Abstract
Forty-nine morphological characters were scored or measured on 44 populations (376 individuals) of Viola subsect. Viola from the West Carpathians and adjacent areas (Slovakia, Czech Republic, Austria and Hungary). The presence of six species, namely V. alba (represented by subsp. alba), V. ambigua, V. collina, V. hirta, V. odorata and V. suavis s.l. was revealed based on pollen fertility, cytological and morphometric analyses. The morphological characters traditionally used to delimit taxa within the subsection and those revealed by our study as most reliable are widely discussed. A key for identifying the taxa and most common hybrids of subsection Viola occurring in the West Carpathians is presented. Chromosome counting and flow cytometry were used to determine the ploidy levels of the populations studied. All individuals of V. alba subsp. alba, V. collina, V. hirta and V. odorata were tetraploid, while those of V. ambigua and V. suavis s.l. were octoploid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Viola L., the largest of the Violaceae family, comprises 525–600 species distributed throughout most parts of the world (Ballard et al. 1999; Yockteng et al. 2003). It is divided into ca. 14 sections and many infrasectional groups (for a review of infrageneric classification see Ballard et al. 1999). The genus probably originated in South America but recent centers of morphological and taxonomic diversity are found mainly in the Northern Hemisphere (Ballard et al. 1999; Yockteng et al. 2003). The section Viola, one of the largest infrageneric groups of violets in Europe, is traditionally divided into five subsections: Viola, Rostratae Kupffer (including Repentes (Kupffer) W. Becker), Stolonosae Kupffer, Adnatae W. Becker, and Boreali-Americanae W. Becker (Valentine et al. 1968; Marcussen et al. 2007), with the last one represented in Europe only by the alien species V. sororia Willd., native to North America.
The subsection Viola, members of which are treated here, includes approximately 25 species native to the temperate zones of Eurasia and adjacent parts of North Africa (Marcussen and Borgen 2000; Marcussen 2006). The only actual autapomorphy of this subsection is the unique capsule morphology (capsules globose, inexplosive, on decumbent peduncles). Nevertheless, it can also be delimited from other subsections within V. sect. Viola by combinations of the following characters: absence of stem, presence of short and stout rhizome, rooting stolons (which may be reduced or absent), stipules free, sepals almost rounded, obtuse or obtusely acute at apex, style beaked at apex, ovate seeds with conspicuous elaiosome adapted to myrmecochory (Valentine 1962; Valentine et al. 1968; Kirschner and Skalický 1990; Okamoto et al. 1993; Mereďa et al. 2008).
The subsection Viola has traditionally been divided into two series, Viola (the species occurring in the West Carpathians include V. alba Besser, V. odorata L., and V. suavis M. Bieb.) and Eflagellatae Kitt. (in the West Carpathians represented by V. ambigua Waldst. & Kit., V. collina Besser, and V. hirta L.), based on the presence or absence of stolons (cf. Becker 1925; Gams 1925; Marcussen and Borgen 2000; Dinç et al. 2003). This classification, however, may be artificial, not reflecting true phylogenetic relationships within this group (Marcussen and Borgen 2000). For example, V. collina occasionally produces short stolons (Gams 1925; Marcussen and Nordal 1998). A study of allozyme markers has also shown that e.g., the stoloniferous V. suavis in its allozymic pattern is more similar to the non-stoloniferous V. pyrenaica Ramond than to any stoloniferous subsection member (Marcussen and Borgen 2000).
In the subsection Viola two cytotypes, 2n = 20 (the more common cytotype) and 2n = 40 (found in V. ambigua and V. suavis), have been reported (for numerous references see Mereďa et al. 2006). Traditionally, the base chromosome number of V. sect. Viola was believed to be x = 10; however, Marcussen and Nordal (1998) and Marcussen and Borgen (2000), interpreting isoenzyme phenotypes, suggested that the base chromosome number within this subsection is x = 5. So true diploids are not known in this subsection, and plants with 2n = 20 should be considered as palaeotetraploid, and those with 2n = 40 as palaeooctoploid.
Species of the subsection Viola are notorious for their taxonomic complexity, and their delimitation has been the topic of many studies (for references see Marcussen and Borgen 2000). In general, problems in taxonomy of this group arise from the facts that (1) there are only a few morphological characters used to delimit taxa, with most of them overlapping across recognized species, (2) some taxa exhibit strong phenotypic plasticity, and (3) interspecific hybridization is frequent (e.g. Schmidt 1961; Kuta 1981; Marcussen and Borgen 2000; Marcussen et al. 2001). A combination of karyological, morphological and molecular approaches, such as DNA sequences (Ballard et al. 1999; Ballard and Sytsma 2000; Nadot et al. 2000; Yockteng et al. 2003) and allozyme markers (Marcussen and Nordal 1998; Marcussen and Borgen 2000; Marcussen et al. 2001, 2005; Marcussen 2003, 2006), has substantially contributed to the elucidation of phylogenetic relationships within the genus and within sect. Viola, as well as to the understanding of intraspecific variation in a number of related species.
Six species of Viola subsect. Viola have been reported from the West Carpathians, of which V. alba, V. ambigua, V. collina, and V. hirta are considered native, and V. odorata and V. suavis are naturalized (for more details see Mereďa et al. 2008).
Viola alba (2n = 20) is well known for its infraspecific variation. As shown by Marcussen (2003) and Marcussen et al. (2005), it comprises three ± vicarious subspecies: (1) V. alba subsp. alba (including two colour morphotypes: alba and scotophylla), occurring from the Caucasus and the Middle East westwards to Central Europe and northern Spain, (2) V. alba subsp. dehnhardtii (Ten.) W. Becker, growing in the Mediterranean region from Turkey westwards to the Iberian Peninsula and Morocco, and (3) V. alba subsp. cretica (Boiss. & Heldr.) Marcussen, endemic to Crete. In the West Carpathians V. alba reaches the northern limit of its native distribution range. It occurs rather rarely from planar to submontane belt. Its main habitats are colline oak-hornbeam and beech woods and shrubberies, mainly on basic substrata.
Viola ambigua (2n = 40) is distributed from the Caucasus westwards to Central Europe. The northwestern limit of its distribution range runs through southern Moravia (Czech Republic). Isolated occurrences exist in northern Bohemia and central Germany (Danihelka and Čeřovský 1999). In Central Europe it is known only from a few localities in the Czech Republic, eastern Austria, southern Slovakia, and northern Hungary. It grows in open, dry and sunny places from the planar to the colline belt and prefers calcareous substrata.
Viola collina (2n = 20) is distributed in most of the temperate parts of Eurasia (Marcussen et al. 2001), but morphologically it is rather uniform (Marcussen and Borgen 2000). It is common in the West Carpathians, growing mainly in their central part from the colline to the montane belt. It occupies sunny pastures and open places in beech and coniferous woods, mainly on basic substrata.
Viola hirta (2n = 20) is widespread from the Iberian Peninsula and British Isles in the west to Lake Baikal in the east (Marcussen et al. 2001). It is closely related to V. ambigua in its allozymic pattern (Marcussen and Borgen 2000) but both species are morphologically well differentiated. In the West Carpathians V. ambigua and V. hirta can be found growing together in dry grasslands; however, V. hirta has a much broader ecological niche including also moderately shaded places, open woods, shrubberies and forest edges on different substrata. It also has the widest distribution in the West Carpathians of the species considered here and is common throughout the area studied.
Viola odorata (2n = 20) is morphologically rather uniform and is commonly distributed in most parts of Europe and adjacent parts of Asia and North Africa. As the only member of subsection Viola, it is naturalized in North America. According to Marcussen (2006), V. odorata is native only to the Mediterranean region south of the Alps and to some parts of western Europe. The species is frequently cultivated, and numerous ornamental cultivars of V. odorata can be found in temperate zones throughout all continents (Marcussen 2006). In the West Carpathians, V. odorata is naturalized and common from the planar to the submontane belt on different substrata. It occurs in man-made and man-influenced habitats, such as parks and cemeteries, as well as in natural and semi-natural open dry grasslands and shrubberies or in shaded alluvial woods.
Viola suavis (2n = 40) represents a taxonomically critical species with a series of morphologically and geographically defined races treated on different taxonomic levels (for further details see e.g. Becker 1910; Schmidt 1961; Marcussen and Nordal 1998). It is distributed in the Mediterranean region from Morocco eastwards to the Middle East but due to cultivation its distribution area expanded also to Central and northern Europe (Marcussen and Nordal 1998). Though not native and with limited distribution, V. suavis is morphologically the most variable species of the subsection Viola in the West Carpathians. It is common in the southern part of the West Carpathians from the planar to the submontane belt, whereas only a few isolated localities exist in northern Slovakia (Mereďa et al. 2008). Viola suavis, like V. odorata, occurs in man-made habitats, such as gardens, parks and old cemeteries, as well as in natural and semi-natural habitats close to settlements, including shaded parts of dry grasslands, open deciduous woods, shrubberies and forest edges on different substrata.
Despite various biosystematic studies of Viola subsect. Viola, species boundaries and relationships among species are not always clear. In addition, no statistically based morphological study including the West-Carpathian (or Central European) populations of the subsection Viola has yet been done. Thus, we present a combined cytological and morphometric study focusing on populations in this part of Europe. The main objectives of this study were (1) to reconsider the value of morphological characters used to delimit the taxa within the subsection, and (2) to explore their chromosome number variation in the West Carpathians.
In the present study we intended to exclude hybrid individuals as much as possible and to focus only on variation of non-hybrid specimens. The identification of non-hybrid and hybrid specimens was relied on assessing their ploidy levels and pollen fertility. Nevertheless, we are aware that only heteroploid hybridization events (between tetraploid and octoploid parental species) and primary hybrids (mostly F1) in the case of homoploid hybridizations may have been safely identified using this approach. It was documented that backcrossed-hybrid plants (introgressants) derived from homoploid interspecific crosses can restore pollen fertility in subsequent generations (cf. Kuta 1990; Krahulcová et al. 1996; Marcussen and Borgen 2000). A certain percentage of the individuals investigated here, therefore, might be a result of such backcrosses. A detailed morphometric analysis of hybrid populations of V. subsect. Viola will be published separately, based on more extensive sampling.
