Abstract:
Leucoagaricus taniae (Agaricaceae) is described as new to science. It was found growing in sand at Armação beach on Santa Catarina Island in Southern Brazil covered by sand among Canavalia rosea (Fabaceae) and Cyperus pedunculatus (Cyperaceae). The new species has a cream white pileus that is up to 50 mm in diameter and exudes golden drops when fresh. The stipe is up to 60 mm height, with rhizomorphs at the end of the volvate base. Photographs of fresh basidiomes in situ and illustrations of the main micromorphological features are presented. A molecular-phylogenetic analysis that includes the species is also presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Leucoagaricus Locq. ex Singer is a genus of Agaricaceae and comprises approximately 135 species (He et al., 2019). Representatives of the genus are saprotrophic and grow in soil, mostly in tropical and subtropical regions (Singer, 1986; Vellinga, 2004a). The genus is characterized morphologically by an entire or slightly striate pileus margin that is never plicate, free lamellae, metachromatic basidiospores in Cresyl blue, and absence of clamp connections and pseudoparaphyses (Singer, 1948, 1986).
Several molecular studies have demonstrated that the genus is heterogeneous and may not be monophyletic since species of Leucoagaricus and Leucocoprinus Pat. were recovered intermixed in a single clade (Johnson & Vilgalys, 1998, Johnson, 1999; Vellinga, 2004b; Vellinga et al., 2011). Because of the large number of species in the genus, the small number of taxa included in molecular studies and the low support in inferred phylogenies, the circumscription of Leucoagaricus (and conversely Leucocoprinus) remains unclear (Vellinga, 2004b; Vellinga et al., 2011). Neotropical taxa are poorly represented in these studies and most sequenced samples are of European and North American species (Johnson, 1999; Vellinga, 2004b; Vellinga et al., 2011). However, recent papers have examined phylogenetic relationships among New World species, describing new taxa from Brazil (Heisecke et al., 2021) and the Dominican Republic (Justo et al., 2015, 2021). The ant-fungus mutualism between La. gongylophorus (Möller) Singer and the leaf-cutter ants has also been studied with phylogenetic approaches (Chapela et al., 1994; Mueller et al., 1998, 2001; Pagnocca et al., 2001; Silva-Pinhati et al., 2004; Ješovnik et al., 2017).
Knowledge of Brazilian fungal diversity has improved in the last decade (Maia & Carvalho-Jr., 2010; Forzza et al.,2010, 2012; Maia et al.,2015). As has the quantity of DNA sequences from Brazilian fungal samples deposited in GenBank (Menolli & Sánchez-Garcia, 2019). Even so, Leucogaricus remains understudied and its current taxonomic understanding in Brazil is based on regional treatments or species lists (Menolli & Sánchez-Garcia 2019). Twenty species of Leucoagaricus have been reported from Brazil (Spegazzini, 1889; Möller, 1893; Rick, 1920, 1937, 1961; Singer, 1949, 1953, 1989; Grandi et al., 1984; Batista, 1957; Muchovej, et al., 1991; Pegler, 1997; Spielmann & Putzke, 1998; Maia et al., 2002; Sobestiansky, 2005; De Meijer, 2006; Albuquerque et al., 2007; Rother & Silveira, 2008, 2009; Wartchow et al., 2008; Rosa & Capelari, 2009; Ferreira & Cortez, 2012; Magnago et al., 2013; Heisecke et al., 2021). Among them, seven species (Leucoagaricus abbreviated below as La.) have been described from specimens collected in Brazilian territory: La. confusus (Rick) Singer (Rick, 1937, 1961; Singer, 1949, 1953), La. gongylophorus (Möller) Singer (Möller, 1893; Spielmann & Putzke, 1998), La. nzumbae C. Heisecke, A.A. Carvalho & M.A. Neves (Heisecke et al., 2021), La. olivaceomamillatus Singer (Rick, 1920, 1961; Singer, 1949, 1953), La. tricolor Singer (Singer, 1989), La. imperialis (Speg.) Pegler (Spegazzini, 1889; Pegler, 1997), and La. weberi Muchovej, Della Lucia & R. Muchovej (Muchovej et al., 1991).
Here we describe Leucoagaricus taniae, a new species from Santa Catarina State, Brazil, based on morphological and molecular data. This is the first species of the genus known to grow in sand in the coastal restinga ecosystem in Brazil (Araujo et al., 1998). This is unusual because species of Leucoagaricus are reported to need a higher availability of nutrients than other Lepiotaceous fungi (Vellinga, 2004b), and restinga ecosystem soils are chemically poor with the main sources of nutrients being salt spray deposition (Leão & Dominguez, 2000; Scarano, 2002; Barcelos et al., 2012) and flooding (Magnago et al., 2010). Only four species of Leucoagaricus have been reported from sandy coastal environments to date: La. gaillardii Bon & Boiffard from France (Bon & Boiffard, 1974; Gennari et al., 1995), La. menieri (Sacc.) Singer also from France (Saccardo, 1891), La. singeri (Bon ex Contu & Signor.) Consiglio & Contu from Italy and Uruguay (Singer, 1968; Bon, 1993; Consiglio & Contu, 2004), and La. subvolvatus (Malençon & Bertault) Bon from Italy, Morocco, and Spain (Gennari et al., 1995). Another species that could be related to this sand inhabiting group of species is Amanita cystidiosa O.K. Mill. & Lodge, which also was recorded for sand dunes in Puerto Rico (Miller et al., 2000), and was cited by Vellinga (2010) as a Lepiotaceous fungus. However, it was not formally transferred to Leucoagaricus.
Material and methods
Throughout this manuscript the abbreviation L. is used for Lepiota (Pers.: Fr.) S. F. Gray, La. for Leucoagaricus and Lc. for Leucocoprinus.
