Abstract
The worldwide growing demand for energy permanently increases the pressure on industrial and scientific community to introduce new alternative biofuels on the global energy market. Besides the leading role of biodiesel and biogas, bioethanol receives more and more attention as first- and second-generation biofuel in the sustainable energy industry. Lately, microalgae (green algae and cyanobacteria) biomass has also remarkable potential as a feedstock for the third-generation biofuel production due to their high lipid and carbohydrate content. The third-generation bioethanol production technology can be divided into three major processing ways: (i) fermentation of pre-treated microalgae biomass, (ii) dark fermentation of reserved carbohydrates and (iii) direct “photo-fermentation” from carbon dioxide to bioethanol using light energy. All three technologies provide possible solutions, but from a practical point of view, traditional fermentation technology from microalgae biomass receives currently the most attention. This study mainly focusses on the latest advances in traditional fermentation processes including the steps of enhanced carbohydrate accumulation, biomass pre-treatment, starch and glycogen downstream processing and various fermentation approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MicroalgaeFootnote 1 are economically important due to their biological role in ecosystems and as a source of commercially significant high-value compounds (Matsubara 2004). They have a broad application in the food and feed industry, aquaculture, cosmetics and pharmacy. Moreover, microalgae have attracted attention as a renewable resource of energetic compounds, particularly as an alternative carbon source for subsequent processing in chemical industry.
The global demand for renewable energy sources like biofuels has been continuously growing. It is promoted by the contrast between the intensifying of energy utilization, decreasing amounts of traditional energy sources and the threat of global warming. Biofuels are generally referred to solid, liquid or gaseous fuels derived from organic matter (Brennan and Owende 2010b; Dragone et al. 2010; Scott et al. 2010). The first- generation biofuels (e.g. biodiesel, bio-oil or bioethanol produced from crops like rape, sugar beet, maize or wheat) possess notable economic, environmental and political concern as the mass production of biofuel requires more arable lands resulting in reduced human food production. Moreover, production process of the first-generation biofuels has also been causing environmental damage. As this generation of biofuels is not viable, research and development (R&D) have been focused on the second-generation biofuels whose feedstock are generally not food crops. As of the production of the second-generation biofuels requires expensive and sophisticated technologies, the production has not yet been profitable. Therefore, the researchers focused on the third-generation biofuels which are derived from microalgae. These are currently being promoted as a suitable biofuel feedstock because of their rapid growth rate, CO2 sequestration ability (zero emission balance), waste nutrient use and high production capacity of storage compounds (lipids, polysaccharides) (Bellou et al. 2014). They also do not compete with food or feed crops, as they can grow in vast and not used areas on non-arable land.
The third-generation biofuels produced from microalgae biomass have potential to overcome these challenges (Behera et al. 2015; Chew et al. 2017; Lakatos et al. 2014, 2017). The vast majority of the alternative biofuel research has been focused on the biogas and biodiesel production (Brennan and Owende 2010a; Wirth et al. 2015). Beside these two, bioethanol has received more and more attention because of the recent advances in its production technologies (de Farias Silva and Bertucco 2016; John et al. 2011). Bioethanol as the first- and second-generation biofuel is currently the most widely used worldwide. However, from the economical aspect, it still faces with challenges (Zhu et al. 2014). The production costs of the upstream and downstream processes are still significantly above the production prices of the fossil fuels (Jones and Mayfield 2012; Kumar and Murthy 2011; Norsker et al. 2011). The basic principles of the various production ways has been well described; further research is needed for increasing production efficiency and reducing production costs.
Bioethanol of the third generation produced from microalgae biomass may represent an environmentally friendly fuel. It has many advantages in view of first- and second-generation biofuels produced from higher plants (Singh et al. 2011; Zabed et al. 2017), mostly due to the rapid generation rate. Microalgae cells have short doubling time; thus, they can reach high productivity and short harvesting cycle (1–10 days) compared to other crop feedstock (once or twice per year). Industrial-scale microalgae cultivation does not need arable lands. The most common production systems employed for microalgae cultivation are outdoor open ponds and closed photobioreactors (PBRs) (Benavides et al. 2017; Bharathiraja et al. 2015; Gupta et al. 2015), as these are artificial systems for extensive production which can be to a certain degree optimized as concerns growth conditions of selected production strains. Utilization of various nutrient sources, the cultivating in fresh and marine water is possible as well as in municipal wastewater which contributes to water remediation and nutrient use and makes microalgae an easy-to-grow feedstock (Dourou et al. 2018; Malibari et al. 2018; Nigam and Singh 2011). In particular, these organisms have been proven effective in removing nutrients/pollutants, especially nitrogen (N) and phosphate (P), from agro-industrial wastewaters (Tsolcha et al. 2018), such as raisin and winery wastewaters (Tsolcha et al. 2017), brewery wastewater (Boboescu et al. 2016) and second cheese whey effluents (Tsolcha et al. 2016), as well as from urban wastewaters (Caporgno et al. 2015). Microalgae can reach higher photosynthetic efficiency of 4–5% under natural conditions as compared to higher plants (1–2%) (Richmond and Hu 2013; Tredici 2010). The microalgae biomass primarily contain proteins (30–50%), carbohydrates (20–40%) and lipids (8–15%) (Hu 2004; Singh et al. 2011; Wang et al. 2013; Zhu et al. 2014). Besides their advantageous features, these photosynthetic organisms are mostly not suitable for bioethanol production due to low polysaccharide content under normal cultivation conditions. The efforts to increase bioethanol production yields are underway, like increasing biomass growth rate, manipulation of cultivation conditions to induce higher carbohydrate content or improving carbohydrate to ethanol conversion efficiency (Bharathiraja et al. 2015; Kumar and Murthy 2011; Zhu et al. 2014). Some studies have documented that the content of carbohydrates can be increased under stress conditions resulting for example in higher starch or glycogen content under nitrogen depletion (Cheng and He 2014; Juneja et al. 2013). Microalgae also do not contain lignin and have low hemicellulose levels which facilitate hydrolysis efficiency and fermentation yields. Milder technics for hydrolysis and fermentation can also reduce the overall cost of bioethanol production (Choi et al. 2012; Eshaq et al. 2011).
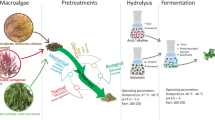
Three possible ways of microalgae biomass processing for bioethanol production are well described in the literature (Fig. 1). Firstly, it is the traditional process in which the carbohydrate-rich biomass undergoes pre-treatment steps, enzymatic hydrolysis and yeast fermentation (Hernández et al. 2015). The second way uses the metabolic pathways under dark conditions. During dark fermentation, photosynthesis is redirected to produce hydrogen, acids and alcohols (such as ethanol) (Magneschi et al. 2012). The third way is “photo-fermentation” (Dexter et al. 2015). It requires the use of genetic engineering to redirect the regular biochemical pathways in microalgae, preferably cyanobacteria, for a more subjective and efficient production of bioethanol. Genetically modified strains use light as energy source to generate bioethanol from carbon dioxide and water in a single step (Dexter and Fu 2009).
The purposes of this article is to review the published results concerning environmental physico-chemical conditions which enhance polysaccharide accumulation in microalgae cells and technological procedures of subsequent downstream processing for bioethanol production including pre-treatments, carbohydrate hydrolysis and various fermentation methodologies.
Bioethanol production by dark fermentation
In phototrophic microorganisms, polysaccharides and lipids are accumulated in cells during daylight hours when photosynthetic oxygen production and carbon dioxide fixation are the dominant metabolic process. Dark fermentation proceeds in the absence of light (Heyer and Krumbein 1991; Hirano et al. 1997) when most of the starch reserves can be hydrolysed to sugars by amylase activity and then via glycolysis to pyruvate (Catalanotti et al. 2013). The main benefit of the fermentation from the aspect of the photosynthetic organism is to generate ATP necessary to drive metabolic and energy requiring processes (Ueno et al. 1998). Eukaryotic algae such as Chlamydomonas reinhardtii (further as C. reinhardtii), Chlamydomonas moewusii, Chlorogonium elongatum and Chlorella fusca can ferment intracellular starch. Carbohydrate polymers (starch in eukaryotic microalgae) can be converted to pyruvate as a major intermediate compound and then to a variety of end products including acetate, ethanol, formate, glycerol, lactate, H2 and CO2 via various fermentative pathways (Ben-Amotz 1975; Gaffron and Rubin 1942; Grossman et al. 2007; Klein and Betz 1978; Mus et al. 2007). The final products vary among eukaryotic algae species and can also significantly vary with changes in environmental conditions. Pyruvate fuels fermentation processes, serving as substrate for acetyl coenzyme A (acetyl-CoA) which can be metabolized to generate ATP by conversion to acetate or to help maintain redox balance by conversion to ethanol in Chlamydomonas (Hemschemeier and Happe 2005; Mus et al. 2007). During this final stage, the putative dual-function alcohol/acetaldehyde dehydrogenase (ADHI) converts the acetyl-CoA into acetaldehyde or into ethanol (Magneschi et al. 2012).