Material and Methods
Field Sampling
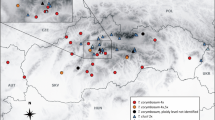
A total of 465 individuals from 57 populations of Viola subsection Viola were collected in the West Carpathians and its adjacent areas in 2003–2005 (see Appendix, Fig. 1). The intra-populational homogeneity known among species within subsection Viola (Marcussen and Nordal 1998) allowed us to collect only one to two plants with spring chasmogamous flowers for cytological and 10 (1–12) plants for morphometric analyses at each site.
Map showing sample sites of the studied populations of Viola sect. Viola subsect. Viola from the West Carpathians and adjacent areas. Viola alba subsp. alba (including alba morphotype (asterisk) and scotophylla morphotype (triangle)), V. ambigua (square), V. collina (star), V. hirta (plus sign), V. odorata (circle), V. suavis (including violet-flowered morphotype (spade) and white-flowered morphotype (trefoil)), hybrid populations of V. alba × V. hirta, V. alba × V. odorata, V. ambigua × V. odorata, V. hirta × V. odorata and V. odorata × V. suavis s.str. (all represented by the symbol “×”) (for more details see Appendix)
Plants for cytological analyses were cultivated in the experimental garden of the Institute of Botany, Slovak Academy of Sciences, Bratislava, Slovakia. For morphometric analyses all vegetative (stolons, leaves, stipules) and floral parts (peduncles, calyx, corolla) per individual were attached to paper using adhesive tape, dried, and preserved as herbarium specimens. Voucher specimens for all analyses are deposited in the herbarium in the Institute of Botany, Slovak Academy of Sciences, Bratislava (SAV).
Pollen Fertility
To eliminate hybrid individuals, a step necessary for the reconsideration of diagnostic morphological characters of taxa within the subsection, pollen fertility was analyzed in all tetraploid and octoploid specimens studied (in total 460 plants; see Appendix). Pollen fertility analyses were not performed on hexaploid individuals (results of heteroploid hybridization events, five plants).
Pollen fertility, indicated by pollen stainability, was examined in one chasmogamous flower of each plant studied. Pollen grains were removed from anthers of open flowers or flower buds and mounted on slides in aceto-carmine jelly (Radford et al. 1974). One hundred pollen grains per individual were observed. The unstained grains, usually shrunk and empty, were considered as sterile and well-stained and regularly developed grains were considered as fertile.
Cytological Analyses
Chromosome numbers and/or ploidy level estimations were determined using chromosome counts in root tips and flow cytometry in all 57 populations studied.
Chromosome numbers
Root-tip meristems of potted plants were used for chromosome counts. They were pre-treated in 0.1% water solution of colchicine for about 3 h, fixed in 98% acetic acid:96% ethanol mixture (ratio 1:3) for 1–24 h, washed in distilled water, macerated in 1 N HCl at the temperature 60°C for 5–6 min and washed in distilled water. The squashes were made using a cellophane square (Murín 1960), stained in the 10% solution of Giemsa stock dye in Sörensen phosphate buffer for about 1 h, washed, dried, and observed.
Flow cytometry
Samples were prepared from the fresh tissues of petals and/or flower peduncles and/or young leaf petioles. Probably because of pronounced slime production, no FCM signal was detected in the samples prepared from leaf laminas. A two-step procedure (Otto 1990; Doležel and Göhde 1995) was used for sample preparation. Fresh material was chopped with a sharp razor blade in a glass Petri dish containing 0.5 ml of ice-cold Otto I buffer (0.1 M citric acid, 0.5% Tween 20). The nuclei suspension was filtered through a 42 μm nylon mesh and centrifuged at 150 g for 5 min. The supernatant was removed and nuclei were resuspended in 100 μl of fresh ice-cold Otto I buffer and incubated for 20 min at room temperature with occasional shaking. For DNA staining a solution of 1 ml of Otto II buffer (0.4 M Na2HPO4 ·12H2O) supplemented with 50 μg/ml propidium iodide and 50 μg/ml RNAse was added and analyzed after 10 min. Relative DNA content was estimated with a Becton Dickinson FACSCalibur flow cytometer using BD Cellquest Pro Software. A spontaneous plant of V. reichenbachiana Jord. with 2n = 20 (counted by L. Mártonfiová and P. Mártonfi) from the decorative area of the Botanical Garden in Košice, Slovakia, 223 m, 48°44′06″ N, 21°14′18″ E (17 Apr 2003 P. Mártonfi 2685 KO s.n.; plant cult. no. M23 in Botanical Garden, P. J. Šafárik University in Košice) was used as the reference plant (standard). Internal standardization (the nuclei of the standard were isolated, stained and analyzed simultaneously with the nuclei of a sample), or, in some cases, pseudo-internal standardization (the nuclei of the standard and a sample were isolated and stained separately, but they were mixed and analyzed together) were employed (Noirot et al. 2005; Greilhuber et al. 2007).
Morphometric Analyses
Apparent hybrid individuals identified by significantly decreased pollen fertility or hexaploid level (see Results) were excluded from the morphometric analyses. Forty-one characters (24 vegetative and 17 floral) were measured or scored on 376 flowering plants from 44 populations collected in spring in the field, and eight ratios were derived from them (together 49 characters, see Table 1, Fig. 2). Most characters were quantitative, nine were binary, and two semi-quantitative. Characters StN, StUL, StAL, StW, StP, LP, SFI, KP, CO, CEN, CP, CSS, CSP, CPSP, and CaVN were scored immediately in the field on fresh plants, vegetative and floral parts were subsequently attached to paper using adhesive tape, and dried. The remaining characters were evaluated on such dried herbarium specimens. Characters on lamina, petioles and stipules were scored on three (if present) well-developed leaves of each plant; characters on peduncle, calyx and corolla on two (if present) largest chasmogamous flowers of each plant. Average values were then entered into the data matrix. Out of the 49 characters scored or measured, only 37 were used in morphometric analyses (Table 1). Altogether 14 characters were excluded from the morphometric analyses: 11 were used solely for calculating ratios, one character (CaVN) was constant across all populations and taxa, and one character (StN) was not included because the information expressed was already given by the characters StAL, StUL and StW. Character CP was excluded from the canonical discriminant analyses, because it was constant within most of the groups.
Morphological characters scored and measured for morphometric analyses. For character explanations see Table 1. Drawings by P. Mereďa Jr.
Characters in the morphometric analyses included those traditionally used for the delimitation of taxa within the subsection Viola (e.g. Kirschner and Skalický 1990; Fischer et al. 2005), or used in modern taxonomic treatments and morphometric papers (e.g. Marcussen and Nordal 1998; Marcussen et al. 2001; Marcussen 2003), as well as those found useful in our preliminary screening of Carpathian populations.
Spearman correlation coefficients (Sneath and Sokal 1973; Krzanowski 1990) were computed for the matrix including all plants studied to eliminate pairs of highly correlated characters from further analyses.
Cluster analysis (CA; UPGMA – average clustering; Everitt 1986) based on populations (characterized by average values of characters) as operational taxonomic units (OTUs) was performed to generate a hypothesis on population groupings. The characters in the primary matrix were standardized by zero mean and unit standard deviation, and the Euclidean coefficient was used to compute the secondary distance matrix.
Principal component analyses (PCA; Sneath and Sokal 1973) based on populations as OTUs and a correlation matrix between the characters were performed on subsets of (I) tetraploids and (II) octoploids. Principal component analyses were used to determine non-hierarchical structure within both tetraploid and octoploid populations.
Canonical discriminant analyses (CDA; Klecka 1980) based on individual plants as OTUs were performed to test the results from cluster and principal component analyses that were based on population averages. Six groups, resolved by UPGMA and PCA (see Results), were defined as groups for CDA 1–8 (see Results).
Mean, standard deviation, minimum, maximum, 10 and 90 percentiles were computed for all quantitative characters.
Analyses were performed using the SAS version 9.1 statistical package (SAS Institute 2000) and the SYN-TAX 2000 package (Podani 2001).
Results
Pollen Fertility
Pollen stainability of individuals attributed to putatively pure V. alba subsp. alba (including alba and scotophylla morphotypes) was 93–100%, to those of V. ambigua 95–100%, V. collina 94–100%, V. hirta 92–100%, V. odorata 95–100%, and of V. suavis 91–100%. In addition, the following hybrid populations showing low fertility were found: V. alba × V. hirta with 0–17% fertility, V. alba × V. odorata with 0–20%, and V. hirta × V. odorata with 10–60%.
Individuals displaying low pollen stainability (less than 70% fertility, altogether 84 individuals, 10 populations) were considered as primary (F1) hybrids and they were excluded from the morphometric analyses.
Cytological Analyses
Chromosome numbers and DNA ploidy levels of 57 populations studied are listed in Appendix. Viola alba, V. collina, V. hirta, V. odorata and their hybrids (V. alba × V. hirta, V. alba × V. odorata, V. hirta × V. odorata) were found to be tetraploid with 2n ∼ 4x ∼ 20; V. ambigua and V. suavis s.l. were octoploid with 2n ∼ 8x ∼ 40. These counts agree with previously published chromosome numbers (for further references see Mereďa et al. 2006).
The hexaploid chromosome number (2n ∼ 6x ∼ 30) was found in five plants (two populations) growing in contact zones of tetraploid and octoploid species V. odorata and V. ambigua or V. odorata and V. suavis s.l. Four of them, determined on the basis of their morphology and vicinity of pure populations, represented the parental combination V. odorata × V. suavis s.l., and one represented V. ambigua × V. odorata.
Morphometric Analyses
Based on the results of pollen fertility and cytological analyses, from the total of 465 individuals (57 populations) only 376 individuals (44 populations) were considered as non-hybrid and were used for the multivariate morphometric analyses.
Spearman Correlation Coefficients
Spearman correlation coefficients of any pair of characters in the whole dataset, used in morphometric analyses, did not exceed the value of 0.9. Thus, all characters were used in subsequent morphometric analyses.