Field Sampling and Morphological Studies
A total of 5 collections of La. taniae were made at Armação beach on the south of Santa Catarina Island. Additionally, 11 other collections of Lepiota s.l. from Santa Catarina State were included in this study (Table 1). The basidiomes were collected and characterized as follows. A small portion of the basidiome was preserved in silica gel for DNA extraction. Macromorphological characters were studied from fresh material following Lodge et al. (2004) and Largent (1986). Color codes (e.g., 1A1) were assigned according to Kornerup and Wanscher (1978). Micromorphological studies were made from hand cut sections of dried specimens mounted in water, or 3% KOH, or 3% KOH plus Congo red, Cresyl blue, or Melzer’s reagent (Largent, 1977; Singer, 1986). At least 20 of each structure were measured for each collection. The length-width ratio of the basidiospores is indicated by Q, and its average by avQ (Largent et al., 1977; Vellinga, 1988). Descriptive terminology follows Vellinga (1988) and Vellinga and Noordeloos (2001).
DNA Extraction, Amplification and Sequencing
DNA was extracted using the CTAB method (Dentinger et al., 2010). PCR amplifications of the nuclear rITS1–5.8S-ITS2 (ITS) loci were made with the primers ITS8F and ITS6R, following the procedures described by Dentinger et al. (2010). PCR products were purified with Polyethylene glycol (PEG) 20%. Sequencing was undertaken at Fundação Oswaldo Cruz (Fiocruz), Minas Gerais State, Brazil. The generated sequences and their respective chromatograms were manually checked and edited with Geneious 6.1.8 (Kearse et al., 2012), and then verified with BLAST searches. The sequences were deposited in GenBank (accession numbers MT952873–MT952884).
Taxon Sampling, Alignment and Phylogenetic Analyses
Sequences of the internal transcribed spacer (ITS) from 57 specimens were used for molecular analyses and are detailed in Table 1. Twelve new sequences of ITS were generated for this work, one corresponds to an accession of new species and the others to accessions of Chlorophyllum (1), Leucoagaricus (5) and Leucocoprinus (5) from Santa Catarina, Brazil (see Table 1). The ingroup includes representatives of Leucoagaricus and Leucocoprinus. The sequences chosen for comparison were selected based on results of a BLAST search for the new species sequence; only returned sequences with Expect (e) values of 0.0 were chosen. In addition, sequences of Leucoagaricus species that exhibit drops on the pileus surface and other putatively related species were included (Vellinga & Balsley, 2010, Yuan et al., 2014; Dutta et al., 2021); as were Leucoagaricus species that occur on sand (Gennari et al., 1995; Vellinga et al., 2011; Munoz et al., 2015); whitish and fleshy species of the genus (Vellinga, 2004b; Vellinga & Davis, 2006); and La. nzumbae, recently described from Brazil (Heisecke et al., 2021). Sequences of Lepiota atrodisca Zeller, L. besseyi H.V. Sm. & N.S. Weber, L. subclypeolaria (Berk. & M.A. Curtis) Sacc. and L. roseolivida Murrill were included in the ingroup because they fall into the Leucoagaricus clade even though the new combinations transferring them into Leucoagaricus have not yet been made. Three sequences of Chlorophyllum were chosen as outgroups (Vellinga et al., 2011).
Sequences were aligned using the MAFFT 7 (Katoh, 2013) plugin in the Geneious software package with the E-INS-I strategy. The aligned matrix is available in Suppl. Material 1. Phylogenetic analyses using Maximum Likelihood (ML) and Bayesian Inference (BI) were performed. The aligned matrix was divided in three partitions corresponding to ITS1, 5.8S and ITS2. ML analysis was carried out in RAxML 8.2 (Stamatakis, 2014) using the rapid bootstrap algorithm with 1000 replicates associated with a search of the best-scoring ML tree, the GTRGAMMA model, and other default parameters. The best nucleotide substitution model for each partition was estimated using jModelTest 2.1 (Darriba et al., 2012) and selected based on the Akaike Information Criterion (AIC). The best models were GTR+G+I for ITS1 and ITS2, and GTR+I for 5.8S. BI analysis was performed using MyBayes 3.2 (Ronquist et al., 2011) with two independent analyses, each with four Monte Carlo Markov Chains, 20,000,000 generations, sampling frequency of 1000, and burn-in of 10%. The best nucleotide substitution models as described above were applied for each partition. Results from BI were examined in Tracer 1.6 (Rambaut et al., 2014) to ensure convergence of the analyses and sufficient sample size of each parameter (>200). The results were visualized in FigTree 1.4 (http://tree.bio.ed.ac.uk/software/figtree). All analyses were done using the CIPRES Science Gateway 3.3 (Miller et al., 2010).
Results
Phylogeny
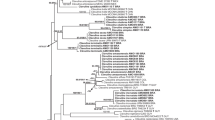
The ITS matrix was 858 characters in length; 54% of the characters were variable and 43.6% were parsimony informative. The ML and BI analyses recovered trees with congruent topologies. Only the ML tree with both BS and BPP values is shown here (Fig. 1). ITS sequences from representatives of Leucoagaricus and Leucocoprinus were mixed together in a single well-supported clade (PP 1.0, BS 99%), suggesting, as reported previously by Johnson and Vilgalys (1998), Johnson (1999), Vellinga (2004b), and Vellinga et al. (2011), that the two genera are not reciprocally monophyletic. Leucoagaricus taniae was placed in a strongly supported clade (PP 1.0, BS 83%), together with two other undetermined Brazilian species of Leucocoprinus (CHC101/MT952882 and CHC102/MT952883).
Phylogenetic tree based on Maximum Likelihood analysis of ITS sequences showing relationships among Leucoagaricus taniae and related species. Posterior probability (PP) (above 0.7) and bootstrap (BS) (above 50%) support values are shown above branches (PP/BS). The sequences generated in this work are in bold. GenBank accession numbers are given for each terminal followed by the country of origin of the specimen (2-letter codes given by the ISO). Chlorophyllum species were used as the outgroup.
