoplasmic male sterility that often occurs when the S. tuberosum cytoplasm is present (Grun 1979), because the cytoplasm of Saikai 35 was derived from S. phureja. Thus, the crossability in F1 and BC1 progenies can be regarded totally as effects of interaction between the three factors. The present aneuploids can be maintained vegetatively as tubers, which would be an advantage over the model plant Arabidopsis.
References
Bamberg, J.B. 1994. Allelism of endosperm balance number (EBN) in Solanum acaule Bitt. and other wild potato species. Theoretical and Applied Genetics 89: 68–686.
Bamberg, J.B., and R.E. Hanneman Jr. 1990. Allelism of Endosperm Balance Number (EBN) in Mexican tuber-bearing Solanum species. Theoretical and Applied Genetics 80: 161–166.
Bassam, B.J., G. Caetano-Anolles, and P.M. Gresshoff. 1991. Fast and sensitive silver staining of DNA in polyacrylamide gels. Analytical Biochemistry 196: 80–83.
Borges, F., G. Gomes, R. Gardner, N. Moreno, S. McCormick, J.A. Feijó, and J.D. Becker. 2008. Comparative transcriptomics of Arabidopsis sperm cells. Plant Physiology 148: 1168–1181.
Camadro, E.L., and R.W. Masuelli. 1995. A genetic model for the endosperm balance number (EBN) in the wild potato Solanum acaule Bitt. and two related diploid species. Sexual Plant Reproduction 8: 283–288.
Dilkes, B.P., and L. Comai. 2004. A differential dosage hypothesis for parental effects in seed development. The Plant Cell 16: 3174–3180.
Dionne, L.A. 1961. Cytoplasmic sterility in derivatives of Solanum demissum. American Potato Journal 38: 117–120.
Ehlendfeldt, M.K., and R.E. Hanneman Jr. 1988. Genetic control of Endosperm Balance Number (EBN): Three additive loci in a threshold-like system. Theoretical and Applied Genetics 75: 825–832.
Ehlendfeldt, M.K., and R. Ortiz. 1995. Evidence on the nature and origins of endosperm dosage requirements in Solanum and other angiosperm genera. Sexual Plant Reproduction 8: 189–186.
Grun, P. 1979. Evolution of the cultivated potato: A cytoplasmic analysis. In The biology and taxonomy of the Solanaceae, ed. J.G. Hawkes, R.N. Lester, and A.D. Skelding, 655–665. London: Academic.
Gutiérrez-Marcos, J.F., L.M. Costa, M.D. Prà, S. Scholten, E. Kranz, P. Perez, and H.G. Dickinson. 2006. Epigenetic asymmetry of imprinted genes in plant gametes. Nature Genetics 38: 876–878.
Hanneman Jr., R.E. 1994. Assignment of Endosperm Balance Numbers to the tuber-bearing Solanums and their close non-tuber-bearing relatives. Euphytica 74: 19–25.
Hosaka, K. 1986. Who is the mother of the potato?—restriction endonuclease analysis of chloroplast DNA of cultivated potatoes. Theoretical and Applied Genetics 72: 606–618.
Hosaka, K., and R.E. Hanneman Jr. 1998. Genetics of self-compatibility in a self-incompatible wild diploid potato species Solanum chacoense. 2. Localization of an S locus inhibitor (Sli) gene on the potato genome using DNA markers. Euphytica 103: 265–271.
Irikura, Y. 1976. Cytogenetic studies on the haploid plants of tuber-bearing Solanum species. 2. Cytological investigations on haploid plants and interspecific hybrids by utilizing haploidy (in Japanese). Research Bulletin of the Hokkaido National Agricultural Experiment Station 115: 1–80.
Johnston, S.A., and R.E. Hanneman Jr. 1980. Support of the endosperm balance number hypothesis utilizing some tuber-bearing Solanum species. American Potato Journal 57: 7–14.
Johnston, S.A., T.P.M. den Nijs, S.J. Peloquin, and R.E. Hanneman Jr. 1980. The significance of genetic balance to endosperm development in interspecific crosses. Theoretical and Applied Genetics 57: 5–9.
Josefsson, C., B. Dilkes, and L. Comai. 2006. Parent-dependent loss of gene silencing during interspecies hybridization. Current Biology 16: 1322–1328.
Katsiotis, A., R.E. Hanneman Jr., and R.A. Forsberg. 1995. Endosperm Balance Number and the polar-nuclei activation hypotheses for endosperm development in interspecific crosses of Solanaceae and Gramineae, respectively. Theoretical and Applied Genetics 91: 848–855.