Cluster Analysis
Cluster analysis of the complete dataset indicated that all 44 non-hybrid populations can be divided into six main clusters (Fig. 3). The first cluster includes two populations, which can be classified as V. alba subsp. alba (alba morphotype). The second cluster contains five populations that can be classified as V. alba subsp. alba (scotophylla morphotype). The third cluster includes four population samples traditionally recognized as V. collina. The fourth cluster is composed of two groups: the first group (A) is formed of eight populations generally corresponding to V. hirta, and the second group (B) includes four populations classifiable as V. ambigua. The fifth cluster contains seven populations of V. odorata. The sixth cluster is formed by 14 populations that correspond to two colour morphotypes of V. suavis s.l. occurring in the Carpathians: populations with blue to (bluish-) violet corollas (further referred to as V. suavis s.str.), and populations with white corollas (further referred to as white-flowered morphotype of V. suavis).
Cluster analysis (UPGMA) based on 37 morphological characters and all 44 studied populations from the West Carpathians and its adjacent areas: ALB – Viola alba subsp. alba (alba morphotype), SCO – V. alba subsp. alba (scotophylla morphotype), AMB – V. ambigua, COL – V. collina, HIR – V. hirta, ODO – V. odorata, SUB – V. suavis s.str., SUW – white-flowered morphotype of V. suavis; abbreviations of taxa names are followed by population numbers (see Appendix) and ploidy levels
Principal Component Analyses
The studied populations were divided into two groups according to their chromosome numbers (tetraploids and octoploids), and these two groups were separately subjected to PCA. To get a better resolution within the group of tetraploids, another PCA based on a subset of tetraploid populations was performed. Thus, three different data subsets (matrices) were assembled and analyzed separately (in all cases only two-dimensional ordination graphs are shown, because the third axis did not contribute to further differentiation):
-
Matrix A (PCA 1) – all 26 tetraploid populations (clusters 1, 2, 3, 4A, 5 in Fig. 3)
-
Matrix B (PCA 2) – 18 tetraploid populations of V. alba, V. collina and V. odorata (clusters 1, 2, 3, 5 in Fig. 3)
-
Matrix C (PCA 3) – all 18 octoploid populations (clusters 4B, 6 in Fig. 3).
Principal Component Analyses of Tetraploids
PCA 1, based on matrix A, resulted in two distinct groups (Fig. 4): (1) on the left side of the ordination graph there is a group corresponding to populations traditionally understood as V. hirta, (2) on the right side of the diagram populations corresponding to V. alba subsp. alba (including alba and scotophylla morphotypes), V. collina and V. odorata are found intermingled. Characters most correlated with the first axis are (with the values of eigenvectors in brackets): CO (0.267), LAA (0.262), SBGN (0.261), StW (0.260), LL/LW (−0.256), LHL (−0.251), LL1/LL (0.249) and LSL/LL (0.242), while characters CAL (0.328), CLL (0.320), CPL (0.315) and SFI (−0.300) are most strongly correlated with the second axis. In an effort to portray differences among V. alba subsp. alba (including alba and scotophylla morphotypes), V. collina and V. odorata, a separate PCA (PCA 2; based on matrix B) was performed. PCA 2 (Fig. 5) showed three clear groups corresponding to the species V. alba subsp. alba (including alba and scotophylla morphotypes), V. collina and V. odorata. On the contrary to cluster analysis (cf. Fig. 3) the scatter plot of PCA did not show any tendency towards a further separation of V. alba subsp. alba into alba and scotophylla morphotypes; both morphotypes were grouped together without any discontinuity. The clusters are delimited by SFI (−0.258), CO (0.236), CPL (0.234), CLL (0.234), LHL (−0.230), CAL (0.227) and LAA (0.223) (with the highest eigenvector values for the first axis) and by CPSP (0.318), CPW (0.306), CLW (0.300), CP (−0.293), CPL1/CPL (−0.275) and SL/SW (−0.253) (with the highest values for the second axis).
Principal component analysis (PCA 1) based on 37 morphological characters and 26 tetraploid populations: Viola alba subsp. alba, including alba morphotype (asterisk) and scotophylla morphotype (triangle), V. collina (star), V. hirta (plus sign) and V. odorata (circle). The first two axes explain 30.35% and 21.59% of variation among OTUs
Principal component analysis (PCA 2) based on 37 morphological characters and 18 tetraploid populations: Viola alba subsp. alba, including alba morphotype (asterisk) and scotophylla morphotype (triangle), V. collina (star) and V. odorata (circle). The first two axes explain 34.89% and 18.47% of variation among OTUs
Principal Component Analysis of Octoploids
The result of PCA 3, based on matrix C, is shown in Fig. 6. Two groups separated along the first principal component can be seen: a group on the left side of the diagram corresponding to plants traditionally understood as V. ambigua, and a group on the right side corresponding to V. suavis s.l. The scatter plot showed some tendency towards a further, but incomplete separation of V. suavis s.l. into two subgroups along the second principal component: (1) plants with blue to (bluish-) violet corollas (V. suavis s.str.) are located in the upper right corner of the diagram and (2) plants with white corollas (white-flowered morphotype of V. suavis) in the lower right corner. The first axis, contributing most to the separation of the groups, was highly correlated (eigenvector values) with the characters LAA (0.252), LSA (0.251), LSL/LL (0.246), LL/LW (−0.246), CAL (0.230), SFN (0.224), PL1/PL (−0.208) and StAL (0.201); the second axis, expressing a slight shift of the V. suavis s.l. specimens, was highly correlated with SL/SW (−0.328), CP (0.319), LP (0.318), SGN/SFN (0.245) and CSP (0.242).
Canonical Discriminant Analyses
Several CDA were run to reveal the differentiation among the groups resolved by cluster analysis and PCA. On the contrary to the latter analyses, CDA were based on individual plants as OTUs. CDA 1 was performed on the whole dataset, with six groups (taxa) pre-defined, as they were delimited by the cluster analysis and principal component analyses (PCA 1–3): V. alba subsp. alba (including alba and scotophylla morphotypes), V. ambigua, V. collina, V. hirta, V. odorata and V. suavis s.l. (including V. suavis s.str. and white-flowered morphotype of V. suavis). The scatter plot affirms the phenetic distinctness of the taxa studied, although they partly overlap morphologically. The ordination diagram of CDA 1 (Fig. 7) showed relatively distinct clusters of V. alba subsp. alba (including alba and scotophylla morphotypes), V. ambigua, V. hirta and V. odorata individuals, while there was an overlap between the clusters of V. collina and V. suavis s.l. (including V. suavis s.str. and white-flowered morphotype of V. suavis). Correlation values of the characters (total canonical structure) with the first and second axis are presented in Table 2.
Results of CDA 1 including 36 morphological characters and 376 individuals as OTUs. The six groups defined on the basis of UPGMA and PCA represent: Viola alba subsp. alba (triangle), V. ambigua (square), V. collina (star), V. hirta (plus), V. odorata (circle) and V. suavis s.l. (spade). The first two axes explain 12.6% and 8.66% of variation among OTUs
To reveal differences between V. collina and V. suavis s.l., a separate CDA (CDA 2) with two groups corresponding to the two taxa was run. The histogram of CDA 2 showed clear separation between the species (Fig. 8). They differ in a number of characters; correlation values with the discriminant axis are presented in Table 2.
A series of six CDA (diagrams not shown) were further performed to identify morphological characters most suitable for the recognition of a particular species from the other species studied. They were computed using datasets with specimens divided into two groups: one including specimens of a particular species and the other including specimens of all the remaining species studied (e.g. CDA 3 was computed using individuals of V. alba subsp. alba as group 1 and individuals of V. ambigua, V. collina, V. hirta, V. odorata and V. suavis s.l. as group 2, etc.). The characters pigmentation of corolla in contrast to pigmentation of spur (CPSP), lamina dentations (LCN) and length of petals (CLL, CAL, CPL) were identified to be the best characters for the definition of V. alba subsp. alba from the remaining species studied; shape of lamina (LSA, LL/LW, LSL/LL) for the recognition of V. ambigua; pigmentation of corolla in contrast to pigmentation of spur (CPSP) and size of flower (CPL, CPW, CLL, CLW, CAL) for the recognition of V. collina; maximum length of petiole hairs (LHL), odour of flower (CO), shape of lamina (LAA, LL/LW), and shape of spur (CSS) for the recognition of V. hirta; lamina apex angle (LAA) and indument of stipule (SBGN, SFI) for the recognition of V. odorata; and fimbriation and indument of stipule (SFL, SFI), insertion of bracteoles on peduncle (PL1/PL), and length of anterior sepals (KAL) for the recognition of V. suavis s.l.
Exploratory Data Analysis
Means, standard deviations, minima, maxima, 10 and 90 percentiles of quantitative characters are presented in Table 3, and frequencies of qualitative characters in Table 4. Although there is a more or less continuous variation across the whole dataset in many characters, the combination of characters allows unambiguous species identification.
Discussion
Our morphological analyses support the existence of six well-delimited and morphologically distinct taxa within Viola subsect. Viola in the West Carpathians: V. alba subsp. alba (including alba and scotophylla morphotypes), V. ambigua, V. collina, V. hirta, V. odorata and V. suavis s.l. Each of the above-mentioned taxa can be clearly distinguished by a unique set of morphological features (see identification key below, and Fig. 9).
In most identification keys to the West-Carpathian or Central-European violets, the presence or absence of stolons is considered a crucial character (e.g. Valentine et al. 1968; Dostál 1989; Kirschner and Skalický 1990; Suda 2002; Fischer et al. 2005), and the species of this subsection are described either as non-stoloniferous (V. ambigua, V. collina and V. hirta; series Eflagellatae) or as stoloniferous (V. alba, V. odorata and V. suavis; series Viola). However, V. collina can also form stolons up to 3.5 cm long, and in contrary, stolons can sometimes be lacking in V. alba, V. odorata and V. suavis, as already pointed out e.g. by Gams (1925), Marcussen and Nordal (1998), and Marcussen (2003). In the present study, four plants (10%) of V. collina were observed having short stolons, whereas stolons were lacking in 8% of V. odorata specimens studied, in 33% of V. suavis s.l., and in 37% of V. alba subsp. alba specimens (cf. character StN, Table 4).