Taxonomic Treatment
Leucoagaricus taniae C.Heisecke & M.A.Neves sp. nov. MycoBank: MB838221.—Type: Brazil: Santa Catarina, Florianópolis, Área de Preservação Permanente Praia da Armação, 27°44′39”S, 48°30′28.3”W, 3 June 2018, Neves MAN1266 (holotype: FLOR [!]; isotype: BR [!]). (Figs. 2 and 3.)
Diagnosis—Most similar to Leucoagaricus amanitoides R.M.Davis & Vellinga, but distinguished by the presence of yellow-brown drops on the pileus when fresh (versus the absence of any exudate drops in La. amanitoides), a relatively narrower stipe that can reach approximately 11 mm wide in the center and 22 mm wide at the bulbous base (vs. 20 mm wide in the center and ca. 30–35 mm wide in the turbinated bulbous base in La. amanitoides), and cheilocystidia that are broadly ellipsoidal to subglobose (vs. narrowly clavate and often slightly capitate in La. amanitoides).
Description.—Pileus 27–50 mm diameter, hemispherical when young, later expanding to plano-convex or applanate, sometimes slightly depressed at the center; pileus surface dry, smooth to slightly radially fibrillose, white to yellowish white (1A1, 1A2) or light orange to yellowish brown (5A4–5, 5D5) when fresh, producing liquid exudates; margin often exceeding the lamellae, straight, entire; context 5 mm broad, white, with no change in color. Lamellae, crowded, free, ventricose, 3–5 mm wide, white becoming cream to light yellow in older basidiomes; edges entire to slightly serrulate. Stipe 65–80 × 10–11 mm, central, cylindrical with a bulbous base 17–22 mm wide; context fistulose; surface fibrillose. Annulus inferior, white, membranous, fragile, easily detachable, and ascending. Basidiospore print pale yellow (1A3). Smell and taste not recorded.
Basidiospores [375, 10, 5] 7.2–10 × 5.3–7.3 μm, on average 8.5 × 6 μm, Q = 1.38–1.64, avQ = 1.37, ellipsoid to broadly ellipsoid to obovoid, aguttulate or uniguttulate, yellowish in water, pale to hyaline in KOH, dextrinoid, metachromatic, thick-walled, without germ pore but an inconspicuous germ pore is visible in Melzer. Basidia 22.2–38.4 × 9.6–12.8 μm, clavate, 4-spored. Lamella edges sterile. Cheilocystidia 23.6–31.3 × 10.3–17 μm, ellipsoidal, broadly ellipsoidal to subglobose, generally short-pedunculate, hyaline, slightly thick-walled. Pleurocystidia absent. Hymenophoral trama subregular, subhymenium cellular. Pileus covering a cutis made up of interwoven, cylindrical to slightly articulated repent hyphae, up to 13 μm diameter, pale yellowish in water and paler in KOH, thick-walled. Stipe covering a cutis made of repent cylindrical hyphae, hyaline in water and KOH, thin-walled. Scattered oleiferous hyphae present in all parts of the basidiome, most numerous in the pileus context. Clamp connections absent.
Habitat and distribution.—Scattered or in small groups over a large area of the beach. It can be difficult to spot because it is almost completely buried in the sand with only part of the pileus above the sand layer, among bushes of Canavalia rosea and Cyperus pedunculatus. Even though both plant species have a wide distribution along the coast of Brazil, they are restricted to beach habitats (Brazil Flora Group, 2021), and the coarse sand found in the Armação beach is uncommon in other areas of the coast. In the last ten years, several mycologists have undertaken mycological surveys at other sites where the same plants occur, but this new species has not been seen. Armação beach is subjected to tidal fluctuations, and almost every year the ocean invades the sand area, disturbing the vegetation that grows there and creating a novel set of conditions (Neves, personal observation).
Etymology.—In honor of Tania Cuturi, whose name was suggested by one of the Synchronicity Earth supporters, a UK conservation charity, which donated financial support that helped this research.
Common name.—Seashell mushroom (cogumelo-concha-da-praia) because it looks like a seashell, and it grows on the sand at the beach, among seashells.
Conservation status.—As discussed above, Leucoagaricus taniae is only known from one small strip of beach in Southern Brazil, on Santa Catarina Island, Florianópolis, in the Área de Preservação Permanente Praia da Armação. The authors know of no other sites in Santa Catarina or Southern Brazil with similar characteristics. So, even though La. taniae is difficult to find as its basidiomes grow buried in the sand, its very restricted distribution is likely due to habitat specificity and not low detectability. The beach is approximately 3.2 km long and 5–20 m wide, and the species has been recollected at this site over several years. The species is assessed as Critically Endangered under the IUCN Red List D criterion (IUCN, 2012; IUCN Standards and Petitions Committee, 2019), given that (1) the number of sites with suitable habitat is small, (2) survey work along the coast of Southern Brazil has been ongoing for over ten years, and (3) the total population size of the species is estimated to be less than 50 based on recommendations for inferring population size by Dahlberg & Mueller (2011). It does not meet criterion B as there is no evidence of continuing decline in population size. While the species appears resilient to tidal fluctuations, increased tourism, ongoing illegal development, and rising sea level due to climate change are threats (Barcelos et al., 2012, Clark et al., 2015). As with almost all fungi, continued fieldwork to confirm its range and better understand its ecological specificity is needed.
Additional specimens examined.—BRAZIL. Santa Catarina: Florianópolis: Área de Preservação Permanente Praia da Armação, 27°44′39”S, 48°30′28.3”W, 07 May 2016, Smith & Drewinski MAN1206 (FLOR, RB); 22 May 2016, Neves & Smith MAN1187 (FLOR); 26 May 2016, Neves & Caddah MAN1188 (FLOR); 03 June 2018, Neves & Smith MAN1267 (FLOR).