Köhler, C., O.M. Scheid, and A. Erilova. 2010. The impact of the triploid block on the origin and evolution of polyploidy plants. Trends in Genetics 26: 142–148.
Kurata, N., and T. Omura. 1978. Karyotype analysis in rice. 1. A new method for identifying all chromosome pairs. Japanese Journal of Genetics 53: 251–255.
Lössl, A., N. Adler, R. Horn, U. Frei, and G. Wenzel. 1999. Chondriome type characterization of potato: mt α, β, γ, δ, ε and novel plastid-mitochondrial configurations. Theoretical and Applied Genetics 99: 1–10.
Lössl, A., M. Götz, A. Braun, and G. Wenzel. 2000. Molecular markers for cytoplasm in potato: male sterility and contribution of different plastid-mitochondrial configurations to starch production. Euphytica 116: 221–230.
Matsubayashi, M. 1991. Phylogenetic relationships in the potato and its related species. In Chromosome engineering in plants: Genetics, breeding, evolution, Part B, ed. T. Tsuchiya and P.K. Gupta, 93–118. Amsterdam, Oxford, New York, Tokyo: Elsevier.
Nishiyama, I., and T. Yabuno. 1978. Causal relationships between the polar nuclei in double fertilization and interspecific cross-incompatibility. Cytologia 43: 453–466.
Okada, T., M.B. Singh, and P.L. Bhalla. 2006. Histone H3 variants in male gametic cells of lily and H3 methylation in mature pollen. Plant Molecular Biology 62: 503–512.
Ortiz, R., and M.K. Ehlenfeldt. 1992. The importance of Endosperm Balance Number in potato breeding and the evolution of tuber-bearing Solanum species. Euphytica 60: 105–113.
Plaisted, R.L., and R.W. Hoopes. 1989. The past record and future prospects for the use of exotic potato germplasm. American Potato Journal 66: 603–627.
Ribeiro, T., W. Viegas, and L. Morais-Cecílio. 2009. Epigenetic marks in the mature pollen of Quercus suber L. (Fagaceae). Sexual Plant Reproduction 22: 1–7.
Ross, H. 1986. Potato breeding-problems and perspectives. Berlin: Verlag Paul Parey.
Sanetomo, R., and K. Hosaka. 2011. Reciprocal differences in DNA sequence and methylation status of the pollen DNA between F1 hybrids of Solanum tuberosum × S. demissum. Euphytica. doi:10.1007/s10681-011-0444-8.
Singh, M.B., P.L. Bhalla, and S.D. Russell. 2008. Molecular repertoire of flowering plant male germ cells. Sexual Plant Reproduction 21: 27–36.
Sitch, L.A., and J.W. Snape. 1987. Factors affecting haploid production in wheat using the Hordeum bulbosum system. 1. Genotypic and environmental effects on pollen grain germination, pollen tube growth and the frequency of fertilization. Euphytica 36: 483–496.
Slotkin, R.K., M. Vaughn, F. Borges, M. Tanurdzic, J.D. Becker, J.A. Feijo, and R.A. Martienssen. 2009. Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136: 461–472.
Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. van de Lee, M. Hornes, A. Frijters, J. Pot, J. Peleman, M. Kuiper, and M. Zabeau. 1995. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Research 23: 4407–4414.
Walia, H., C. Josefsson, B. Dilkes, R. Kirkbride, J. Harada, and L. Comai. 2009. Dosage-dependent deregulation of an AGAMOUS-LIKE gene cluster contributes to interspecific incompatibility. Current Biology 19: 1128–1132.
Xiong, L.Z., C.G. Xu, and M.A. Saghai Maroof. 1999. Patterns of cytosine methylation in an elite rice hybrid and its parental lines, detected by a methylation-sensitive amplification polymorphism technique. Molecular and General Genetics 261: 439–446.
Acknowledgements
We thank the US Potato Genebank at Sturgeon Bay, Wis. for providing Solanum seeds. This research was supported in part by a Grant-in-Aid (No. 18580005) for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan, and Calbee Potato Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanetomo, R., Ono, S. & Hosaka, K. Characterization of Crossability in the Crosses between Solanum demissum and S. tuberosum, and the F1 and BC1 Progenies. Am. J. Pot Res 88, 500–510 (2011). https://doi.org/10.1007/s12230-011-9217-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-011-9217-0