In Central Europe the morphological separation of taxa within subsection Viola has also commonly relied on the presence or absence of glands on stipules. Many previous authors stated that stipules of V. alba (cf. Gams 1925; Dostál 1989; Kirschner and Skalický 1990); V. collina, V. hirta (e.g. Kirschner and Skalický 1990) and V. suavis (e.g. Dostál 1989) are eglandular, whereas stipules of V. ambigua and V. odorata are glandular. Our study demonstrated a large variation in this character and gave reason to doubt its importance in the infrasubsectional classification. All members of subsection Viola studied by us possessed sparsely or densely glandular stipules (cf. characters SFN and SGN/SFN, Table 3), with a proportion of glandular fimbriae to the total number of fimbriae in V. alba subsp. alba (0–)25–88(–100)%, in V. ambigua (0–)27–100%, in V. collina (0–)7–63(–83)%, in V. hirta (0–)29–100%, V. odorata (21–)48–100% and in V. suavis s.l. (0–)18–91(–100)%.
Although the insertion of bracteoles on peduncles is a relevant diagnostic character, it may vary much more than given in most identification keys and floras. According to many authors bracteoles in V. alba, V. collina, V. odorata (e.g. Gams 1925; Valentine et al. 1968; Dostál 1989; Kirschner and Skalický 1990; Marcussen and Nordal 1998; Suda 2002; Fischer et al. 2005), and in V. ambigua (e.g. Gams 1925; Dostál 1989) should be inserted at or above the middle of the peduncle. In fact, the insertion place varied considerably in the plants studied, and bracteoles of these species were relatively often inserted below the middle of the peduncle (cf. character PL1/PL, Table 3).
Viola alba subsp. alba
The concept of two subspecies in V. alba = subsp. alba and subsp. scotophylla (Jord.) Gremli – has been traditionally accepted in Central Europe (e.g. Dostál 1989). Division of the species into these races was based mainly on pigmentation of plants: subsp. scotophylla possesses strongly pigmented stolons, leaves, peduncles, sepals and capsules and its spur is purplish; all organs of subsp. alba are without anthocyan pigmentation and its spur is white with a yellowish-green apex.
The results of our morphological analyses (cluster analysis and principal component analyses, PCA 1–2) are quite discordant for V. alba. The specimens identified as alba and scotophylla morphotypes formed two distinct clusters in the CA, however, they did not show any tendency towards a separation in the PCA 1–2. The most important characters influencing the position of alba and scotophylla morphotypes in the CA are connected with the pigmentation of stolons, lamina, sepals, corolla and spur (StP, LP, KP, CP, CSP, CPSP). Nevertheless, we have not observed any other significant differences between morphological characters of these two morphotypes, and their morphological and ecological ranges broadly overlap.
This result is fully in agreement with the morphometric and allozymic studies by Marcussen and Borgen (2000) and Marcussen (2003). They demonstrated that the above-mentioned characters are taxonomically rather unimportant, and the populations of “subsp. scotophylla” should be included into the nominate subspecies. There is a note by Marcussen (2003) concerning differences in colour: “the genetic basis for such polymorphism is probably simple and may be explained by bi-allelic variation in two loci, one coding for anthocyan production, and another for its expression in the corolla (thus, giving rise to three possible morphotypes: the alba morphotype, and the white- and lilac-flowered scotophylla morphotype)”. In the West Carpathians mixed populations of both morphotypes occur and morphological differences between them are likely to break down. Thus, following the results of Marcussen (2003) we included “subsp. scotophylla” in the synonymy of subsp. alba.
In some parts of the distribution range of V. alba subsp. alba, a scotophylla morphotype with completely violet corollas (classified by Gams (1925) as V. alba var. scotophylla f. violacea Wiesb.) is rather common. Mainly in southern parts of the distribution range of V. alba subsp. alba, violet-flowered individuals may even prevail in comparison to the white-flowered individuals (cf. Marcussen 2003; Hodálová and Mereďa Jr., unpublished). The violet-flowered morphotype occurs in the West Carpathians as well (Hodálová and Mereďa Jr., unpublished), but we did not include specimens with violet flowers in the present analyses because of their rarity.
According to numerous previous studies (e.g. Gams 1925; Kirschner and Skalický 1990; Suda 2002; Marcussen et al. 2005), V. alba subsp. alba should possess only aboveground stolons. However, our study showed that V. alba subsp. alba develops both aboveground and underground stolons (in proportion of ca. 2:1; cf. character StN, Table 4). So we consider this character unsuitable for its delimitation from other (mostly stoloniferous) members of subsection Viola.
The results of our morphometric study are not in full agreement with those of Marcussen (2003) and Marcussen et al. (2005) regarding limits of some morphological traits within V. alba subsp. alba. Discrepancies concern mainly lamina dentations and maximum petiole hair length: both characters are shown in our study as most important for the separation of V. alba within subsection Viola. Marcussen (2003) also uses these characters for the delimitation of subspecies within V. alba, i.e., subsp. alba, subsp. cretica and subsp. dehnhardtii. However, Marcussen (2003) reported only interquartile ranges as follows: maximum hair length on laminas 0.5–1 mm in subsp. alba, 1–1.3 mm in subsp. cretica, and 0.4–0.6 mm in subsp. dehnhardtii; and number of crenulae along one leaf margin 19–26 in subsp. alba, 13–17 in subsp. cretica, and 14–18 in subsp. dehnhardtii. In our material, maximum hair length on petiole in V. alba subsp. alba was (0.5–)0.7–1.4(–1.8) mm and number of crenulae along one lamina margin was (10–)13–19(–25) (cf. characters LHL and LCN, Table 3).
Viola ambigua
Dostál (1989), Kirschner and Skalický (1990), and Suda (2002) considered the number of veins on capsule valves as one of the most important diagnostic characters for the delimitation of V. ambigua from the other species of subsection Viola: according to them, V. ambigua should have three veins on each valve, whereas the other species of subsection Viola should have only one vein. However, in fact, all individuals of this subsection from the West Carpathians that we had the opportunity to study had 3–5-veined capsule valves (cf. character CaVN, Table 3).
Viola collina and V. hirta
The morphological separation of V. collina and V. hirta is often based on the number of emarginated petals per corolla. Many authors (e.g. Gams 1925; Dostál 1989; Kirschner and Skalický 1990; Suda 2002) reported that V. collina tends to have only one emarginated petal (the anterior one), whereas in V. hirta all five petals are usually emarginated. However, much greater variation in this character has been observed on our material and V. collina partly shared the number of emarginated petal with V. hirta. Among the plants of V. collina studied, 11% of the flowers possessed two emarginated petals, 15% three emarginated petals, 7% four emarginated petals, and 13% five emarginated petals. In contrary, 39% of flowers of V. hirta possess four or less emarginated petals (cf. character CEN, Table 3). Thus, it is clear that the numbers of emarginated petals per corolla partly overlap, and the species cannot be unambiguously distinguished by this trait.
Viola odorata and V. suavis s.l.
The morphological separation of V. odorata and V. suavis is, in addition to other characters, commonly based on the presence or absence of aboveground and underground stolons: V. odorata should have only aboveground stolons (e.g. Gams 1925; Dostál 1989), whereas V. suavis only underground ones (e.g. Dostál 1989; Fischer et al. 2005). Based on results of this study, it is apparent that there are only slight differences between those two in this respect, and both species possess aboveground as well as underground stolons (in proportion of 2:1; cf. character StN, Table 4).
Viola suavis s.l.
It represents a taxonomically critical species, and its morphological variation has been repeatedly discussed (e.g. Becker 1910; Gams 1925, Marcussen and Nordal 1998). Recently, many European authors have expressed doubts as to the further subdivision of V. suavis because of a lack of reliable characters, but they have emphasized that further investigations of infraspecific variation of this taxon are necessary (cf. Marcussen and Nordal 1998). According to Marcussen and Borgen (2000) V. suavis is enzymatically highly variable but geographic patterns are not seen. It was hypothesized that V. suavis originated recurrently from V. pyrenaica (distributed from the Atlas and Pyrenees to the Caucasus) and other unidentified tetraploids (Marcussen and Borgen 2000).
According to our study, V. suavis s.l. is morphologically the most variable species of subsection Viola also in the West Carpathians. Like in V. alba subsp. alba, we found in V. suavis s.l. two colour morphotypes: (1) blue to (bluish-) violet-flowered plants (V. suavis s.str.) and (2) white-flowered plants (white-flowered morphotype of V. suavis). These morphotypes can be unambiguously distinguished by flower colour: V. suavis s.str. has blue to (bluish-) violet petals (excluding spur) with a large conspicuous white throat at base (reaching 1/3–1/2 of the length of lateral and anterior petals) and a pale blue to deep (bluish-) violet spur; the white-flowered morphotype of V. suavis has white petals with a pale to deep (bluish-) violet spur. In addition to this character, stolons, laminas, and sepals are, on average, more intensively anthocyanine-tinted in V. suavis s.str. than in the white-flowered morphotype. On the basis of our observation, V. suavis s.str. and its white-flowered morphotype differ (apart from characters connected with pigmentation of vegetative and generative parts) also in other morphological characters. Generally, the white-flowered morphotype of V. suavis has more narrow stipules, longer fimbriae on stipules, and bracteoles are inserted in lower parts of peduncles. The populations of the white-flowered morphotype of V. suavis are commonly cultivated in the gardens and parks, but in contrast to V. suavis s.str. they are not often established in natural and semi-natural habitats. Up to now the white-flowered morphotype of V. suavis has never been reported from Central Europe.
The taxonomic structure of V. suavis s.l. in the West Carpathians might be much more complicated as mentioned above and the taxonomy of this species should be studied within the whole species range. For this reason the taxonomic status and origin of the white-flowered morphotype of V. suavis should be further studied, based on more plant material.
Key to the Species of Viola Sect. Viola Subsect. Viola Occurring in the West Carpathians
Notes: When identifying species of the subsection Viola it is necessary to remember that many important diagnostic characters are changing after the period of flowering in the course of late spring and summer (e.g. leaf shape, lamina consistence, and the length of hairs on petioles) or they disappear totally (characters in stipules and flowers). Therefore most plants can be reliably identified only in spring when chasmogamous flowers are present. The chasmogamous flowers appearing sometimes in summer or autumn (reflorescence) are usually smaller, poorly developed and lack some attributes. For these reasons, the descriptions of leaves in the key refer to vernal flowering plants, and the descriptions of flowers refer exclusively to open (chasmogamous) vernal flowers.