Notes.—Leucoagaricus taniae is characterized macromorphologically by fleshy basidiomes, a smooth and white to yellowish pileus with yellow-brown drops on the surface when fresh, and a bulbous stipe. Micromorphogically it is characterized by broad basidiospores, broadly ellipsoidal to subglobose cheilocystidia, and a pileus covering composed of pale to hyaline repent hyphae. Morphologically, the most similar species is La. amanitoides described from the vast Central Valley of California, U.S.A. Both species have whitish pilei, bulbous stipes and cuticular pileus coverings (Vellinga & Davis, 2006). Leucoagaricus amanitoides was not reported as producing drops on its pilei. It also differs by the wider stipe that can reach approximately 20 mm wide in the center and around 30–35 mm in the turbinated bulbous base, and narrowly clavate to narrowly lageniform cheilocystidia (Vellinga & Davis, 2006).
Leucoagaricus menieri, La. subvolvatus, and La. volvatus Bon & A.Caball. (Bon, 1993; Vellinga, 2001) from Europe also have some similarities to La. taniae. These three European species have fleshy and whitish basidiomes with a bulbous stipe and cutis-like pileus covering, but they can be easily distinguished by their cheilocystidia with crystals on the apex (Bon, 1993; Gennari et al., 1995; Vellinga, 2001). Leucoagaricus gaillardii resembles La. taniae as it also grows in the sand, has a bulbous stipe and basidiospores of the same size as La. taniae, however the pileus is pinkish, and the cheilocystidia are narrowly lageniform to cylindrical and wavy (Bon & Boiffard, 1974; Gennari et al., 1995).
Amanita cystidiosa from Puerto Rico (Miller et al., 2000) and La. singeri reported from Uruguay (Singer, 1968; Bon, 1993; Consiglio & Contu, 2004) are sand inhabiting species and have similar basidiomes as La. taniae. However, A. cystidiosa can be distinguished by its white basidiospore print, cylindrical to flexuose cheilocystidia and inamyloid basidiospore (Miller et al., 2000). While La. singeri has variable basidiospores, pseudoparaphyses are present and cheilocystidia were not observed (Singer, 1968; Bon, 1993; Consiglio & Contu, 2004).
Other fleshy white or whitish species with widespread distributions that have been reported from Brazil are: Leucoagaricus barssii (Zeller) Vellinga, which was described from the U.S.A. and is also known from Europe, and La. leucothites (Vittad.) Wasser, which although it was described from Italy is also known from the Southern Hemisphere (Rick, 1937, 1961; Grandi et al., 1984; De Meijer, 2006; Rosa & Capelari, 2009). Leucoagaricus barssii differs from La. taniae by the whitish to greyish fibrillose pileus surface, stipe tapering downward, narrower basidiospores, and narrower cheilocystidia with greyish content (Zeller, 1934; Vellinga, 2000, 2001). Leucoagaricus leucothites has a cylindrical stipe, sometimes with a small bulbous base, cylindrical and narrowly clavate to irregular cheilocystidia and trichodermal pileus covering (Bon, 1993; Vellinga, 2001).
The presence of exudate drops is considered an important diagnostic character for the taxonomy of Lepiotaceous fungi (Kumar & Manimohan, 2004; Vellinga & Balsley, 2010; Yuan et al., 2014). However, the pigmentation and portion of the basidiome where the exudate is produced varies greatly in Lepiota s.l. (Vellinga & Balsley, 2010; Yuan et al., 2014). The chemical composition of the drops and factors involved in their production are still unclear (Vellinga & Balsley, 2010; Yuan et al., 2014). In La. taniae, the drops are golden and were observed only on the pileus surface of some fresh basidiomes. They were not observed on the stipe or annulus.
Other Leucoagaricus species that exhibit drops on their basidiomes are La. brunneodiscus A.K.Dutta & K.Acharya from India, La. dacrytus Vellinga from the U.S.A., La. tangerinus Y.Yuan & J. F.Liang from China and La. tener (P.D.Orton) Bon from Great Britain (Vellinga & Balsley, 2010; Yuan et al., 2014; Orton, 1960; Bon, 1993; Dutta et al., 2021). These four species are closely related to each other and were placed by the phylogenetic analysis in a clade, together with L. cf. atrodisca (ecv237 and ecv3265), which also forms droplets on the pileus, but this clade was relatively far removed from that containing La. taniae (Fig.1; Vellinga & Balsley, 2010; Yuan et al., 2014; Dutta et al., 2021). Morphologically, those species differ from La. taniae by the smaller and more delicate basidiomes, stipe without a bulbous base, darker pileus surface, and narrower basidiospores (Vellinga & Balsley, 2010; Yuan et al., 2014; Orton, 1960; Bon, 1993; Dutta et al., 2021).
Our phylogenetical analyses (Fig.1) recovered an unexpected relationship among La. taniae and related species within the Leucoagaricus/Leucocoprinus clade. Based on morphology, it was expected to be closely related to La amanitoides, La. leucothites or La. subvolvatus and allies. Instead, La. taniae was recovered in a group with Leucocoprinus species with delicate and fragile basidiomes and dark fibrils on the pileus. However, to better elucidate relationships in this group, more taxonomic and phylogenetic studies that include other similar species and more markers are needed.
Literature cited
Araujo, D. S. D., F. R. Scarano, C. F. C. Sá, B. C. Kurtz, H. L. T. Zaluar, R. C. M. Montezuma & R. C. Oliveira. 1998. Comunidades vegetais do Parque Nacional da Restinga de Jurubatiba. Pp. 39–62in: Esteves, F. A. (ed.), Ecologia das lagoas costeiras do Parque Nacional da Restinga de Jurubatiba e do Município de Macaé (RJ). Departamento de Ecologia, Instituto de Biologia, Universidade Federal do Rio de Janeiro, Rio de Janeiro.
Albuquerque, M. P., A. B. Pereira & A. A. de Carvalho Jr. 2007. Novas ocorrências de Agaricales para o Brasil. Revista Brasileira de Biociências 5(S2): 1143–1145.