Photofermentative bioethanol production
Cyanobacteria are able to produce ethanol directly in photosynthetic process. It is called the “photofermentative’” or “photanol” routes (Deng and Coleman 1999; Dexter et al. 2015). Recently, genetically modified cyanobacterial strains (Synechocystis sp. PCC 6803, Synechococcus sp. PCC 7942, Synechococcus sp. PCC 7002; Pasteur Culture Collection, Paris) have been constructed to carry out the “photofermentative” way of bioethanol production (Dexter and Fu 2009; Dienst et al. 2014; Gao et al. 2012). Firstly, pyruvate decarboxylase (PDC) and alcohol dehydrogenase II (ADHII) genes from Zymomonas mobilis (further as Z. mobilis) were introduced into the cyanobacterium Synechococcus sp. PCC 7942 (Deng and Coleman 1999). The same PDC/ADHII cassette was used in this model system like previously in Escherichia coli for demonstrating heterologous expression of these genes (Ingram et al. 1987). In the newly established metabolic pathway, the carbon dioxide is fixed in the Calvin-Benson cycle using the reducing power of photosynthesis to generate pyruvate which is converted to acetaldehyde and ethanol via the inserted PDC and ADHII. The initial results of genetic modifications were promising, but the ethanol production rate and volume were inefficient. Further investigations attempted to understand its limitations. The concentration of ethanol about 2.5 and 4.5 g/L inhibited the growth of Synechococcus sp. PCC 7942 and Synechocystis sp. PCC 6803, respectively (Kämäräinen et al. 2012). The phototrophic metabolism during ethanol production in Synechocystis sp. PCC 6803 was extensively examined by proteomic analysis to elaborately understand the operation of this artificially inserted pathway (Pade et al. 2017; Song et al. 2014). Besides, systems analysis was performed in Synechocystis sp. PCC 6803 and Synechococcus sp. PCC 7002 cultures (Dienst et al. 2014). The study revealed that the progressive intracellular organic carbon limitation is caused by its excessive drain from central metabolism via ethanol loss (Kopka et al. 2017). Other studies combined genetic modifications with stress conditions showing that nitrogen starvation together with the combinatorial deletions of some parts of the glycogen synthesis pathway and the poly(3-hydroxybutyrate) (PHB) synthesis pathway enhanced the ethanol production rate in Synechocystis sp. PCC 6803 (Namakoshi et al. 2016). To test theoretical metabolic modifications, in silico simulations were developed for Synechocystis sp. PCC 6803 strain (Yoshikawa et al. 2017). It was predicted that the knockout of NAD(P) H dehydrogenase (ndhF1) can enhance ethanol production under photoautotrophic conditions using ammonium as nitrogen source. Ethanol titre was significantly increased in the ΔndhF1 mutant compared to the wild type. The mutant cultivation was conducted indoor at laboratory scale, but the main purpose of the “photofermentation” concept has been the large-scale one-step bioethanol production. Despite the initial success, serious practical problems have arisen already at pilot scale. The construct Synechocystis Syn-HZ24 was successfully prepared and cultivated in the laboratory, but ethanol production was aborted by Pannonibacter phragmitetus during outdoor cultivation (Zhu et al. 2017) as the construct was overgrown by the bacterium and accumulated ethanol was consumed. pH-rising strategy (NaHCO3) was applied to reduce P. phragmitetus population and accumulate produced ethanol. Final ethanol concentration was about 0.9 g/L after 10-day cultivation resulting in about 80% recovery.
Fermentative bioethanol production
Polysaccharide composition of microalgae
In the fermentative method, the carbohydrate content of microalgae biomass serves as the feedstock for the bioethanol production. Among green microalgae, Chlorella, Scenedesmus, Chlorococcum and Tetraselmis have mostly been studied (Figueroa-Torres et al. 2017). Cyanobacterial strains like Synechococcus and Arthrospira have also been investigated as a polysaccharide feedstock for bioethanol production (Aikawa et al. 2018; Arias et al. 2018; Markou et al. 2013; Möllers et al. 2014). Microalgae capture solar energy and convert it into chemical energy through a number of complex photosynthetic reactions. There are two main types of reactions, the light and the dark reactions. In the light reactions, the solar energy is used to split water into protons, electrons and oxygen. The electrons and protons are then used to generate energy carriers (NADPH and ATP) which support the metabolic needs of the organism. In the dark reactions, carbon dioxide is reduced to carbohydrates by the Calvin-Benson cycle, using NADPH and ATP (Richmond and Hu 2013).
Polyglucans comprise the major carbohydrate content and serve two main purposes for microalgae. They are either accumulated in the plastids as reserve components (starch, glycogen) or become the main structural component of the cell wall (cellulose, sulphated polysaccharides). Carbohydrates, as storage compounds, provide the energy needed for metabolic processes and allow temporary survival in dark conditions (Raven and Beardall 2003). The reserve carbohydrates are species dependent (Kadouche et al. 2016). Cyanobacteria synthesize glycogen which is a water-soluble α-polyglucan; the size of particles is less than 0.04 μm in diameter and contains α-1,4-glucosidic and α-1,6-glucosidic bonds between the monomers (De Porcellinis et al. 2017; Melendez-Hevia et al. 1993). Green microalgae synthesize starch molecules whose size is between 2 and 100 μm and is composed of 72–82% of amylopectin (containing α-1,4-glucosidic and α-1,6-glucosidic bonds) and 18–28% of amylose (containing α-1,4-glucosidic bonds) (Buléon et al. 1998; Busi et al. 2014; Nakamura et al. 2005). Starch has α-1,6-glucosidic bonds between the amylose chains, creating the branched amylopectin. Glycogen and amylopectin have similar structure, but the former has about one branch point per ten 1,4-alpha bonds, compared to about one branch point per 30 1,4-alpha bonds in the latter.
Red algae synthesize floridean starch which is practically identical with that of potato and maize starch, indicating that it is a glucose polymer linked by α-1,4- and α-1,6-glucosidic bonds (hybrid of starch and glycogen) (Nyvall et al. 1999). In addition, the Euglenophyta and Chlorarachniophyta genera accumulate as storage substances, β-polyglucans, where the glucose residues are linked only at the β-1,3 glucosidic bonds (Suzuki and Suzuki 2013). The cellulose present in the microalgae cell wall is also suitable as a feedstock for bioethanol production (Wang et al. 2014), and the microorganisms have different cell wall structures from lignocellulose of terrestrial plants. Especially the lack of lignin in microalgae results in less harsh pre-treatments for releasing the biodegradable organic matter. Major carbon storage compounds like starch or glycogen need hydrolytic degradation to glucose (Bibi et al. 2017). Then, the glucose from the treated biomass can be fermented with yeast or bacteria to obtain bioethanol. But before that, the cells must be firstly disintegrated (physically, chemically or enzymatically) to access the storage compounds (Table 1). The most common microorganisms used for alcoholic fermentation are yeasts of the genus Saccharomyces or bacteria of the genus Zymomonas.
Culturing conditions affecting polysaccharide accumulation
Irradiance
Light is the main energy source for the photosynthetic organisms and therefore it is essential for the phototrophic growth of microalgae. The quality and quantity of light affects the growth rate and also the biomass composition. The efficiency of light energy supply thus becomes one of the major limiting factors for large-scale microalgae cultivation. The growth rates increase as the irradiance increases up to a level in which the light-saturation occurs. The majority of the microalgae are saturated under cell irradiance of 200–400-μmol photons m−2 s−1. If the irradiance increases above a certain level, then it becomes harmful for the photosynthetic apparatus and such excessive irradiance can cause photoinhibition (Richmond et al. 2003).
Dihydroxyacetone phosphate, sucrose and starch synthesis precursor is stimulated by light (Champigny 1985) and therefore the light intensity affects positively the carbohydrate accumulation in microalgae. It was demonstrated that the light intensity in the range of 300–400-μmol photons m−2 s−1 could slightly increase the accumulation of carbohydrates (Carvalho et al. 2009). A significant increase in starch content from 8.5 to 40% (dry mass basis) was observed when the mean light intensity was increased from 215- to 330-μmol photons m−2 s−1 (Fernandes et al. 2010). However, the accumulation of microalgae carbohydrates depends not only on light intensity, but also on other environmental parameters, mainly temperature and nutrient content (e.g. nitrogen or sulphur limitation).