Because in most species the first leaves appearing in early spring have glabrous or subglabrous petioles, the indument should be observed on petioles of younger, more hairy and still developing “summer” leaves. At that time, the longest hairs can be sometimes found also on the petioles of over-wintering leaves, developed in summer or autumn of the previous year.
The stipule shape described in the key refers to outer stipules of the main leaf rosette. Towards the middle of the main rosette and in filial leaf rosettes stipules get narrower and are less characteristically fimbriate or glandular and lack characters typical of different species.
Flower length is measured from the apex of the spur to the apex of the anterior petal (character CAL, Fig. 2).
The fragrance of flowers should be inspected when the weather is warm and sunny and the flowers are in full bloom. On cold or rainy days flowers partly or completely lose their odour.
In the key the main diagnostic characters (i.e., those sufficient for the safe identification of a particular species) are separated by the symbol • from the supplementary ones (which are less reliable and have no complementary statements consistently given in the other branch of the key). Main characters are arranged according to their importance, the order of supplementary characters corresponds to the usual arrangement of morphological descriptions. In the key, corolla colour is described in more detail than in the coded descriptions used in morphometric studies; so besides violet colour also its tints that could not be reliably coded are given in the key.
The most common hybrids are described in notes.
Character values given in the key represent 10 and 90 percentiles, those in brackets minima and maxima.
-
1a
Plants without stolons (but often with a thick many-headed rhizome), or with aboveground or underground stolons up to 3.5 cm long……………………… 2
-
1b
Plants at least with one aboveground or underground stolon more than 3.5 cm long……………………………………………………………………………. 8
-
2a
Lamina truncate to shallowly cordate at base, with sinus angle (80–)120–180(–190)°, lamina sinus depth reaching (0–)0.3–7(–11)% of lamina length……. 3
-
2b
Lamina shallowly to deeply cordate at base, with sinus angle [(−38)–]9–110(–155)°, lamina sinus depth reaching (3–)8–26(–41)% of lamina length…… 4
-
3a
Petioles with short hairs, the longest hairs (0.15–)0.2–0.4(–0.5) mm long. Flowers strongly fragrant. • Lamina fleshy. Bracteoles inserted in (25–)30–52(–56)% of peduncle length from the base. Petals (including spur) usually deep (bluish-) violet, flowers with (1–)2–5 emarginated petals. Spur ± straight or rarely hook-shaped, pointing upwards……………………………… V. ambigua
Note: Plants with shallowly cordate laminas at base, the longest hairs on petioles 0.3–1 mm long, and pale (bluish-)violet corollas may be of hybrid origin between V. ambigua and V. hirta. However, such plants can often represent only extreme variants of V. ambigua or V. hirta and cannot usually be exactly identified without a cytological analysis.
-
3b
Petioles with long hairs, the longest hairs (0.7–)0.9–1.5(–1.7) mm long. Flowers non-fragrant. • Lamina not fleshy……………… V. hirta (see also 4a)
-
4a
Flowers non-fragrant. Stipules elongated to narrowly triangular, 2–5(–7.5)× longer than wide, short-fimbriate or entire, the longest fimbriae (0.2–)0.3–1(–1.5) mm long, ± glabrous or near apex of the stipule sparsely ciliate, most of glandular fimbriae yellow or yellowish-brown. The longest hairs on petioles (0.7–)0.9–1.5(–1.7) mm long. Lamina shallowly to deeply cordate, rarely truncate at base, with sinus angle (30–)65–140(–180)° and sinus depth reaching (0–)4–12(–21)% of lamina length. Spur hook-shaped at apex, pointing upwards, rarely ± straight, pinkish-violet, rarely whitish. • Lamina (transversely) rotundate-ovate to elongate-triangular, (0.8–)1.2–1.7(–2.2)× longer than wide. Bracteoles inserted in (10–)18–36(–49)% of peduncle length from the base. Petals pale (bluish-) violet with a pink tint, rarely pink or white, flowers with (0–)3–5 emarginated petals………………………………………………V. hirta
Note: Plants forming stout tufts, occasionally with short stolons; the longest hairs on petioles 0.25–1 mm long, laminas ovate-lanceolate to rotundate-ovate, 0.7–1.8× longer than wide, stipules often ovate-lanceolate, flowers sometimes fragrant and spur deep (bluish-) violet belong to hybrid V. hirta × V. odorata. It is the most frequent hybrid among the species of subsection Viola, which occurs almost anywhere where parental species grow together.
-
4b
Flowers gently or strongly fragrant, rarely non-fragrant. Stipules either (1) ovate to lanceolate, 1.5–3.5(–5)× longer than wide, short-fimbriate, the longest fimbriae (0.2–)0.3–0.9(–1.6) mm long, ± glabrous, most of glandular fimbriae blackish (V. odorata); or (2) stipules ovate-lanceolate to narrowly triangular, 3–6(–10)× longer than wide, long-fimbriate, the longest fimbriae (0.2–)0.4–2.5(–3.7) mm long, stipules (and fimbriae) along the whole margin or at least near apex ciliate, glandular fimbriae yellow, yellowish-brown or blackish. The longest hairs on petioles (0.1–)0.15–1.4(–1.8) mm long. Lamina deeply cordate, with sinus angle [(−38)–]9–110(–155)°, and sinus depth reaching (3–)8–26(–41)% of lamina length. Spur ± straight, evenly curved up at full length or rarely hook-shaped at apex, either (1) pinkish-violet (V. collina) or (2) pale to deep (bluish-)violet, or yellowish-green……………………………………… 5
-
5a
Stipules short-fimbriate, the longest fimbriae (0.2–)0.3–0.9(–1.6) mm long, (0.02–)0.06–0.2(–0.33)× as long as the width of the undivided part of stipule, stipules and fimbriae ± glabrous. The longest hairs on petioles (0.1–)0.15–0.4(–0.5) mm long……………….…………………………V. odorata (see also 8b)
-
5b
Stipules long-fimbriate, the longest fimbriae (0.2–)0.4–2.5(–3.7) mm long, (0.06–)0.19–0.78(–1.31)× as long as the width of the undivided part of stipule; stipules in the upper half (including fimbriae) usually sparsely ciliate. The longest hairs on petioles 0.1–1.8 mm long…………………………………… 6
-
6a
Stipules ovate-lanceolate to narrowly lanceolate, (1.9–)2.4–3.7(–4) mm wide, long-fimbriate, the longest fimbriae (0.6–)0.9–1.8(–2.2) mm long. Bracteoles inserted in (30–)37–55(–62)% of peduncle length from the base. • The longest hairs on petioles (0.2–)0.4–1.2 mm long. Stipules and fimbriae ciliate along the whole margin or at least near apex. Lamina (transversely) rotundate-ovate to ovate, (0.8–)1–1.5(–1.8)× longer than wide, deeply cordate at base. Flowers small, (9.5–)11.5–16.5(–18) mm long. Petals (excluding spur) pale pinkish-violet. Spur whitish- to pale pinkish-violet, ± paler than petals……… V. collina
Note: Plants with short- to long-fimbriate stipules and ±ovate lamina are hybrids V. collina × V. hirta.
-
6b
Stipules either (1) narrowly triangular, (0.9–)1.6–2.9(–4.5) mm wide, the longest fimbriae (0.2–)0.4–1.1(–2.3) mm long and bracteoles inserted like in 6a (V. alba subsp. alba); or (2) stipule shape like in 6a, (1.6–)2.6–4.7(–6.2) mm wide, the longest fimbriae (0.2–)0.7–2.5(–3.7) mm long and bracteoles inserted in (5–)9–38(–51)% of peduncle length from the base (V. suavis s.l.)………… 7
-
7a
The longest hairs on petioles (0.5–)0.7–1.4(–1.8) mm long. Stipules narrowly triangular, (0.9–)1.6–2.9(–4.5) mm wide; the longest fimbriae (0.2–)0.4–1.1(–2.3) mm long. Bracteoles inserted in (20–)28–50(–59)% of peduncle length from the base. • Stolons slender, (3–)6–17(–19) cm long and (0.7–)0.9–1.5(–1.9) mm thick. Lamina apex obtusely acute to obtuse, with apex angle (60–)70–120(–170)°. Glandular fimbriae on stipules yellow, yellowish-brown or blackish. Flowers (8.5–)11.4–16.9(–19.1) mm long, posterior petals usually markedly asymmetric, 1.34–1.8(–2)× longer than wide, petals (excluding spur) white or yellowish-white, rarely pale to deep (bluish-)violet or violet. Spur white with yellowish-green colour at apex, or purplish… V. alba subsp. alba (including alba and scotophylla morphotypes)
Note: Plants with short stolons, laminas elongate-triangular, stipules 2–4 mm wide, spur usually purplish, and petals white, usually with purplish spots (mainly on the outside of upper petals) and/or violet venation near the base of the anterior petal belong to hybrid V. alba × V. hirta.
Note: Plants with numerous and very long stolons, lamina ±rounded or triangular-ovate, petiole hairs 0.4–1 mm long, stipules ovate to narrowly lanceolate (2–4× longer than wide), 2.5–5 mm wide, petals inside paler (pinkish) than outside (purplish) with violet venation near the base of the anterior petal and spur pinkish-violet belong to hybrid V. alba × V. odorata.
-
7b
Longest hairs on petioles 0.1–0.7(–1.1) mm long. Stipules ovate to narrowly lanceolate, (1.6–)2.6–4.7(–6.2) mm wide; the longest fimbriae (0.2–)1.1–2.5(–3.7) mm long. Bracteoles inserted in (5–)11–31(–51)% of peduncle length from the base. • Stolons stout, (1–)2–19(–28) cm long and (1–)1.4–2.7(–3.7) mm thick. Lamina apex obtusely acute to obtuse, with apex angle (65–)85–130(–180)°. Glandular fimbriae on stipules yellow or yellowish-brown. Calycine appendages appressed to the peduncle. Flowers (13–)15.5–19(–21) mm long, petals (excluding spur) either blue to (bluish-)violet, with large conspicuous white throat (reaching to ±1/3 of petal length from the base) or entirely white. Spur pale blue to deep (bluish-)violet……………………………… V. suavis s.l.