Barcelos, M. E., J. R. Riguete, L. T. Silva & P. F. Ferreira Jr. 2012. Uma visão panorâmica sobre os solos das restingas e seu papel na definição de comunidades vegetais nas planícies costeiras do sudeste do Brasil. Natureza online 10(2): 71–76.
Batista, A. C. 1957. Alguns Agaricaceae saprofitos de Pernambuco. Mycopathologia et Mycologia Applicata 8: 127–134. https://doi.org/10.1007/BF02130068
Bon, M. 1993. Flore mycologique d’Europe 3: Les Lepiotes. CRDP de Picardie, Amiens Cedex.
Bon, M. & J. Boiffard. 1974. Lépiotes de Vendée et de la Côte atlantique française (1). Bulletin Trimestriel de la Societe Mycologique de France 90: 287–306.
Brazil Flora Group. 2021. Brazilian Flora 2020 project - Projeto Flora do Brasil 2020. v393.274. Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Dataset/Checklist. https://doi.org/10.15468/1mtkaw
Chapela, I. H, S. A. Rehner, T. R. Schultz & U. G. Mueller. 1994. Evolutionary history of the symbiosis between fungus-growing ants and their fungi. Science 266(5191): 1691–1694. https://doi.org/10.1126/science.266.5191.1691
Clark, P. U., J. A. Church, J. M. Gregory & A. J. Payne. 2015. Recent progress in understanding and projecting regional and global mean sea level change. Current Climate Change Reports 1: 224–246. https://doi.org/10.1007/s40641-015-0024-4
Consiglio, G. & M. Contu. 2004. Some rare, interesting species of Leucoagaricus subgen. Sericeomyces. Micologia e Vegetazione Mediterranea 19(1): 57–72.
Dahlberg, A. & G. M. Mueller. 2011. Applying IUCN red-listing criteria for assessing and reporting on the conservation status of fungal species. Fungal Ecology 4(2): 147–162. https://doi.org/10.1016/j.funeco.2010.11.001
Darriba, D., G. L. Taboada, R. Doallo & D. Posada. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9(8): 772–772. https://doi.org/10.1038/nmeth.2109
De Meijer, A. A. R. 2006. Preliminary list of the macromycetes from the Brazilian state of Paraná. Boletim do Museu Botânico Municipal. Prefeitura Municipal de Curitiba. Secretaria Municipal do Meio Ambiente. Departamento de Produção Vegetal 68: 1–53.
Dentinger, B. T. M., S. Margaritescu & J. M. Moncalvo. 2010. Rapid and reliable high-throughput methods of DNA extraction for use in barcoding and molecular systematics of mushrooms. Molecular Ecology Resources 10: 628–633. https://doi.org/10.1111/j.1755-0998.2009.02825.x
Dutta, A. K., J. K. Stallman, S. Bera, E. Hoque, S. Paloi & K. Acharya. 2021. Lepiotaceous fungi of West Bengal, India: two new species of Leucoagaricus. Mycological Progress 20: 493–507. https://doi.org/10.1007/s11557-021-01685-w
Ferreira, A. J. & V. G. Cortez. 2012. Lepiotoid Agaricaceae (Basidiomycota) from São Camilo State Park, Paraná State, Brazil. Mycosphere 3(6): 962–976.
Forzza, R. C., J. F. A. Baumgratz, C. E. M. Bicudo, D. A. L. Canhos, A. A. de Carvalho Jr., M. A. N. Coelho, A. F. Costa, D. P. Costa, M. G. Hopkins, P. M. Leitman, L. G. Lohmann, E. N. Lughadha, L. C. Maia, G. Martinelli, M. Menezes, M. P. Morim, A. L. Peixoto, J. R. Pirani, J. Prado, L. P. Queiroz, S. Souza, V. C. S. Souza, J. R. Stehmann, L. S. Sylvestre, B. M. T. Walter & D. C. Zappi. 2012. New Brazilian floristic list highlights conservation challenges. BioScience 62(1): 39–45. https://doi.org/10.1525/bio.2012.62.1.8
Forzza, R. C., J. F. A. Baumgratz, C. E. M. Bicudo, D. A. L. Canhos, A. A. de Carvalho Jr., A. F. Costa, D. P. Costa, M. Hopkins, P. M. Leitman, L. G. Lohmann, L. C. Maia, G. Martinelli, M. Menezes, M. P. Morim, M. A. Nadruz-Coelho, A. L. Peixoto, J. R. Pirani, J. Prado, L. P. Queiroz, V. C. Souza, J. R. Stehmann, L. Sylvestre, B. M. T. Walter & D. Zappi. (eds.). 2010. Catálogo de plantas e fungos do Brasil. 2 vols. Andrea Jakobsson Estúdio / Jardim Botânico do Rio de Janeiro, Rio de Janeiro.
Gennari, A., V. Migliozzi & J. L. Siquier Virgòs. 1995. Tre interessanti Leucocoprinee sabulicole. Leucoagaricus gaillardii Bon & Boiffard, Sericomyces subvolvatus (Malençon & Bertault) Contu fo. subvolvatus e S. subvolvatus fo. pictus (Malençon & Bertault) Bon. Micologia e Vegetazione Mediterranea 10(1): 9–21.
Grandi, R. A. P., G. Guzmán & V. L. R. Bononi. 1984. Adições às Agaricales (Basidiomycetes) do Parque Estadual das Fontes do Ipiranga, São Paulo, SP, Brasil. Rickia 11: 27–33.