During light periods, photosynthesis produces carbohydrates that are consumed by respiration in the dark when starch consumption required to maintain metabolic functions can reduce the biomass by about 35% (Ogbonna and Tanaka 1996). In order to avoid these losses, alternative energy sources (such as acetate) are fed to the culture over dark periods (heterotrophic growth). On the other hand, the phototrophic mode takes place during illumination periods when photosynthesis is active. Under this mode, microalgae consume CO2 and allocate it mainly in carbohydrates (John et al. 2011).
High light intensities result in an increase in the carbohydrate content (Hu 2004). In cultures of Porphyridium species, a 3-fold increase in carbohydrate content was observed, while in the cultures of Spirulina (Arthrospira) maxima an increase from 7–10% up to 34% in carbohydrates was obtained, when light increased (De Philippis et al. 1992; Friedman et al. 1991). High light intensity (700-μmol photons m−2 s−1) in combination with nitrate limitation to produce glycogen (about 1 g/L) from the cyanobacterium Arthrospira platensis (further as A. platensis) were used (Aikawa et al. 2012).
Overall, irradiance periods are crucial for carbohydrate accumulation and content. Once again, light exposure effects are variable among microalgae strains. Carbohydrate accumulation may take place upon particular irradiance regimes. Hence, biomass harvest should be planned strategically in order to avoid biomass loss or changes in macromolecular composition.
Temperature
Rising culture temperature can increase microalgae growth, until favourable range is reached. Changes of this variable also play an important role in carbohydrate accumulation. Sugar content can be enhanced by 25% by shifting cultivation temperature at a particular growth stage of microalgae culture. For example, allowing exponential growth at 20 °C until nitrate depletion, followed by a drastic decrease of temperature to 14 °C, leads to sugar accumulation. When Chlorella vulgaris (further as C. vulgaris) is cultivated at 20 °C, amylopectin synthesis is preferred over amylase synthesis which normally predominates at 38 °C. Moderate temperatures (25 °C) favour carbohydrate synthesis in Spirulina (Torzillo et al. 1991). In the case of Anabaena, extracellular polysaccharides are preferentially released at low temperature (20 °C) (Sangar and Dugan 1972). Likewise, increasing temperature to 35 °C during culturing of Synechocystis sp. shifts the natural accumulation of glucosyl-glycerol toward sucrose production (Warr et al. 1985).
In addition, there exists an interplay between temperature and light intensity as their effects on the biomass composition seem to be synergistic (Carvalho et al. 2009; Jensen and Knutsen 1993). The temperature affects the level where the light inhibition occurs as low temperatures enhance photostress (Jensen and Knutsen 1993). Additionally, temperature affects the uptake of nutrients and the structure of cell membranes and it also influences the oxygen evolving activity of the PSII complex (Vonshak 1997). The habitat where a strain was isolated is related to optimal temperature regime that results in remarkable differences among the species. In Arthrospira cultures, the temperature rise from 25 to 40 °C resulted in a slight increase of the biomass carbohydrates from about 14 to 21% (Ogbonda et al. 2007), while in the diatom Chaetoceros the carbohydrate content was higher at the lowest temperatures tested (de Castro and Garcia 2005). The same effect was observed in Chlorella where growth rate dropped with decreasing temperatures and the sugar content increased (Hosono et al. 1994).
The significant differences in biochemical composition have not always been observed under temperature variations in some microalgae species (Renaud et al. 2002). Thus, the effect of temperature on carbohydrate accumulation in microalgae seems to be highly species-specific and has to be better clarified (De Oliveira et al. 1999).
pH
The pH value is an important environmental variable for the metabolism of microorganisms including microalgae as it affects not only cell growth but also biochemical composition. In general, the suitable pH for carbohydrate accumulation differs based on the group of microalgae species used. The pH value could significantly affect the accumulation of total carbohydrates in both Dunaliella bardawil and Chlorella ellipsoidea as their maximum carbohydrate accumulation was reached at pH 7.5 and 9.0, respectively (Khalil et al. 2010). For example, in the culture of marine diatom Skeletonema costatum, extracellular carbohydrate production was dramatically increased from 2.1 to 17.7% when it was grown at pH 9.4 (Taraldsvik and Myklestad 2000).
Carbon dioxide (and bicarbonate) supply
Despite the ability of some microalgae to utilize carbon in both inorganic (CO2) and organic form (glucose, acetate etc.), their basic disposition is phototrophic growth and therefore the supply of CO2 is required for growth of microalgae in dense mass cultures. A sufficient supply of CO2 is considered to be positively related to the efficiency of photosynthesis, resulting in the synthesis of carbohydrates as the end product; CO2 is thus one of the key factors influencing the accumulation of carbohydrate in microalgae. Some studies found that carbohydrate accumulation in microalgae is improved by increasing the percentage of CO2 in the inlet gas (Giordano 2001; Xia and Gao 2005). For example, increasing dissolved CO2 concentration from 3 up to 186 μmol/L in cultures of Chlorella pyrenoidosa and Chlamydomonas reinhardtii could elevate the carbohydrate content from 9 to 21% and 3 to 7.4% (w/w), respectively. Due to carbon concentrating mechanisms (CCM), some microalgae extract inorganic carbon from the extracellular environment. This mechanism is induced under low carbon dioxide concentrations. In addition, intracellular starch location is also ruled by CO2 concentration. At high CO2 (3% v/v) levels, starch accumulates in the chloroplast stroma. However, when CO2 supply decreases, the storage location shifts to the pyrenoid (Izumo et al. 2007). Thus, CO2 can be used to enhance starch content and to control starch deposition. The morphological differences of the compartments mediate this increase. In the case of Dunaliella salina, high concentration of CO2 and ammonium N-source resulted in decreased pigment content and slight increase of carbohydrates and proteins (Giordano and Bowes 1997). On the other hand, high concentration (5% v/v) of CO2 and nitrate N-source diminished soluble sugars, and increased starch slightly, while protein increased markedly. Therefore, it can be concluded that there is a clear relation among N-source, CO2 supply concentration and carbohydrate profile. In some microalgae species, increases in the CO2 concentration result in an increase in the protein content, but a decrease or no obvious change in the carbohydrate content (Brown et al. 1997). However, under nitrogen starvation conditions and with an adequate supply of CO2 and light energy, the protein content in microalgae can be consumed as a nitrogen source, and the carbohydrate content may increase significantly during this process. Therefore, suitable addition of CO2 is a key step to improve the phototrophic growth of microalgae cells (biomass productivity and protein content), although it may not directly enhance carbohydrate accumulation in microalgae, unless appropriate stress conditions are employed.
Apart from the phototrophic growth, some microalgae are also capable to grow on organic substances, such as glucose, glycerol and acetate. The transport of such organic substances is achieved by several mechanisms, like phosphorylation (in the case of sugars), simple diffusion into the cells (in the case of glycerol) or by membrane transporter proteins (in the case of organic acids) (Perez-Garcia et al. 2011). The carbon metabolic mode in general affects the growth rates which are increased under mixotrophic or heterotrophic mode compared to phototrophic (Chojnacka and Marquez-Rocha 2004). Additionally, the carbon metabolic mode seems to affect the microalgae biomass composition. For instance, Chlorella accumulates more carbohydrates under mixotrophic (Abreu et al. 2012) or heterotrophic growth regime (Choix et al. 2012) than under phototrophic growth. However, the majority of the studies dealing with mixotrophic or heterotrophic modes of microalgae metabolism focus on lipid production and therefore not as much is known about the effect of these modes on carbohydrate accumulation.
Macronutrients
Microalgae often respond to unfavourable conditions (low or extremely high irradiance intensity, nutrient limitation, temperature extremes, salinity) by modifying key biochemical pathways toward the production of various storage compounds. Manipulation of culture conditions that directly affect biomass composition can promote polysaccharide productivity (Bracken et al. 2015). In many microalgae, the accumulation of storage compounds can be commonly enhanced through macronutrient (N, P, S) limitation (insufficient supply) or starvation (no supply) but at the expense of biomass productivity (Chen et al. 2013; Dragone et al. 2011; Jerez et al. 2016; Ji et al. 2018; Markou et al. 2012a). Nutrient limitation like nitrogen, sulphur or phosphorus deprivation has been commonly used and until now the most efficient way to enhance starch and glycogen accumulation (de Farias Silva et al. 2018).