Note: Plants with short-fimbriate stipules, bracteoles inserted in 1/3 to 1/2 of peduncle and calycine appendages slightly patulous from peduncle represent hybrids V. odorata × V. suavis s.str. This hybrid can be very difficult to recognize, especially from some individuals of V. suavis s.str. representing extreme variation, and cytological analysis is necessary for their safe identification.
-
8a
Stipules long-fimbriate, the longest fimbriae (0.2–)0.4–2.3(–3.7) mm long, (0.06–)0.19–0.78(–1.31)× as long as the width of the undivided part of stipule, stipules and fimbriae ciliate along the whole margin or least near apex. The longest hairs on petioles 0.1–1.8 mm long…………………………………… 7
-
8b
Stipules short-fimbriate, the longest fimbriae (0.2–)0.3–0.9(–1.6) mm long, (0.02–)0.06–0.2(–0.33)× as long as the width of the undivided part of stipule, stipules and fimbriae ± not ciliate. The longest hairs on petioles (0.1–)0.15–0.4(–0.5) mm long. • Stolons slender, (2–)4–19(–28.5) cm long and (0.9–)1–1.9(–2.5) mm thick. Lamina apex obtuse to rounded, rarely obtusely acute, with apex angle (80–)95–140(–180)°. Bracteoles inserted in (15–)33–61(–71)% of peduncle length. Stipules ovate to narrowly triangular, (1.5–)2–3.5(–5)× longer than wide, (3–)3.6–5.2(–7) mm wide, most of glandular fimbriae blackish. Calycine appendages straight, not appressed to the peduncle. Petals (including spur) (bluish-)violet, deep-violet, rarely whitish or (in cultivars) pink, yellow etc. with small white throat at base (reaching up to ±1/4–1/5 of petal length from the base)………………………….……………………………… V. odorata
References
Ballard HE Jr, Sytsma KJ (2000) Evolution and biogeography of the woody Hawaiian violets (Viola, Violaceae): Arctic origins, herbaceous ancestry and bird dispersal. Evolution 54:1521–1532
Ballard HE Jr, Sytsma KJ, Kowal RR (1999) Shrinking the violets: phylogenetic relationships of infrageneric groups in Viola (Violaceae) based on internal transcribed spacer DNA sequences. Syst Bot 23:439–458
Becker W. (1910) Violae Europaeae. Verlag von C. Heinrich, Dresden
Becker W (1925) Viola. In Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien 2. Verlag von Wilhelm Engelmann, Leipzig, pp 363–376
Cortini Pedrotti C (2001) New check-list of the Mosses of Italy. F1 Medit 11:23-107
Danihelka J, Čeřovský J (1999) Viola ambigua. In Čeřovský J, Feráková V, Holub J, Maglocký Š, Procházka F (eds) Červená kniha ohrozených a vzácnych druhov rastlín a živočíchov SR a ČR 5, Vyššie rastliny. Príroda, Bratislava, p 403
Dınç M, Bağci Y, Yildirimli S. (2003) A new species of Viola L. (Violaceae) from South Anatolia. Bot J Linn Soc 141:477–482
Doležel J, Göhde W (1995) Sex determination in dioecious plants Melandrium album and M. rubrum using high-resolution flow cytometry. Cytometry 19:103–106
Dostál J (1989) Nová květena ČSSR 1. Academia, Praha
Everitt BS (1986) Cluster analysis. Ed. 2 (2nd reprint), Gower, Halsted Press, New York
Fischer MA, Adler W, Oswald K, Karrer G (2005) Veilchen u. Stiefmütterchen/Viola. In Fischer MA, Adler W, Oswald K Exkursionsflora für Österreich, Liechtenstein und Südtirol. Ed 2, Land Oberösterreich, Biologiezentrum der OÖ Landesmuseen, Linz, pp 428–434
Futák J (1984) Fytogeografické členenie Slovenska. In Bertová L (ed), Flóra Slovenska 4/1. Veda, Bratislava, pp 418–420
Gams H (1925) Violaceae. In Hegi G, Illustriete Flora von Mitteleuropa, Band 5. J. F. Lehmanns Verlag, München, pp. 585–657
Greilhuber J, Temsch EM, Loureiro JCM (2007) Nuclear DNA content measurement. In Doležel J, Greilhuber J, Suda J (eds) Flow cytometry with plant cells. Wiley-VCH, Weinheim, pp 67–101
Kirschner J, Skalický V (1990) Violaceae Batsch. In Hejný S, Slavík B (eds) Květena České republiky 2. Academia, Paraha, pp 394–431
Klecka WR (1980) Discriminant analysis. (Sage University Papers, Series: quantitative applications in the social sciences, no. 19). Sage Publications Inc, Sage, Beverly Hills, London
Krahulcová A, Krahulec F, Kirschner J (1996) Introgressive hybridization between native and an introduced species: Viola lutea subsp. sudetica versus V. tricolor. Folia Geobot Phytotax 31:219–244
Krzanowski WJ (1990) Principles of multivariate analysis. Clarendon Press, Oxford
Kuta E (1981) Further cyto-embryological studies on Viola L., section Viola L. Acta Biol Cracov, Ser Bot 23:69–85
Kuta E (1990) Biosystematic studies on the genus Viola L., section Plagiostigma Godr. II. Embryological analyses of V. epipsila Ledeb., V. palustris L. and their hybrids from Poland. Acta Biol Crac, Ser Bot 31:45–62
Marcussen T (2003) Evolution, phylogeography, and taxonomy within the Viola alba complex (Violaceae). Plant Syst Evol 237:51–74
Marcussen T (2006) Allozymic variation in the widespread and cultivated Viola odorata (Violaceae) in western Eurasia. Bot J Linn Soc 151:563–571
Marcussen T, Borgen L (2000) Allozymic variation and relationships within Viola subsection Viola (Violaceae). Plant Syst Evol 223:29–57
Marcussen T, Nordal I (1998) Viola suavis, a new species in the Nordic flora, with analyses of the relation to other species in the subsection Viola (Violaceae). Nord J Bot 18:221–237
Marcussen T, Borgen L, Nordal I (2001) Viola hirta (Violaceae) and its relatives in Norway. Nord J Bot 21:5–17
Marcussen T, Borgen L, Nordal I (2005) New distributional and molecular information call into question the systematic position of the West Asian Viola sintenisii (Violaceae). Bot J Linn Soc 147:91–98
Marcussen T, Wind P, Jonsell B, Karlsson T (2007) Violaceae. In Jonsell B (ed), Flora Nordica 6, (version 4b, 20060529, in review): http://www.floranordica.org/internt/-Review/-Review_editors/editrev.html.
Mereďa P Jr, Hodálová I, Mártonfi P, Kolarčik V (2006) [Reports (17–22)]. In Mráz P (ed) Chromosome number and DNA ploidy level reports from Central Europe - 2. Biologia (Bratislava) 61:116–117
Mereďa P Jr, Mártonfi P, Hodálová I, Šípošová H, Danihelka J (2008) Viola L. In Goliašová K, Šípošová H (eds) Flóra Slovenska 6/1. Veda, Bratislava (in press)
Murín A (1960) Substitution of cellophane for glass covers to facilitate preparation of permanent squashes and smears. Stain Technol 35:351–353
Nadot S, Ballard HE Jr, Creach JB, Dajoz I (2000) The evolution of pollen hetermorphism in Viola: A phylogenetic approach. Plant Syst Evol 223:155–171
Nimis PL, Martellos S (2008) The Information System on Italian Lichens. Version 4.0. University of Trieste, Dept. of Biology, Trieste, IN4.0/1 (http://dbiodbs.univ.trieste.it/)
Noirot M, Barre P, Duperray C, Hamon S, De Kochko A (2005) Investigation on the causes of stochiometric error in genome size estimation using heat experiments: Consequences on data interpretation. Ann Bot (Oxford) 95:111–118
Okamoto M, Okada H, Ueda K (1993) Morphology and chromosome number of Viola pilosa, and its systematic position. Taxon 42:781–787
Otto F (1990) DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. In Crissman HA, Darzynkiewicz Z (eds) Methods in cell biology 33. Academic, New York, pp 105–110
Podani J (2001) SYN-TAX 2000. Computer programs for multivariate data analysis in ecology and systematics. Users’s manual. Scientia, Budapest
Radford AE, Dickinson WC, Massey JR, Bell CR (1974) Vascular plant systematics. Harper & Row, New York
SAS Institute (2000) SAS online Doc®, Version 8 (available online). SAS Institute, Cary
Schmidt A (1961) Zytotaxonomische Untersuchungen an europäischen Viola-Arten der Sektion Nomimium. Oesterr Bot Z 108:20–88
Skalický V (1988) Regionálně fytogeografické členení. In Hejný S, Slavík B (eds) Květena České socialistické republiky 1. Academia, Praha, pp 103–121
Sneath PHA, Sokal RR (1973) Numerical taxonomy. W. H. Freeman, San Francisco
Soó R (1964) A magyar flóra és vegetáció rendszertani-kézikönyve I. Akadémiai Kiadó, Budapest
Suda J (2002) Viola L. – violka. In Hrouda L, Chrtek J Jr, Kaplan Z, Kirschner J, Kubát K, Štěpánek J (eds) Klíč ke květeně České republiky. Academia, Praha, pp 207–214
Valentine DH (1962) Variation and evolution in the genus Viola. Preslia 34:190–206
Valentine DH, Merxmüller H, Schmidt A (1968) Viola L. In Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (eds) Flora Europaea 2 (Rosaceae to Umbelliferae). Cambridge University Press, Cambridge, pp 270–282
Yockteng R, Ballard HE Jr, Mansion G, Dajoz I, Nadot S (2003) Relationships among pansies (Viola section Melanium) investigated using ITS and ISSR markers. Plant Syst Evol 241:153–170
Acknowledgements
The authors wish to express their thanks to K. Marhold for valuable discussions and to three anonymous reviewers for their useful comments on the manuscript. We are much obliged to P. Mereďa sen. for his help with plant collecting and during our study. We are thankful to V. Kolarčik and Ľ. Majeský for help with flow cytometric analyses, V. Polakovičová and J. Kučera for technical help, and to H. Šípošová and D. Dítě for their assistance in the field. This study was supported by the Grant Agency of Ministry of Education of the Slovak Republic and Slovak Academy of Sciences VEGA (grant no. 6054) and by Research and Development Support Agency of Slovak Republic (grant no. 6404). The participation of J. D. was supported by the Ministry of Education, Youth and Sports of the Czech Republic, project no. MSM 0021622416, and by the long-term research plan no. AV0Z60050516 of the Institute of Botany, Czech Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
List of the studied populations of Viola sect. Viola subsect. Viola. Each record is given as follows: population number, country, locality description, geographic coordinates (WGS84), altitude, date of collection, name of collector(s); mitotic chromosome number and/or DNA ploidy level, name(s) of the author(s) of the chromosome count(s) or author(s) of the measurements of DNA ploidy level; in parenthesis the total number of plants studied: number of plants studied for pollen fertility/for chromosome numbers/in flow cytometry/in morphometric analyses. Phytogeographical division of the Czech Republic follows Skalický (1988), that of Slovakia Futák (1984) and that of Hungary Soó (1964). Abbreviations of collectors, authors of the chromosome counts and authors of the measurements of DNA ploidy level: JD – J. Danihelka, DD – D. Dítě, IH – I. Hodálová, PMA – P. Mártonfi, LM – L. Mártonfiová, PMj – P. Mereďa Jr., PMs – P. Mereďa sen., HŠ – H. Šípošová.