He, M. Q., R. L. Zhao, K. D. Hyde, D. Begerow, M. Kemler, A. Yurkov, E. H. C. McKenzie, O. Raspe, M. Kakishima, S. Sánchez-Ramírez, E. C. Vellinga, R. Halling, V. Papp, I. V. Zmitrovich, B. Buyck, D. Ertz, N. N. Wijayawardene, B. K. Cui, N. Schoutteten, X. Z. Liu, T. H. Li, Y. J. Yao, X. Y. Zhu, A. Q. Liu, G. J. Li, M. Z. Zhang, Z. L. Ling, B. Cao, V. Antonín, T. Boekhout, B. D. B. Silva, E. De Crop, C. Decock, B. Dima, A. K. Dutta, J. W. Fell, J. Geml, M. Ghobad-Nejhad, A. J. Giachini, T. B. Gibertoni, S. P. Gorjón, D. Haelewaters, S. H. He, B. P. Hodkinson, E. Horak, T. Hoshino, A. Justo, Y. W. Lim, N. Menolli Jr., A. Mešić, J. M. Moncalvo, G. M. Mueller, L. G. Nagy, R. H. Nilsson, M. Noordeloos, J. Nuytinck, T. Orihara, C. Ratchadawan, M. Rajchenberg, A. G. S. Silva-Filho, M. A. Sulzbacher, Z. Tkalčec, R. Valenzuela, A. Verbeken, A. Vizzini, F. Wartchow, M. Weiß, C. L. Zhao & P. M. Kirk. 2019. Notes, outline and divergence times of Basidiomycota. Fungal Diversity 99(1): 105–367. https://doi.org/10.1007/s13225-019-00435-4
Heisecke, C., J. A. D. Barbosa, M. A. Neves & A. A. de Carvalho Jr. 2021. Taxonomic and nomenclatural novelties in Leucoagaricus (Agaricaceae) from Brazil. Phytotaxa 494(1): 42–58. https://doi.org/10.11646/phytotaxa.494.1.2
IUCN. 2012. IUCN Red List Categories and Criteria. Version 3.1, Second edition. IUCN, Gland, Switzerland and Cambridge, UK.
IUCN Standards and Petitions Committee. 2019. Guidelines for using the IUCN Red List Categories and Criteria. Version 14. http://www.iucnredlist.org/documents/RedListGuidelines.pdf.
Ješovnik, A., J. Sosa-Calvo, M. W. Lloyd, M. G. Branstetter, F. Fernandez & T. R. Schultz. 2017. Phylogenomic species delimitation and host-symbiont coevolution in the fungus-farming ant genus Sericomyrmex Mayr (Hymenoptera: Formicidae): ultraconserved elements (UCEs) resolve a recent radiation. Systematic Entomology 42(3): 523–542. https://doi.org/10.1111/syen.12228
Johnson, J. 1999. Phylogenetic relationships within Lepiota sensu lato based on morphological and molecular data. Mycologia 91(3): 443–458. https://doi.org/10.2307/3761345
Johnson, J. & R. Vilgalys. 1998. Phylogenetic systematics of Lepiota sensu lato based on nuclear large subunit rDNA evidence. Mycologia 90(6): 971–979. https://doi.org/10.2307/3761269
Justo, A., C. Angelini & A. Bizzi. 2021. The genera Leucoagaricus and Leucocoprinus in the Dominican Republic. Mycologia 113(2): 348–389. https://doi.org/10.1080/00275514.2020.1819142
Justo, A., C. Angelini, A. Bizzi & A. Vizzini. 2015. Leucoagaricus sabinae (Agaricaceae), a new species from the Dominican Republic. North American Fungi 10: 1–15. https://doi.org/10.2509/naf2015.010.005
Katoh, K. & D. M. Standley. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30(4): 772–780. https://doi.org/10.1093/molbev/mst010
Kearse, M., R. Moir, A. Wilson, S. Stones-Havas, M. Cheung, S. Sturrock, S. Buxton, A. Cooper, S. Markowitz, C. Duran, T. Thierer, B. Ashton, P. Mentjies & A. Drummond. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12): 1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kornerup, A. & J. H. Wanscher. 1978. Methuen Handbook of Colour. 3rd Edition. Eyre Methuen, London.
Kumar, T. A. & P. Manimohan. 2004. A new species of Leucocoprinus from India. Mycotaxon 90(2): 393–397.
Largent, D. L. 1986. How to Identify Mushrooms to Genus I: Macroscopic Features. 3rd Edition. Mad River Press Inc., Eureka.
Largent, D. L., D. Johnson & R. Watling. 1977. How to Identify Mushrooms to Genus III: Microscopic Features. 3 ed. Mad River Press Inc., Eureka.
Leão, Z. M. A. N. & J. M. L. Dominguez. 2000. Tropical coast of Brazil. Marine Pollution Bulletin 41: 112–122.
Lodge, D. J., J. F. Ammirati, T. E. O’Dell & G. M. Mueller. 2004. Collecting and describing macrofungi. Pp. 128–158in: G. M. Mueller, G. F. Bills & M. S. Foster, Biodiversity of Fungi. Inventory and Monitoring Methods. Elsevier Academic Press.
Magnago, A. C., J. J. S. Oliveira, A. N. M. Furtado, S. Urrea-Valencia & M. A. Neves. 2013. Basidiomycota. Mushrooms. Pp. 23–50in: M. A. Neves, I. G. Beseia, E. R. Drechsler-Santos & A. Góes-Neto, Guide to the Common Fungi of the Semiarid Region of Brazil, TECC.
Magnago, L. F. S., S. V. Martins, C. E. G. R. Schaefer & A. V. Neri. 2010. Gradiente fitofisionômico-edáfico em formações florestais de restinga no sudeste do Brasil. Acta Botânica Brasílica 24: 734–746.
Maia, L. C. & A. A. de Carvalho Jr. 2010. Fungos do Brasil. Pp. 43–48in: R. C. Forzza et al. (orgs.), Catálogo de Plantas e Fungos do Brasil. Vol.1. Andrea Jakobsson Estúdio /Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, Rio de Janeiro.
Maia, L. C., A. M. Yano-Melo & M. A. Q. Cavalcanti. 2002. Diversidade de Fungos no Estado de Pernambuco. Pp. 15–50in: M. Tabarelli & J. M. C. Silva (eds.), Diagnóstico da Biodiversidade de Pernambuco. Ed. Massangana, Recife.