Nitrogen
Nitrogen is an essential nutrient for microalgae growth. It is crucial for the synthesis of proteins, amino acids, DNA, pigments, enzymes, coenzymes and many other compounds (Turpin 1991). Nitrogen starvation affects firstly the photosynthetic systems, mainly the PSII complex, having negative impact on the synthesis of proteins participating in both photosystems (PSI and PSII) (Berges et al. 1996). Under nitrogen starvation, the flow of the photosynthetically fixed carbon is turned from the protein synthesis pathway to the reserve components (lipids or carbohydrates), resulting in either polysaccharide or lipid accumulation (Bellou and Aggelis 2013; Hu 2004; Huo et al. 2011). There are many examples in the literature showing that nitrogen limitation is the most effective way to trigger carbohydrate accumulation in microalgae (Huo et al. 2011; Shang et al. 2017). As an example, a 55% carbohydrate content was achieved when cultivating C. vulgaris in a low-nitrogen medium. Also the cultivation of Tetraselmis suecica under nitrogen starvation could dramatically enhance the cellular carbohydrate content from 10 to 57% (D'Souza and Kelly 2000). When subjected to nitrogen limitation, C. reinhardtii can mixotrophically accumulate starch from 35 to 58% of dry mass (Li et al. 2010). The main problem connected with the use of nitrogen-deficient medium to accumulate carbohydrates in microalgae is serious stress leading to a gradual inhibition of growth and cell lysis in a relatively short time (Eriksen et al. 2007). Some authors tried to solve the problem by limiting the concentration of nitrogen in growth medium instead of the total deficiency (Yao et al. 2012). By this approach, they succeeded in increasing the maximum biomass and starch content.
However, some studies indicated that there was a correlation between lipid and carbohydrate synthesis under nitrogen limitation because the metabolic pathways associated with biosynthesis and degradation of energy-rich compounds (lipids and carbohydrates) are closely linked (Bellou et al. 2014; Ho et al. 2013; Siaut et al. 2011). Starch biosynthesis can directly come from lipid synthesis on one side and vice versa degradation of starch provides the metabolites for producing acetyl-CoA which is the precursor in fatty acid biosynthesis (Li et al. 2010). CO2 is converted to glycerate-3-phosphate through photosynthesis, then acetyl-CoA is generated via pyruvate by the pyruvate dehydrogenase complex. This initiates the lipid biosynthetic pathway which occurs in the plastid. Acetyl-CoA can also be generated via a biochemical pathway that permits the conversion of polysaccharides into lipids. This is commonly utilized by oleaginous heterotrophs during sugar assimilation (Bellou and Aggelis 2013). As an alternative way, glycerate-3-phosphate can be converted into polysaccharides, molecules used for structural purposes and storage material. Energy from sugars is generated during their catabolism, commonly via glycolysis in the cytosol followed by citric acid cycle in the mitochondrion. However, under specific growth conditions (i.e. under nitrogen or phosphate limitation), citric acid cycle could be disturbed, due i.e. to the inhibition of isocitrate dehydrogenase enzyme that catalyses the conversion of isocitrate to α-ketoglutarate. In this case, citrate is accumulated in the mitochondrion and then excreted in the cytosol where, in the presence of ATP-dependent citrate lyase, it is ceased into acetyl-CoA and oxaloacetate. Cytosolic acetyl-CoA carboxylase converts acetyl-CoA into malonyl-CoA and becomes available for fatty acid elongation in the membranes of the endoplasmic reticulum (Mühlroth et al. 2013). These pathways lead to the conclusion that the effect of nitrogen starvation is probably more complex and also depends on additional factors, although this mechanism has only been shown in Nannochloropsis salina and Chlorella sp. cultivated in a lab-scale open pond-simulating PBR (Bellou and Aggelis 2013).
In cyanobacteria, the majority of the studies dealing with nitrogen limitation reported predominantly carbohydrate (glycogen) accumulation, i.e. A. maxima accumulated 60–70% (De Philippis et al. 1992) and A. platensis 55–65% of carbohydrates (Sassano et al. 2010).
Phosphorus
Phosphorus (P) is an essential macronutrient necessary for growth and it is essential for compounds like DNA, RNA, ATP, proteins and phospholipids (Geider and La Roche 2002). This element also affects carbon uptake and hence carbon allocation in the microalgae cells. In general, the P-deficient microalgae cultivation results in increased content of intracellular reserve compounds (storage carbohydrates or lipids) similar to limitations of other macroelements. This enhancement is strain specific; for example Chlamydomonas showed a stronger response to P deficiency when compared to Scenedesmus (Dean et al. 2008b), and depended on the length of the starvation period.
Carbohydrates are synthesized in the chloroplasts and/or in the cytosol. In the case of eukaryotes, when the cytosolic P concentration is low, ATP is kept within the chloroplast and carbohydrates are synthesized (Taiz and Zeiger 2010). Moreover, it is known that the carbohydrate synthesis is not a process consuming inorganic P, and that the ADP-glucose pyrophosphorylase, the enzyme which controls the carbohydrates synthesis, is activated by the 3-phosphoglycerate and is inhibited by inorganic P. Therefore, the level of accumulated carbohydrates is determined by the ratio of 3-phosphoglycerate to inorganic P. The effect of P starvation on the microalgae biomass composition is the simultaneous carbohydrate and lipid accumulation against the decrease of protein synthesis (Dean et al. 2008b; Lynn et al. 2000). Carbohydrates are more significantly affected by the P starvation and their accumulation is more notable than that of lipids (Dean et al. 2008a; Guerrini et al. 2000). Furthermore, it was found that the accumulation of energy-rich storage compounds is controlled by a critical intracellular P concentration (P quota) rather than its extracellular one (Sigee et al. 2007). In P-starved Chlorella, the carbohydrates amounted 55% of biomass (Brányiková et al. 2011), while in cultures of A. platensis the carbohydrates amounted about 63% (Markou et al. 2012a) when the P starvation was used for the production of carbohydrate-rich microalgae for energy purposes. The extracellular P deprivation caused significant starch accumulation also in marine green microalga Tetraselmis subcordioformis (Yao et al. 2013). It is known that carbohydrates start to accumulate when the intracellular P drops below a threshold limitation level (Cade-Menun and Paytan 2010).
Sulphur
Sulphur (S) is another essential macronutrient required for key cellular processes and normal development and growth of microalgae. This element participates in the synthesis of S-containing amino acids (cysteine, methionine) and thus proteins, sulpholipids, thiol compounds (glutathione), cell wall components, vitamins (thiamine, biotin), thioether and thioester compounds (coenzyme A), polysaccharides and electron transport carriers.
The S starvation results in the inhibition of cell division (Roopnarain et al. 2014; Yao et al. 2013). This may be a response to the S deficiency for synthesis of amino acids and proteins or other cell components required for growth, resulting further in accumulation of energy storage compounds, such as carbohydrates. Microalgae have low-storage capability for S, their growth and development is dependent upon a continuous supply of this element from the environment and thus its starvation affects the microalgae very fast (Zhang et al. 2004). On the other hand, cell recovery from S deficiency is also very quick (Arad et al. 1992; Ariño et al. 1995). During the first days of S starvation, carbohydrate accumulation occurs. This perhaps is because the carbohydrate formation acts as the only sink of PSII electrons and on the other hand the S starvation may induce the activity of the carboxylic acid cycle. Through the carboxylic acid cycle, the degraded products of ribulose-1,5-bisphosphate (RuBisCO) are recycled and thus leading to the accumulation of carbohydrates (Fouchard et al. 2005; Melis 2007). In cultures of C. reinhardtii, a 10-fold carbohydrate content increase was reported (Melis 2007). The starch overproduction under S limitation was extensively studied in freshwater species, e.g. C. vulgaris (Brányiková et al. 2011; Dragone et al. 2011), and also in marine species, e.g. Tetraselmis (Yao et al. 2012). Both species showed similar starch productivities under S deprivation. A detailed description of the response mechanism to S limitation is described by Takahashi et al. (2011)).
S starvation is, therefore, technologically the most feasible nutrient limitation strategy for producing biomass rich in carbohydrates because the interval between maximum production and cell death is the longest in comparison to other nutrients (nitrogen and phosphorus) and also because the S-limited cells reach the highest starch content, best viability and stable starch content. Consequently, the interval is sufficient for comfortable biomass harvesting, while in the case of N and P limitation, cell death occurred rapidly (Brányiková et al. 2011).