Viola alba Besser subsp. alba (incl. subsp. scotophylla (Jord.) Gremli)
109A – Slovakia, Devínska Kobyla Mts., city quarter of Bratislava-Dúbravka, E of the elevation point 406, near the red-marked tourist path, 48°11′15″ N, 17°00′25″ E, 390 m, 14 Apr 2003, coll. PMj; 2n = 20, det. IH & PMj (1:1/1/0/1); alba morphotype.
109B – Slovakia, Devínska Kobyla Mts., city quarter of Bratislava-Dúbravka, E of the elevation point 406, near the red-marked tourist path, 48°11′15″ N, 17°00′25″ E, 390 m, 14 Apr 2003, coll. PMj; 2n = 20, det. IH & PMj (8:8/1/0/8); scotophylla morphotype.
6 – Slovakia, Podunajská nížina Lowlands, SW of the settlement of Čenkov, 47°45′51″ N, 18°31′39″ E, 110 m, 2 Apr 2003, coll. IH & PMj; 2n = 20, det. IH & PMj (10:10/1/0/10); scotophylla morphotype.
111A – Slovakia, Strážovské and Súľovské vrchy Mts., village of Omšenie, 0.5 km NW of the top of Omšenská Baba Hill, near the red-marked tourist path, 48°54′56″ N, 18°13′56″ E, 530 m, 19 Apr 2003, coll. PMj & PMs; 2n = 20, det. IH & PMj (8:8/1/0/8); alba morphotype.
111B – Slovakia, Strážovské and Súľovské vrchy Mts., village of Omšenie, 0.5 km NW of the top of Omšenská Baba Hill, near the red-marked tourist path, 48°54′56″ N, 18°13′56″ E, 530 m, 19 Apr 2003, coll. PMj & PMs; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (4:4/0/1/4); scotophylla morphotype.
112 – Slovakia, Strážovské and Súľovské vrchy Mts., town od Nová Dubnica, Markovica Hill, 0.5 km SW of the summit, 48°55′25″ N, 18°10′43″ E, 450 m, 21 Apr 2003, coll. PMj; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (10:10/0/1/10); scotophylla morphotype.
123 – Hungary, Sokoró Mts., SE of village of Györújbarát, near the camp Ifjuságy, 47°35′12″ N, 17°39′09″ E, 242 m, 1 Apr 2004, coll. PMj; 2n = 20, det. IH & PMj (10:10/1/0/10); scotophylla morphotype.
Viola ambigua Waldst. & Kit.
9 – Czech Republic, Moravia, Pavlovské kopce Mts., Pálava Hill, SE facing slope N of limestone quarry, 48°51′26″ N, 16°38′37″ E, 400 m, 4 Apr 2003, coll. IH & PMj; 2n = 40, det. LM (8:8/2/0/8).
21 – Slovakia, Devínska Kobyla Mts., city quarter of Bratislava-Devín, 1.2 km SW of the top of Devínska Kobyla Hill, 48°10′52″ N, 16°59′12″ E, 250 m, 8 Apr 2003, coll. IH & PMj; 2n = 40, det. IH & PMj (2:2/1/0/2).
151 – Hungary, Gerecse Mts., village of Csolnok, Magos-hegy Hill (elevation point 317 m), 47°41′20″ N, 18°42′10″ E, 314 m, 14 Apr 2005, coll. IH & PMj; 2n = 40, det. IH & PMj (8:8/1/0/8).
149 – Hungary, Gerecse Mts., village of Dág (SSE of town of Dorog), SE slope of Kecske-hegy Hill, 47°40′31″ N, 18°42′36″ E, 207 m, 14 Apr 2005, coll. IH & PMj; 2n = 40, det. IH & PMj (9:9/1/0/9).
Viola collina Besser
30 – Austria, Lower Austria, Eastern Alps, town of Baden Ali Wien, slope of Rauheneck Castle Hill, 48°00′34″ N, 16°12′25″ E, 350 m, 15 Apr 2003, coll. IH; 2n = 20, det. LM (10:10/2/0/10).
128 – Slovakia, Strážovské and Súľovské vrchy Mts., village of Omšenie, 0.5 km NW of the top of Omšenská Baba Hill, W facing slope, 48°54′40″ N, 18°14′07″ E, 580 m, 11 Apr 2004, coll. PMj & PMs; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (8:8/0/1/8).
127 – Slovakia, Strážovské and Súľovské vrchy Mts., town of Nová Dubnica, Markovica Hill, 0.5 km SW of the summit, 48°55′30″ N, 18°10′40″ E, 425 m, 10 Apr 2004, coll. PMj; 2n = 20, det. IH & PMj (10:10/1/0/10).
130 – Slovakia, Nízke Tatry Mts., village of Kráľova Lehota, near the settlement of Hlboké, 49°02′06″ N, 19°47′15″ E, 605 m, 15 Apr 2004, coll. IH & PMj; 2n = 20, det. IH & PMj (10:10/1/0/10).
Viola hirta L.
15 – Czech Republic, Moravia, Pavlovské kopce Mts., town of Mikulov, Svatý kopeček Hill, 48°48′25″ N, 16°38′56″ E, 330 m, 4 Apr 2003, coll. JD, IH & PMj; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (10:10/0/1/10).
14 – Czech Republic, Moravia, Pavlovské kopce Mts., town of Mikulov, Svatý kopeček Hill, 48°48′26″ N, 16°38′53″ E, 260 m, 4 Apr 2003, coll. JD, IH & PMj; 2n = 20, det. IH & PMj (3:3/1/0/3).
24 – Slovakia, Slovenský kras Karst, village of Silica, 1 km W of the edge of the village, 48°33′20″ N, 20°30′10″ E, 530 m, 9 Apr 2003, coll. IH; 2n = 20, det. LM (5:5/2/0/5).
103 – Slovakia, Devínska Kobyla Mts., city quarter of Bratislava-Devín, 0.8 km SWS of the top of the Devínska Kobyla Hill, near the red-marked tourist path, 48°10′58″ N, 16°59′34″ E, 344 m, 5 Apr 2003, coll. PMj; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (10:10/0/1/10).
116 – Slovakia, Biele Karpaty (southern part) Mts., village of Chocholná-Velčice, Chocholničianska dolina valley, 0.5 km NWW of Urbanová Hill, 48°53′48″ N, 17°54′57″ E, 460 m, 23 Apr 2003, coll. PMj; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (10:10/0/1/10).
113 – Slovakia, Strážovské and Súľovské vrchy Mts., town of Nová Dubnica, valley of Veľkokolačanský potok stream, 0.7 km SWW of the top of Markovica Hill, 48°55′25″ N, 18°10′35″ E, 415 m, 21 Apr 2003, coll. PMj; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (10:10/0/1/10).
114 – Slovakia, Strážovské and Súľovské vrchy Mts., town of Nová Dubnica, upper part of the city quarter of Veľký Kolačín, 48°55′55″ N, 18°10′13″ E, 285 m, 22 Apr 2003, coll. PMj; 2n = 20, det. IH & PMj (10:10/1/0/10).
23 – Slovakia, Spišské kotliny Basin, settlement of Primovce, near E edge of settlement, 49°00′49″ N, 20°23′07″ E, 620 m, 9 Apr 2003, coll. IH & DD; 2n = 20, det. LM (10:10/1/0/10).
Viola odorata L.
31 – Austria, Lower Austria, Eastern Alps, town of Baden Ali Wien, alluvium of the river below the Rauheneck Castle Hill, 48°00′34″ N, 16°12′25″ E, 250 m, 15 Apr 2003, coll. IH; 2n = 20, det. IH & PMj (9:9/1/0/9).
8 – Czech Republic, Moravia, Pavlovské kopce Mts., town of Mikulov, Pálava Hill, SE slope north of limestone quarry, 48°51′28″ N, 16°38′42″ E, 380 m, 4 Apr 2003, coll. IH & PMj; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (10:10/0/1/10).
3 – Slovakia, Burda Mts., settlement of Kováčov, in vicinity of railway station, 47°49′25″ N, 18°46′51″ E, 110 m, 2 Apr 2003, coll. IH & PMj; 2n = 20, det. IH & PMj; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (9:9/1/1/9).
26 – Slovakia, Košická kotlina Basin, town of Turňa nad Bodvou, S foot of Turniansky hradný vrch Castle Hill, 48°36′23″ N, 20°52′19″ E, 200 m, 10 Apr 2003, coll. IH; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (8:8/0/1/8).