Maia, L. C., A. A. de Carvalho Jr., L. H. Cavalcanti, A. M. Gugliotta, E. R. Drechsler-Santos, A. L. M. A. Santiago, M. E. S. Cáceres, T. B. Gibertoni, A. Aptroot, A. J. Giachini, A. M. S. Soares, A. C. G. Silva, A. C. Magnago, B. T. Goto, C. R. S. Lira, C. A. S. Montoya, C. L. A. Pires-Zottarelli, D. K. A. da Silva, D. J. Soares, D. H. C. Rezende, E. D. M. N. Luz, E. L. Gumboski, F. Wartchow, F. Karstedt, F. M. Freire, F. P. Coutinho, G. S. N. de Melo, H. M. P. Sotão, I. G. Baseia, J. Pereira, J. J. S. de Oliveira, J. F. Souza, J. L. Bezerra, L. H. Cavalcanti, L. S. Araujo Neta, L. H. Pfenning, L. F. P. Gusmão, M. A. Neves, M. Capelari, M. C. W. Jaeger, M. P. Pulgarín, N. Menolli Jr., P. S. Medeiros, R. C. S. Friedrich, R. S. Chikowski, R. M. Pires, R. F. Melo, R. M. B. da Silveira, S. Urrea-Valencia, V. G. Cortez & V. F. da Silva. 2015. Diversity of Brazilian Fungi. Rodriguésia 66(4): 1033–1045. https://doi.org/10.1590/2175-7860201566407
Menolli Jr., N. & M. Sánchez-García. 2019. Brazilian fungal diversity represented by DNA markers generated over 20 years. Brazilian Journal of Microbiology 51: 729–749. https://doi.org/10.1007/s42770-019-00206-y
Miller, M. A., W. Pfeiffer & T. Schwartz. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE). San Diego Supercomputer.Center, New Orleans, Louisiana.
Miller, O. K., D. J. Lodge & T. J. Baroni. 2000. New and interesting ectomycorrhizal fungi from Puerto Rico, Mona, and Guana Islands. Mycologia 92: 558–570. https://doi.org/10.1080/00275514.2000.12061193
Möller, A. 1893. Die Pilzgärten einiger südamerikanischer Ameisen (No. 6). Botanische Mitteilungen aus den Tropen 6: 1–127.
Muchovej, J. J, T. M. Della Lucia & R. M. C. Muchovej. 1991. Leucoagaricus weberi sp. nov. from a live nest of leaf-cutting ants. Mycological Research 95(11): 1308–1311.
Mueller, U. G., S. A. Rehner & T. R. Schultz. 1998. The evolution of agriculture in ants. Science 281(5385): 2034–2038. https://doi.org/10.1126/science.281.5385.2034
Mueller, U. G., T. R. Schultz, C. R. Currie, R. M. Adams & D. Malloch. 2001. The origin of the attine ant-fungus mutualism. The Quarterly Review of Biology 76(2): 169–197. https://doi.org/10.1086/393867
Munoz, G., A. Caballero, J. C. Salom, E. Ercole & A. Vizzini. 2015. Leucoagaricus viridariorum (Agaricaceae, Agaricales), a new species from Spain. Phytotaxa 236(3): 226–236
Orton, P. D. 1960 New check list of British Agarics and Boleti: part III. Notes on genera and species in the list. Transactions of the British Mycological Society 43(2): 159–384.
Pagnocca, F. C., M. Bacci Jr., M. H. Fungaro, O. C. Bueno, M. J. Hebling, A. Sant'anna & M. Capelari. 2001. RAPD analysis of the sexual state and sterile mycelium of the fungus cultivated by the leaf-cutting ant Acromyrmex hispidus fallax. Mycological Research 105: 173–176. https://doi.org/10.1017/S0953756200003191
Pegler, D. N. 1997 The Agarics of Sao Paulo: An Account of the Agaricoid Fungi (Holobasidiomycetes) of Sao Paulo State, Brazil. Royal Botanic Gardens, Kew.
Rambaut, A., M. A. Suchard, D. Xie & A. J. Drummond. 2014. Tracer v.1.6. Available at: http://beast.bio.ed.ac.uk/Tracer [accessed 12 Aug. 2020].
Rick, J. 1937. Agarici Riograndenses. Lilloa 1: 307–346.
Rick, J. 1961. Basidiomycetes eubasidii in Rio Grande do Sul – Brasilia. 5. Agaricaceae. Iheringia. Série Botânica 8: 296–450.
Rick, J. 1920. Contributio ad monographiam Agaricearum brasiliensium. Brotéria Série Botânica 18: 48–63
Ronquist, F., M. Teslenko, P. van der Mark, D. Ayres, A. Darling, S. Höhna, B. Larget, L. Liu, M. A. Suchard & J. P. Huelsenbeck. 2011. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3): 539–542. https://doi.org/10.1093/sysbio/sys029
Rosa, L. H. & M. Capelari. 2009. Agaricales fungi from Atlantic Rain Forest fragments in Minas Gerais, Brazil. Brazilian Journal of Microbiology 40: 846–851. https://doi.org/10.1590/S1517-83822009000400015
Rother, M. S. & R. M. B. Silveira. 2008. Família Agaricaceae (Agaricales, Basidiomycota) no Parque Estadual de Itapuã, Viamão, Rio Grande do Sul, Brasil. Revista Brasileira de Biociências: Brazilian Journal of Biosciences 6(3): 259–268.
Rother, M. S. & R. M. B. Silveira. 2009. Leucoagaricus lilaceus (Agaricaceae), a poorly known Neotropical agaric. Mycotaxon 107: 473–481.