Specific inhibitors
Among environmental polysaccharide-enhancing factors, it can also be considered the application of specific inhibitors of DNA replication or proteosynthesis. The basic principle is the inhibition of some cell cycle processes which normally consume polysaccharides and thus cause their accumulation in cells. Chemical treatment allows direct control over the organism’s cellular machinery, but it is technologically demanding and leads to a number of difficulties (e.g. legislative). Examples of such inhibitors include specific inhibitors of DNA replication or proteosynthesis. Among the specific inhibitors of nuclear DNA synthesis, the fluorodeoxyuridine is the most frequently used. It inhibits the enzyme thymidylate synthase which catalyses the reductive methylation of 2′-deoxyuridylate to form deoxythymidylate (Bachmann et al. 1983; Cisneros et al. 1993). The inhibition of nuclear DNA synthesis prevents nuclear division and cytokinesis (Zachleder et al. 1996) and in synchronized culture, daughter cells remained mononuclear with their initial nuclear DNA content and they did not divide but continued to grow to giant size and continuously accumulated starch.
The antibiotic cycloheximide is a specific inhibitor of cytoplasmic proteosynthesis. The inhibition of proteosynthesis prevents microalgae from undergoing nuclear and cell division but starch is intensively synthesized implying that its synthesis in the chloroplast is independent of cytoplasmic protein synthesis and nuclear DNA replication (Brányiková et al. 2011). The application of specific inhibitors is advantageous for basic research because the mechanisms by which they affect cellular processes can be studied, but is unacceptable for large-scale cultivation because of costs and toxicity of the inhibitors as well as the use of biomass.
Biomass treatments
The carbohydrates in microalgae are mainly represented by glycogen, starch and cellulose. Starch and glycogen are important feedstock for the production of bioethanol from microalgae. The cellulose present in cell wall is also suitable as a source for bioethanol production (Wang et al. 2014) as these have different structure from lignocellulose of terrestrial plants. Especially the lack of lignin in microalgae results in less harsh pre-treatments for releasing the biodegradable organic matter. Lignin is not readily fermentable for ethanol production; thus, they need hydrolytic degradation to glucose (Bibi et al. 2017). The glucose from the treated biomass can be then fermented by yeast or bacteria to obtain bioethanol. Before the fermentation, the cells must be first disintegrated (physical, chemical or enzymatic treatment) to access the storage compounds (Table 1).
Physical treatment
Physical pre-treatments are commonly used to enhance cell disintegration and carbohydrate hydrolysis (Lee et al. 2017) (Table 1). Many different energy-demanding methods, including agitation, vigorous mixing, bead milling, high-pressure homogenisation, steam autoclaving, ultrasonication and microwave treatment supported with freezing, air-drying and grinding, have been tested with different microalgae for cell disintegration (Mulchandani et al. 2015). The efficiency is influenced by operational conditions such as intensity, processing time and biomass status (dried/wet and concentration) as well as volume. The final decision on appropriate cell disintegration methods depends mainly on the selected microalgae’s physiology, mainly, and cell wall characteristics.
Cell disruption can be achieved by shear stress created by a tangential force applied to the cell wall using for example vigorous mixing (vortexing). Bead milling is used to disrupt cells through abrasion caused by solid beads moving at high speed; although a generally effective method, it has relatively high energy requirement and a significant amount of heat is generated. This technique is well applicable for microalgae like Chlorella and other Chlorophyta with tough cellulosic cell wall.
Nannochloropsis cells were efficiently broken using high-pressure disintegrator as 98% cell disruption was achieved at a pressure of 1750 bar in wet environment (Taleb et al. 2016). High-pressure homogenisation is a simple technique to rupture microalgae cells containing tough cell walls which depends on the pressure and the cell-suspension properties. Agitation is a simple and low-energy-demand technique for disruption of filamentous species (Table 4). The cells of Arthrospira are susceptible to disruption by physical agitation because of their rather weak, non-cellulosic cell wall, likely to release glycogen into the medium upon stirring. Glucose was released into the medium after 24 h of α-amylase of glucoamylase treatment (Aikawa et al. 2013).
The incubation of Synechococcus sp. PCC 7002 with lysozyme at 37 °C had little effect on the cells. Freezing is an easy-to-use method for cell disruption; at − 20 °C for 1 h combined with subsequent lysozyme treatment, it resulted in proper cell disintegration, but it had no effect as a single treatment (Möllers et al. 2014; Skorupskaite et al. 2017) (Table 4). Besides freezing, air-drying and grinding can also facilitate the cell disruption (Bennamoun et al. 2015; Hossain et al. 2015; Poblete et al. 2018; Show et al. 2015) (Table 2). The wet biomass becomes more fermentable; thus, final ethanol content can be increased. Autoclaving is a harsher and more effective method for cell disruption compared to the previous mild techniques (Tables 2 and 3) as it generates high-pressure steam at 110–160 °C for about 15–60 min. It is suitable for both cell degradation and enhancing hydrolysis process (Hossain et al. 2015) as makes it possible to produce fermentable sugars without acid treatment. After autoclaving for 20–80 min of the red microalga Gelidium amansii, the recovery from biomass was 40–55% for solids, 16–33% for galactose and 82% for glucose compared to untreated biomass. Unlike acid treatment, glucose content was constant despite the increase in treatment time (Kim et al. 2015). The relative content of glucose also increased, while the galactose content decreased with the elongation of treatment time. Acid pre-treatment can inhibit the growth of microorganisms and cause environmental pollution (Lee et al. 2013). Ultrasonication represents high-frequency technique which is based on two major mechanisms that disrupt the cell (cavitation and shock-wave propagation). Shock-wave propagation forms jet streams in the surrounding medium, thereby causing cell disruption by high shear forces. Ultrasonic treatment was used in Chlorella cultures under various power (600–1,000 W) and various treatment time (30–90 min). Maximum glucose yield (about 37 g of 100 g DW) was obtained using 1,000 W for 80 min; then it started to decrease rapidly (Zhao et al. 2013). Microwave treatment (based on electromagnetic field with frequencies between 300 and 300 GHz) is a non-contact method that causes vibration and heating of biomass. This technique has short processing time, high disruption efficiency but relatively high energy consumption. Microwave treatment can produce unstable bonds in the carbon-chain structures thereby altering the quality of the products. Microwave-assisted hydrothermal extraction was applied for production of sulphated polysaccharides from Ulva spp. and Monostroma latissimum (Tsubaki et al. 2016).