203 – Slovakia, Slanské vrchy Mts., village of Kalša, 48°36′25″ N, 21°31′13″ E, 225 m, 8 Apr 2004, coll. PMA; 2n = 20, det. LM; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (10:10/1/1/10).
204 – Slovakia, Slanské vrchy Mts., village of Kalša, 48°36′23″ N, 21°31′11″ E, 236 m, 8 Apr 2004, coll. PMA; 2n = 20, det. LM; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (9:9/1/1/9).
120 – Hungary, Sokoró Mts., SE of village of Györújbarát, near the camp Ifjuságy, 47°35′11″ N, 17°39′12″ E, 246 m, 1 Apr 2004, coll. PMj; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (10:10/0/1/10).
Viola suavis M. Bieb. s.l.
11 – Czech Republic, Moravia, Pavlovské kopce Mts., town of Mikulov, Pálava Hill, 48°51′26″ N, 16°38′37″ E, 400 m, 4 Apr 2003, coll. IH & PMj; 2n = 40, det. LM (8:8/1/0/8).
4 – Slovakia, Burda Mts., settlement of Kováčov, in vicinity of railway station, 47°49′25″ N, 18°46′51″2 E, 110 m, 6 Apr 2004, coll. IH & PMj; 2n = 40, det. LM (10:10/1/0/10); violet-flowered morphotype.
16 – Slovakia, Devínska Kobyla Mts., city quarter of Bratislava-Devín, 1 km SWS of the top of Devínska Kobyla Hill, 48°10′51″ N, 16°59′32″ E, 290 m, 6 Apr 2003, coll. IH; 2n = 40, det. IH & PMj (7:7/1/0/7); violet-flowered morphotype.
7 – Slovakia, Devínska Kobyla Mts., city quarter of Bratislava-Dúbravka, Brižite Hill, 48°11′49″ N, 17°01′29″ E, 240 m, 1 Apr 2003, coll. IH; 2n ∼ 8x ∼ 40, det. PMA, IH & PMj (10:10/0/1/10); white-flowered morphotype.
18 – Slovakia, Podunajská nížina Lowlands, city quarter of Bratislava-Petržalka, Dudova street, 48°06′59″ N, 17°07′09″ E, 135 m, 4 Apr 2003, coll. HŠ & IH; 2n = 40, det. LM (4:4/2/0/4); white-flowered morphotype.
129 – Slovakia, Podunajská nížina Lowlands, town of Nitra, Šibeničný vrch Hill, edge of forest with Pinus nigra, Urbánkova street, 48°18′16″ N, 18°04′30″ E, 180 m, 13 Apr 2004, coll. PMj; 2n = 40, det. IH & PMj (10:10/1/0/10); violet-flowered morphotype.
106 – Slovakia, Podunajská nížina Lowlands, town of Nitra, Kalvária Hill, Pod Borinou street, 48°17′52″ N, 18°05′22″ E, 175 m, 13 Apr 2003, coll. PMj; 2n = 40, det. LM (12:12/2/0/12); white-flowered morphotype.
25 – Slovakia, Košická kotlina Basin, town of Turňa nad Bodvou, S foot of Turniansky hradný vrch Castle Hill, 48°36′23″ N, 20°52′22″ E, 205 m, 10 Apr 2003, coll. IH; 2n = 40, det. LM (10:10/2/0/10); white-flowered morphotype.
201 – Slovakia, Košická kotlina Basin, city of Košice, Botanical garden of P. J. Šafárik University–Faculty of Science, Mánesova street, 48°44′05″ N, 21°14′18″ E, 227 m, 17 Apr 2003, coll. PMA & LM; 2n = 40, det. LM (10:10/1/0/10); white-flowered morphotype.
202 – Slovakia, Košická kotlina Basin, city of Košice, Humenská street, lawn in kindergarten, 48°42′23″ N, 21°14′16″ E, 249 m, 17 Apr 2003, coll. PMA & LM; 2n = 40, det. LM (10:10/1/0/10); white-flowered morphotype.
28 – Slovakia, Východoslovenská nížina Lowlands, village of Hrušov, near the church, 48o26′10″ N, 21o51′41″ E, 105 m, 10 Apr 2003, coll. IH; 2n = 40, det. LM (10:10/2/0/10); violet-flowered morphotype.
22 – Slovakia, Liptovská kotlina Basin, town of Ružomberok, E of railway station, on the foot of Mních Hill, 49°05′00″ N, 19°18′37″ E, 490 m, 9 Apr 2003, coll. DD & IH; 2n = 40, det. LM (6:6/2/0/6); white-flowered morphotype.
125 – Hungary, Pilis Mts., town of Esztergom, 0.4 km NW of the top of Vaskapu Hill, near the red-marked tourist path, 47°47′21″ N, 18°46′11″ E, 340 m, 6 Apr 2004, coll. IH & PMj; 2n = ca. 40, det. IH & PMj; 2n ∼ 8x ∼ 40, det. PMA, IH & PMj (10:10/1/1/10); violet-flowered morphotype.
122 – Hungary, Sokoró Mts., SE of village of Györújbarát, near the camp Ifjuságy, 47°35′12″ N, 17°39′09″ E, 242 m, 1 Apr 2004, coll. PMj; 2n ∼ 8x ∼ 40, det. PMA, IH & PMj (10:10/0/1/10); violet-flowered morphotype.
Viola alba × V. hirta (V. ×adulterina Godr.)
17 – Slovakia, Devínska Kobyla Mts., city quarter of Bratislava-Devín, 1.3 km SW of the top of Devínska Kobyla Hill, near the educational path, 48°10′48″ N, 16°59′08″ E, 220 m, 6 Apr 2003, coll. IH; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (10:10/0/1/0).
101 – Slovakia, Devínska Kobyla Mts., city quarter of Bratislava-Devín, 1 km SWS of the top of Devínska Kobyla Hill, 48°10′57″ N, 16°59′20″ E, 300 m, 5 Apr 2003, coll. PMj; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (10:10/1/0/0).
Viola alba × V. odorata (V. ×pluricaulis Borbás)
102 – Slovakia, Devínska Kobyla Mts., city quarter of Bratislava-Devín, 1 km SWS of the top of Devínska Kobyla Hill, 48°10′57″ N, 16°59′22″ E, 300 m, 5 Apr 2003, coll. PMj; 2n = 20, det. IH & PMj (6:6/1/0/0).
105 – Slovakia, Devínska Kobyla Mts., city quarter of Bratislava-Devín, 0.8 km SES of the top of Devínska Kobyla Hill, near the yellow-marked tourist path, 48°10′56″ N, 16°59′56″ E, 350 m, 6 Apr 2003, coll. PMj; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (5:5/0/1/0).
110 – Slovakia, Devínska Kobyla Mts., city quarter of Bratislava-Dúbravka, E of elevation point 406 m, near the red-marked tourist path, 48°11′15″ N, 17°00′20″ E, 390 m, 14 Apr 2003, coll. PMj; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (8:8/0/1/0).
121 – Hungary, Sokoró Mts., SE of village of Györújbarát, near the camp Ifjuságy, 47°35′12″ N, 17°39′09″ E, 242 m, 1 Apr 2004, coll. PMj; 2n = 20, det. IH & PMj (8:8/1/0/0).
Viola ambigua × V. odorata (V. ×hungarica Degen & Sabr.)
13 – Czech Republic, Moravia, Pavlovské kopce Mts., town of Mikulov, Svatý kopeček Hill, 48°48′26″ N, 16°38′53″ E, 4 Apr 2003, coll. JD, IH & PMj; 2n = 30, det. LM (1:0/1/0/0).
Viola hirta × V. odorata (V. ×scabra F. Braun)
12 – Czech Republic, Moravia, Pavlovské kopce Mts., saddle Nad Soutěskou, 48°52′07″ N, 16°38′18″ E, 4 Apr 2003, coll. IH & PMj; 2n = 20, det. LM (9:9/2/0/0).
104 – Slovakia, Devínska Kobyla Mts., city quarter of Bratislava-Devín, 0.8 km SW of the top of the Devínska Kobyla Hill, near the red-marked tourist path, 48°10′58″ N, 16°59′40″ E, 320 m, 5 Apr 2003, coll. PMj; 2n ∼ 4x ∼ 20, det. PMA, IH & PMj (8:8/0/1/0).
117 – Slovakia, Biele Karpaty (southern part) Mts., village of Chocholná-Velčice, near N edge of part of Malá Chocholná, 48°52′37″ N, 17°56′44″ E, 270 m, 23 Apr 2003, coll. PMj; 2n = 20, det. IH & PMj (10:10/1/0/0).
115 – Slovakia, Strážovské and Súľovské vrchy Mts., town of Nová Dubnica, lower part of the city quarter of Veľký Kolačín, 48°56′22″ N, 18°09′38″ E, 255 m, 22 Apr 2003, coll. PMj; 2n = ca. 20, det. IH & PMj (10:10/1/0/0).
Viola odorata × V. suavis s.str. (V. ×vindobonensis Wiesb.)
118 – Czech Republic, Moravia, Pavlovské kopce Mts., town of Mikulov, Svatý kopeček Hill, 48°48′26″ N, 16°38′53″ E, 340 m, 4 Apr 2003, coll. JD, IH & PMj; 2n = 30, det. IH & PMj; 2n ∼ 6x ∼ 30, det. PMA, IH & PMj (2:0/1/1/0).
124 – Hungary, Pilis Mts., town of Esztergom, 0.4 km NW of the top of Vaskapu Hill, near the red-marked tourist path, 47°47′21″ N, 18°46′11″ E, 340 m, 6 Apr 2004, coll. IH & PMj; 2n ∼ 6x ∼ 30, det. PMA, IH & PMj (2:0/0/2/0).
Rights and permissions
About this article
Cite this article
Hodálová, I., Mereďa Jr., P., Mártonfi, P. et al. Morphological Characters Useful for the Delimitation of Taxa Within Viola Subsect. Viola (Violaceae): A Morphometric Study from the West Carpathians. Folia Geobot 43, 83–117 (2008). https://doi.org/10.1007/s12224-008-9005-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12224-008-9005-x