Saccardo, P. A. 1891. Supplementum Universale, Pars. I. Sylloge Fungorum 9: 1–1141. https://doi.org/10.5962/bhl.title.5371
Scarano, F. R. 2002. Strucuture, function and floristic relationships of plant communities in stressful habitats marginal to the Brazilian Atlantic Rainforest. Annals of Botany 90(4): 517–524.
Silva-Pinhati, A. C. O., M. Bacci Jr, G. Hinkle, M. L. Sogin, F. C. Pagnocca, V. G. Martins, O. C. Bueno & M. J. A. Hebling. 2004. Low variation in ribosomal DNA and internal transcribed spacers of the symbiotic fungi of leaf-cutting ants (Attini: Formicidae). Brazilian Journal of Medical and Biological Research 37(10): 1463–1472. https://doi.org/10.1590/S0100-879X2004001000004
Singer, R. 1948. Diagnoses fungorum novorum Agaricalium. Sydowia 2(1–6): 26–42.
Singer, R. 1949[1951]. The Agaricales (Mushrooms) in modern taxonomy. Lilloa 22: 1–832.
Singer, R. 1953. Type studies on Basidiomycetes. VI. Lilloa 26: 57–159.
Singer, R. 1966[1968]. Sand-dune inhabiting fungi of the South Atlantic coast from Uruguay to Bahía Blanca. Mycopathologia et Mycologia Applicata 34: 129–143. https://doi.org/10.1007/BF02051422
Singer, R. 1986. The Agaricales in Modern Taxonomy, 4th ed. Koeltz Scientific Books, Königstein.
Singer, R. 1989. New taxa and new combinations of Agaricales (Diagnoses fungorum novorum Agaricalium 4). Fieldiana Botany 21: 1–133.
Sobestiansky, G. 2005. Contribution to a macromycete survey of the States of Rio Grande do Sul and Santa Catarina in Brazil. Brazilian Archives of Biology and Technology 48(3): 437–457. https://doi.org/10.1590/S1516-89132005000300015
Spegazzini, C. 1889. Fungi puiggariani. Pugillus I. Boletin de la Academia Nacional de Ciencias en Córdoba, República Argentina 11: 381–622. https://doi.org/10.5962/bhl.title.3624
Spielmann, A. A. & J. Putzke. 1998. Leucoagaricus gongylophorus (Agaricales, Basidiomycota) em ninho ativo de formigas Attini (Acromyrmex Aspersus). Caderno de Pesquisa, ser. Botânica 10(1/2):27–36.
Stamatakis, A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9): 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Vellinga, E. C. 1988. Glossary. Pp. 54–64 in: M. E. Noordeloos, T. W. Kuyper, E. C. Vellinga, Flora Agaricina Neerlandica, vol. 2. AA Balkema Publishers, Lisse.
Vellinga, E. C. 2000. Notes on Lepiota and Leucoagaricus. Type studies on Lepiota magnispora, Lepiota barssii, and Agaricus americanus. Mycotaxon 76: 429–438.
Vellinga, E. C. 2001. Leucoagaricus Locq. ex Singer. Pp. 85–108in: M. E. Noordeloos, T. W. Kuyper & E. C. Vellinga, Flora Agaricina Neerlandica, vol. 5: AA Balkema Publishers, Lisse.
Vellinga, E. C. 2004a. Ecology and distribution of Lepiotaceous fungi (Agaricaceae) – A Review. Nova Hedwigia 78(3): 273–299. https://doi.org/10.1127/0029-5035/2004/0078-0273
Vellinga, E. C. 2004b. Genera in the family Agaricaceae: evidence from nrITS and nrLSU sequences. Mycological Research 108(4): 354–377. https://doi.org/10.3852/10-204
Vellinga, E. C. 2010. Nomenclatural overview of Lepiotaceous Fungi. Version 4.8.
Vellinga, E. C. & R. B. Balsley. 2010. Leucoagaricus dacrytus–a new species from New Jersey, USA. Mycotaxon 113(1): 73–80. https://doi.org/10.5248/113.73
Vellinga, E. C. & R. M. Davis. 2006. Lepiotaceous fungi from California, USA 1-Leucoagaricus amanitoides sp. nov. Mycotaxon 98:197–204.
Vellinga, E. C. & M. E. Noordeloos. 2001. Glossary. Pp. 6–11in: M. E. Noordeloos, T. W. Kuyper, E. C. Vellinga, Flora Agaricina Neerlandica, vol. 5. AA Balkema Publishers, Lisse.
Vellinga, E. C., P. Sysouphanthong & K. D. Hyde. 2011. The family Agaricaceae: phylogenies and two new white-spored genera. Mycologia 103(3): 494–509. https://doi.org/10.3852/10-204
Wartchow, F., J. Putzke & M. A. D. Q. Cavalcanti. 2008. Agaricaceae Fr. (Agaricales, Basidiomycota) from areas of Atlantic Forest in Pernambuco, Brazil. Acta Botânica Brasílica 22(1): 287–299. https://doi.org/10.1590/S0102-33062008000100026
Yuan, Y., Y. K. Li & J. F. Liang. 2014. Leucoagaricus tangerinus, a new species with drops from southern China. Mycological Progress 13: 893–898. https://doi.org/10.1007/s11557-014-0974-2
Zeller, S. M. 1934. A new species of Lepiota. Mycologia 26(3): 210–211. https://doi.org/10.1080/00275514.1934.12020716
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES) – Finance Code 001. The authors thank Ariadne Furtado for help with sequencing, the Synchronicity Earth (https://www.synchronicityearth.org) for choosing this mushroom for financial support, and the editor and two anonymous reviewers for improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
ESM 1
(TXT 62 kb)
Rights and permissions
About this article
Cite this article
Heisecke, C., Lima, D.F., Mueller, G.M. et al. Leucoagaricus taniae sp. nov. (Agaricaceae), a sand-dwelling mushroom from Brazil. Brittonia 74, 18–29 (2022). https://doi.org/10.1007/s12228-021-09693-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12228-021-09693-6