Chemical treatment
By chemical (acid and alkaline) pre-treatments, cell wall disintegration and carbohydrate hydrolysis can be achieved in one step (Table 1). The great advantage of acid hydrolysis is its rapidness, easiness and lower costs compared to other hydrolysis technics. On the other hand, the acidic environment may lead to decomposition of sugars into unwanted compounds that inhibit the fermentation process. Besides, high acid concentrations can inhibit the fermentation step because of salt formation after neutralization of the mixture. Table 2 shows the overview of procedures of acidic/thermal hydrolysis which were used for treatment of microalgae biomass before fermentation to bioethanol. For the hydrolysis of the carbohydrate-enriched Arthrospira biomass, various acids (H2SO4, HNO3, HCl and H3PO4) were used at concentrations from 0.083 to 2.5 mol/L carried out in the temperature range from 40 to 100 °C (Markou et al. 2013). The highest bioethanol yields of 16% (g ethanol per g DW) were obtained in hydrolysates produced by treatment with 0.5 mol/L HNO3 and 0.25 mol/L H2SO4 at 100 °C, respectively. The hydrolysates were fermented by Saccharomyces cerevisiae (further as S. cerevisiae) adapted to salt stress. In another series of experiments, process standardization of bioethanol production from Arthrospira sp. was performed. Various biomass-drying intervals, acid concentrations, time for hydrolysis and fermentation period were tested (Hossain et al. 2015). The ethanol concentration of 1 g/L was achieved from air-dried Arthrospira biomass treated with 2% sulphuric acid at 125 °C. As an example, C. reinhardtii biomass was used for ethanol production by low-cost treatment using hydrothermal acid pre-treatment with high efficiency (Nguyen et al. 2009). Biomass (5% w/v) were pre-treated with sulphuric acid (1–5%) under temperatures of 100–120 °C for 15–120 min. As a result, the glucose yield from the biomass was 58% (w/w) after pre-treatment with 3% sulphuric acid at 110 °C for 30 min. The method of separate hydrolysis and fermentation (SHF) by S. cerevisiae was used resulting in bioethanol yield of 14.6 g/L in 24 h. In another study, biomass of Chlorella sp. was hydrolysed to sugars in the presence of HCl (0.5–6%), H2SO2 (2%) and MgCl2 (1.25, 1.75, 2.5 and 3.75%) in two hydrolysis regimes (180 °C/10 min and 120 °C/60 min) (Zhou et al. 2011). The mixture of MgCl2 (2.5%) and HCl (2%) enhanced the effectiveness of hydrolysis (180 °C/10 min) up to 83% sugar yield resulting in 23 g/L ethanol after 48-h fermentation which was about 90% of the theoretical yield. Acidic treatments with sulphuric acid at various concentrations (0.1–5%) followed by enzymatic treatments with Pseudomonas sp. CL3 extracts (enzyme mixture consisted of endoglucanase, β-glucosidase and amylases) were tested to optimize hydrolysis process. Fermentation of acid-hydrolysed microalgae biomass (1% sulphuric acid at 121 °C for 20 min) by Z. mobilis resulted in the maximum ethanol concentration of about 12 g/L with about 88% efficiency in 12 h. In another trial, Scenedesmus obliquus was cultivated for carbohydrates in outdoor tubular PBRs all year round (August 2012–July 2013) to demonstrate the feasibility of outdoor cultivation for bioethanol production in Taiwan (Ho et al. 2017). Seasonal changes of the carbohydrate content under nitrogen-deficient conditions were also monitored to follow cell growth, CO2 fixation and carbohydrate production. The Scenedesmus culture could accumulate nearly 45–50% of carbohydrates, mainly composed of glucose (70–80% of total carbohydrate content) which makes it appropriate for use as a feedstock for bioethanol fermentation. Fresh biomass (40 g/L) was hydrolysed with 2% sulphuric acid leading to an initial glucose concentration of about 15–18 g/L. Maximum bioethanol concentration of about 8 g/L was achieved within 4 h by using Z. mobilis for separated hydrolysis and fermentation process. Mixed microalgae culture was also chosen for bioethanol production (Shokrkar et al. 2017). Dried biomass was mixed separately with H2SO4, HCl and H3PO3 (0.5, 1 and 2 mol/L, respectively) and the resulting slurries were finally autoclaved. The highest ethanol concentration, about 5 g/L ethanol concentration, was obtained using 0.5 mol/L H2SO4 and 2.5% MgSO4 at 121 °C for 40 min as pre-treatment method and fermentation with S. cerevisiae for 24 h.
Based on experimental results mentioned above, chemical treatment is a well-applicable method in practice. Best results were obtained using H2SO4, HNO3 and HCl acids in 1–3% concentration, at 100–180 °C for 10 min to 3 h. Treatment time and temperature as well as acid concentration are strongly related to each other. The main costs of the method are acid treatment together with neutralization and heating. Considering these aspects, application of longer treatment time and lower acid concentration at lower temperature can be the most feasible technical setup for acid treatment if time is not a crucial point.
For alkaline-based pre-treatment, mostly sodium hydroxide is used (Table 1). This process forms “pores” in the cell wall, thus releasing carbohydrate compounds from the cell. Besides, it decreases the size of the starch polymers. Alkaline hydrolysis cleaves intermolecular linkages between complex polysaccharides, and liberates carbohydrate fibres and other polymeric components to the medium, but does not break down complex carbohydrates into simple sugars. Alkaline (NaOH) pre-treatment method was used to cleave biomass of Chlorococcum infusionum (further as C. infusionum) for bioethanol production (Harun et al. 2011) (Table 3). Three variables were examined: the concentration of NaOH (0.5–3%), pre-treatment temperature (60–140 °C) and time period (15–60 min). The highest bioethanol yield obtained was 26% (w/w; g ethanol per g biomass) resulting from biomass pre-treatment with 0.75% NaOH at 120 °C for 30 min. Hernández et al. (2015)) tested Chlorella sorokiniana (further as C. sorokiniana), Scenedesmus almeriensis (further as S. almeriensis) and Nannochloropsis gaditana (further as N. gaditana) to produce bioethanol due to their ability to grow in wastewater containing high organic matter as well as in high-salt media. Sodium hydroxide (1 and 5 mol/L) was used for chemical treatment and biomass was incubated at 90 °C for 30 min. When treated with 1 mol/L NaOH, the sugar release was lower than 4 mg/g DW in all microalgae samples, while it raised 2–5 times when 5 mol/L NaOH was applied. Mixed cultures of microalgae were also chosen for bioethanol production via alkaline pre-treatment (Shokrkar et al. 2017). Microalgae biomass (50 g/L) was treated by NaOH and autoclaved at 121 °C for 10–40 min. The maximum yield of reducing sugar and glucose reached 76–80% of the theoretical amounts when 2 mol/L NaOH was used for treatment.
As the final conclusion, the hydrolysis by diluted acid is even more efficient compared to strong alkaline addition. Alkaline hydrolysis cleaves only intermolecular linkages between polysaccharide units and results in polymeric components while acid hydrolysis can degrade all complex polysaccharides (starch, glycogen, long cellulose and hemicellulose chains) into short oligomers or monomers (glucose and other monomers) thus increasing sugar yield and ethanol production.
Enzymatic treatment
Enzymatic hydrolysis is an environmentally less harmful, but costly process (compared to chemical treatment) that gives remarkably high glucose yields without producing inhibitory side products (Tables 1 and 4). On the other hand, efficiency is influenced by several factors like temperature, pH and enzyme concentration that has to be optimized. For example, marine cyanobacterium Synechococcus sp. PCC 7002 was used as a carbohydrate-rich feedstock for bioethanol production when enhanced accumulation was induced by nitrate limitation (Möllers et al. 2014). The highest carbohydrate content per dry mass was about 60% (w/w). An efficient release of carbohydrates from cells was facilitated by lysozyme (an antimicrobial enzyme, a glycoside hydrolase catalysing the hydrolysis of 1,4-beta-linkages) that cleaves cell wall containing peptidoglycan. Cell wall disruption process was promoted when freshly harvested biomass was frozen at − 20 °C for 1 h before lysozyme treatment at 37 °C. Freezing itself without lysozyme addition did not change either the cell morphology or cause lysis of cells; it just increased the susceptibility to lysozyme. Intracellular carbohydrates were cleaved using the combined treatment of lysozyme and two α-glucanases and then the hydrolysed mixture was fermented using S. cerevisiae. About 90% of the glucose in the biomass was converted to ethanol when the highest ethanol yield was 0.27 g/g DW.
Application of strains producing fermentative enzymes instead of adding isolated and costly enzymes can reduce bioethanol production costs. Direct ethanol production from A. platensis culture was examined without pre-treatment or enzymatic hydrolytic processes when it was grown under N limitation (Aikawa et al. 2013). The cultivation resulted in 60% glycogen per dry cell mass, about 3-fold more than in the nitrate replete culture. Then, direct conversion of biomass produced 6.5 g/L ethanol after agitation at 30 °C in the presence of lysozyme and α-amylase. The yeast S. cerevisiae was used in which α-amylase from Streptococcus bovis and glucoamylase from Rhizopus oryzae was expressed. Direct ethanol production was further enhanced by the addition of CaCl2 to the cyanobacterial biomass which increased the permeability of the polysaccharide layer surrounding the cells and efficiency of glycogen extraction (Aikawa et al. 2018). The combined application of CaCl2 and lysozyme with the α-amylase on Arthrospira biomass resulted in 48 g/L ethanol (1 g/L per h) reaching the 93% of the theoretical yield.
Mixed culture of microalgae was enzymatically treated by β-glucosidase/cellulase from Talaromyces emersonii (further as T. emersonii), thermostable α-amylase from Bacillus licheniformis (further as B. licheniformis) and amyloglucosidase from Aspergillus niger (further as A. niger), respectively (Shokrkar et al. 2017). Enzymatic treatment by these three enzymes resulted in 97% of reducing sugar yield which was finally converted to 6.4 g/L bioethanol by S. cerevisiae.
An addition of enzymes (lysozyme, cellulase, β-glucosidase, α-amylase and amyloglucosidase) to microalgae biomass can increase the cost of the process, although production price can be lowered by the application of enzyme-producing mutants. Besides, bioethanol yield after enzymatic hydrolysis of microalgae is higher than that of acid hydrolysis (Shokrkar et al. 2017). As a conclusion, enzymatic hydrolysis would be promising, due to higher sugar and bioethanol yields, less corrosion problems, lower utility consumption and more environmentally friendly treatment compared to acid hydrolysis.
Fermentation
Monosaccharides like glucose, xylose, mannose, galactose and arabinose are present in the hydrolysed microalgae biomass used for further fermentation (Hernández et al. 2015). The most abundant sugars are glucose which is derived from the hydrolysis of glycogen, starch or cellulose (Ho et al. 2017; Möllers et al. 2014). The most frequently used microorganisms for bioethanol production from hexoses are the yeast S. cerevisiae and the bacterium Z. mobilis. S. cerevisiae is one of the microorganisms used since ancient times in biotechnology for production of alcoholic beverages with remarkable efficiency in the conversion of sugars (mainly glucose) into ethanol and high tolerance to it. Besides, Saccharomyces is a generally recognized safe (GRAS) microorganism. When growing, it produces flocs in the fermentation media which makes it easy to settle down and separate. Another GRAS microorganism Zymomonas, a gram-negative bacterium used for bioethanol production from starch and glycogen, was also studied in detail (Ajit et al. 2017). Compared to S. cerevisiae, it has higher tolerance to alcohol, higher glucose uptake and higher bioethanol yield (Yang et al. 2013). Various strategies of chemical or enzymatic hydrolysis followed by fermentation are used: separate hydrolysis and fermentation (SHF), simultaneous saccharification and fermentation (SSF) or consolidated bioprocess (CBP) (Rastogi and Shrivastava 2017). In SHF procedure, pre-treated microalgae biomass is hydrolysed to glucose and subsequently fermented to bioethanol in separate units. SSF is a single-step process that combines the saccharification and fermentation processes. Starch or glycogen are hydrolysed to monosaccharides which are further fermented to bioethanol by S. cerevisiae or Z. mobilis. Hydrolysing enzymes as α-amylase and amyloglucosidase are added to the same processing unit (fermenter) with the fermenting microorganisms. The major benefits of SHF are the low cost of chemicals, short residence time and simple technology which allows its large-scale application. The SSF process is a more efficient technique compared to SHF because it reduces the number of necessary operations and higher bioethanol yields can be obtained. The CBP procedure combines enzyme production, saccharification and fermentation in a single step, but it is still in its early stage of establishment (Aikawa et al. 2018, 2013; Hasunuma and Kondo 2012). Costs of capital investment, substances and other raw materials associated with enzyme production can be avoided (Salehi Jouzani and Taherzadeh 2015; Yamada et al. 2010).
Bioethanol in a fermentation broth is usually separated by distillation followed by dehydration through a molecular sieve. From an industrial point of view, high ethanol concentration is also crucial as it lowers energy consumption during distillation and thereby costs of the whole process. Logically, separation costs can be decreased if ethanol concentration is increased. When its concentration is lower than 4% (w/w), regular distillation is not economical (Huang and Zhang 2011). In the bioethanol industry, final ethanol concentrations exceeding 100 g/L are common (Manochio et al. 2017). When bioethanol concentration is 12% (about 95 g/L), distillation energy equals only about 13% of its combustion energy.
Conclusions
Bioethanol production from the third-generation biomass feedstock—microalgae—has not yet been fully developed. Many attempts have been tested to create an economically feasible bioethanol production in which operating costs would be close to that from the first- and second-generation feedstock. Yet, dark fermentation and “photo-fermentation” techniques cannot reach the necessary requirements for industrial bioethanol production, even at laboratory or pilot scale. Nevertheless, significant advancement has been achieved in the field of microalgae biomass fermentation in terms of increasing production efficiency and lowered processing costs at laboratory scale. Carbohydrate content of 50–60% was achieved in various microalgae applying stress conditions which can provide sufficient amount of raw material for fermentation after the hydrolysis step (Aikawa et al. 2013). Production costs were decreased by the cultivation of filamentous strains like Arthrospira sp. that requires less energy-demanding screen filtration for the step of biomass concentration (Markou et al. 2012b). Besides, the fragile structure of filamentous cyanobacteria also allows to apply mild physical disintegration of the fragile cell wall thus further decreasing the processing costs (Märkl et al. 1991). Hydrolysis efficiency can be further improved by applying enzymes (although at higher costs) instead of chemicals. Unique hydrolysis step, like enzymatic hydrolysis without the need for drying and chemical pre-treatment, is eligible and thus pre-treatment costs can be declined. In general, the fermentation of glucose after enzymatic hydrolysis exhibits higher bioethanol yield than that of acid hydrolysis of microalgae (Shokrkar et al. 2017). Economic feasibility of enzyme application can be further improved by the utilization of genetically modified microorganisms which can evolve hydrolysing enzymes during fermentation (Aikawa et al. 2018).
The cost of enzymes is still a significant technological barrier. Further improvement of the direct bioethanol conversion process from cyanobacterial biomass could be obtained by the construction of a recombinant yeast that also expresses lysozyme. The other major bottleneck for an industrial implementation is the cost of microalgae biomass. Culturing of selected fast-growing microalgae producing polysaccharides on wastewaters can further reduce costs as well as culturing at large scale (Hena et al. 2018). Procedures using microalgae remediation are not just cost-effective but also environmentally sustainable because they do not generate additional waste such as sludge, but do provide opportunities for efficient nutrient recycling and the sustainable production of microalgae biofuels (Tsolcha et al. 2017, 2018). In biorefinery approach, the residual organic matter and minerals could be used as biofertilizer after bioethanol stripping (Wuang et al. 2016).
Notes
According to applied phycology, these represent microscopic, unicellular, filamentous or colonial prokaryotic cyanobacteria and eukaryotic algae that are capable of converting inorganic nutrients, water, carbon dioxide and light energy (sunlight) into biomass via photosynthesis.
Abbreviations
- Acetyl-CoA:
-
Acetyl coenzyme A
- ADHI, ADHII:
-
Alcohol dehydrogenase I and II
- ATP:
-
Adenosine triphosphate
- CBP:
-
Consolidated bioprocess
- CCM:
-
Carbon dioxide concentrating mechanism
- DW:
-
Dry mass
- GRAS:
-
Generally recognized as safe
- PBRs:
-
Photobioreactors
- PDC:
-
Pyruvate decarboxylase
- PHB:
-
Poly(3-hydroxybutyrate)
- PSI, II:
-
Photosystem I, II
- RuBisCO:
-
Ribulose-1,5-bisphosphate
- SHF:
-
Separate hydrolysis and fermentation
- SSF:
-
Simultaneous saccharification and fermentation
- NaHCO3 :
-
Sodium bicarbonate
References
Abreu AP, Fernandes B, Vicente AA, Teixeira J, Dragone G (2012) Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour Technol 118:61–66
Aikawa S, Inokuma K, Wakai S, Sasaki K, Ogino C, Chang JS, Hasunuma T, Kondo A (2018) Direct and highly productive conversion of cyanobacteria Arthrospira platensis to ethanol with CaCl2 addition. Biotechnol Biofuels 11:50
Aikawa S, Izumi Y, Matsuda F, Hasunuma T, Chang J-S, Kondo A (2012) Synergistic enhancement of glycogen production in Arthrospira platensis by optimization of light intensity and nitrate supply. Bioresour Technol 108:211–215
Aikawa S et al (2013) Direct conversion of Spirulina to ethanol without pretreatment or enzymatic hydrolysis processes. Energy Environ Sci 6:1844–1849
Ajit A, Sulaiman AZ, Chisti Y (2017) Production of bioethanol by Zymomonas mobilis in high-gravity extractive fermentations. Food Bioprod Process 102:123–135
Arad SM, Lerental YB, Dubinsky O (1992) Effect of nitrate and sulfate starvation on polysaccharide formation in Rhodella reticulata. Bioresour Technol 42:141–148
Arias DM, Uggetti E, García-Galán MJ, García J (2018) Production of polyhydroxybutyrates and carbohydrates in a mixed cyanobacterial culture: effect of nutrients limitation and photoperiods. New Biotechnol
Ariño X, Ortega-Calvo J-J, Hernandez-Marine M, Saiz-Jimenez C (1995) Effect of sulfur starvation on the morphology and ultrastructure of the cyanobacterium Gloeothece sp. PCC 6909. Arch Microbiol 163:447–453
Bachmann B, Hofmann R, Follmann H (1983) Tight coordination of ribonucleotide reduction and thymidylate synthesis in synchronous algae. FEBS Lett 152:247–250
Behera S, Singh R, Arora R, Sharma NK, Shukla M, Kumar S (2015) Scope of algae as third generation biofuels. Front Bioeng Biotechnol 2:90
Bellou S, Aggelis G (2013) Biochemical activities in Chlorella sp. and Nannochloropsis salina during lipid and sugar synthesis in a lab-scale open pond simulating reactor. J Biotechnol 164:318–329
Bellou S, Baeshen MN, Elazzazy AM, Aggeli D, Sayegh F, Aggelis G (2014) Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol Adv 32:1476–1493
Ben-Amotz A (1975) Adaptation of the unicellular alga Dunaliella parva to a saline environment. J Phycol 11:50–54
Benavides AMS, Ranglová K, Malapascua JR, Masojídek J, Torzillo G (2017) Diurnal changes of photosynthesis and growth of Arthrospira platensis cultured in a thin-layer cascade and an open pond. Algal Res 28:48–56
Bennamoun L, Afzal MT, Léonard A (2015) Drying of alga as a source of bioenergy feedstock and food supplement–a review. Renew Sust Energ Rev 50:1203–1212
Berges JA, Charlebois DO, Mauzerall DC, Falkowski PG (1996) Differential effects of nitrogen limitation on photosynthetic efficiency of photosystems I and II in microalgae. Plant Physiol 110:689–696
Bharathiraja B et al (2015) Aquatic biomass (algae) as a future feed stock for bio-refineries: a review on cultivation, processing and products. Renew Sust Energ Rev 47:634–653
Bibi R, Ahmad Z, Imran M, Hussain S, Ditta A, Mahmood S, Khalid A (2017) Algal bioethanol production technology: a trend towards sustainable development. Renew Sust Energ Rev 71:976–985
Boboescu IZ, Gherman VD, Lakatos G, Pap B, Bíró T, Maroti G (2016) Surpassing the current limitations of biohydrogen production systems: the case for a novel hybrid approach. Bioresour Technol 204:192–201
Bracken ME et al (2015) Signatures of nutrient limitation and co-limitation: responses of autotroph internal nutrient concentrations to nitrogen and phosphorus additions. Oikos, 124:113–121
Brányiková I, Maršálková B, Doucha J, Brányik T, Bišová K, Zachleder V, Vítová M (2011) Microalgae - novel highly efficient starch producers. Biotechnol Bioeng 108:766–776
Brennan L, Owende P (2010a) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577
Brennan L, Owende P (2010b) Biofuels from microalgae - a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577
Brown M, Jeffrey S, Volkman J, Dunstan G (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331
Buléon A, Colonna P, Planchot V, Ball S (1998) Starch granules: structure and biosynthesis. Int J Biol Macromol 23:85–112
Busi MV, Barchiesi J, Martín M, Gomez-Casati DF (2014) Starch metabolism in green algae. Starch-Stärke 66:28–40
Cade-Menun BJ, Paytan A (2010) Nutrient temperature and light stress alter phosphorus and carbon forms in culture-grown algae. Mar Chem 121:27–36
Caporgno M, Taleb A, Olkiewicz M, Font J, Pruvost J, Legrand J, Bengoa C (2015) Microalgae cultivation in urban wastewater: nutrient removal and biomass production for biodiesel and methane. Algal Res 10:232–239
Carvalho AP, Monteiro CM, Malcata FX (2009) Simultaneous effect of irradiance and temperature on biochemical composition of the microalga Pavlova lutheri. J Appl Phycol 21:543–552
Catalanotti C, Yang W, Posewitz MC, Grossman AR (2013) Fermentation metabolism and its evolution in algae. Front Plant Sci 4:150
Champigny M (1985) Regulation of photosynthetic carbon assimilation at the cellular level: a review. Photosynth Res 6:273–286
Chen C-Y et al (2013) Microalgae-based carbohydrates for biofuel production. Biochem Eng J 78:1–10
Cheng D, He Q (2014) Assessment of environmental stresses for enhanced microalgal biofuel production–an overview. Front Energy Res 2:26
Chew KW, Yap JY, Show PL, Suan NH, Juan JC, Ling TC, Lee DJ, Chang JS (2017) Microalgae biorefinery: high value products perspectives. Bioresour Technol 229:53–62
Choi W et al (2012) Bioethanol production from Ulva pertusa Kjellman by high-temperature liquefaction. Chem Biochem Eng Q 26:15–21
Choix FJ, de Bashan LE, Bashan Y (2012) Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: II. Heterotrophic conditions. Enzym Microb Technol 51:300–309
Chojnacka K, Marquez-Rocha F-J (2004) Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 3:21–34
Cisneros RJ, Zapf JW, Dunlap RB (1993) Studies of 5-fluorodeoxyuridine 5′-monophosphate binding to carboxypeptidase A-inactivated thymidylate synthase from Lactobacillus casei. J Biol Chem 268:10102–10108
D'Souza FM, Kelly GJ (2000) Effects of a diet of a nitrogen-limited alga (Tetraselmis suecica) on growth, survival and biochemical composition of tiger prawn (Penaeus semisulcatus) larvae. Aquaculture 181:311–329
de Castro AS, Garcia VMT (2005) Growth and biochemical composition of the diatom Chaetoceros cf. wighamii brightwell under different temperature, salinity and carbon dioxide levels. I. Protein, carbohydrates and lipids. Aquaculture 246:405–412
de Farias Silva CE, Bertucco A (2016) Bioethanol from microalgae and cyanobacteria: a review and technological outlook. Process Biochem 51:1833–1842
de Farias Silva CE, Sforza E, Bertucco A (2018) Stability of carbohydrate production in continuous microalgal cultivation under nitrogen limitation: effect of irradiation regime and intensity on Tetradesmus obliquus. J Appl Phycol 30:261–270
De Oliveira M, Monteiro M, Robbs P, Leite S (1999) Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquac Int 7:261–275
De Philippis R, Sili C, Vincenzini M (1992) Glycogen and poly-β-hydroxybutyrate synthesis in Spirulina maxima. Microbiology 138:1623–1628
De Porcellinis A, Frigaard N-U, Sakuragi Y (2017) Determination of the glycogen content in cyanobacteria. J Vis Exp:e56068
Dean AP, Estrada B, Nicholson JM, Sigee DC (2008a) Molecular response of Anabaena flos-aquae to differing concentrations of phosphorus: a combined Fourier transform infrared and X-ray microanalytical study. Phycol Res 56:193–201
Dean AP, Nicholson JM, Sigee DC (2008b) Impact of phosphorus quota and growth phase on carbon allocation in Chlamydomonas reinhardtii: an FTIR microspectroscopy study. Eur J Phycol 43:345–354
Deng M-D, Coleman JR (1999) Ethanol synthesis by genetic engineering in cyanobacteria. Appl Environ Microbiol 65:523–528
Dexter J, Armshaw P, Sheahan C, Pembroke J (2015) The state of autotrophic ethanol production in cyanobacteria. J Appl Microbiol 119:11–24
Dexter J, Fu P (2009) Metabolic engineering of cyanobacteria for ethanol production. Energy Environ Sci 2:857–864
Dienst D et al (2014) Transcriptomic response to prolonged ethanol production in the cyanobacterium Synechocystis sp. PCC6803. Biotechnol Biofuels 7:21
Dourou M, Tsolcha ON, Tekerlekopoulou AG, Bokas D, Aggelis G (2018) Fish farm effluents are suitable growth media for Nannochloropsis gaditana, a polyunsaturated fatty acid producing microalga. Eng Life Sci 18:851–860
Dragone G, Fernandes BD, Abreu AP, Vicente AA, Teixeira JA (2011) Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl Energy 88:3331–3335
Dragone G, Fernandes BD, Vicente AA, Teixeira JA (2010) Third generation biofuels from microalgae, Current research, technology and education topics in applied microbiology and microbial biotechnology. 2:1355–1366
Eriksen NT, Riisgård FK, Gunther WS, Iversen JJL (2007) On-line estimation of O2 production, CO2 uptake, and growth kinetics of microalgal cultures in a gas-tight photobioreactor. J Appl Phycol 19:161
Eshaq FS, Ali MN, Mohd MK (2011) Production of bioethanol from next generation feed-stock alga Spirogyra species. Int J Eng Sci Technol 3:1749–1755
Fernandes BD, Dragone GM, Teixeira JA, Vicente AA (2010) Light regime characterization in an airlift photobioreactor for production of microalgae with high starch content. Appl Biochem Biotechnol 161:218–226
Figueroa-Torres GM, Pittman JK, Theodoropoulos C (2017) Kinetic modelling of starch and lipid formation during mixotrophic, nutrient-limited microalgal growth. Bioresour Technol 241:868–878
Fouchard S et al (2005) Autotrophic and mixotrophic hydrogen photoproduction in sulfur-deprived Chlamydomonas cells. Appl Environ Microbiol 71:6199–6205
Friedman O, Dubinsky Z, Arad SM (1991) Effect of light intensity on growth and polysaccharide production in red and blue-green rhodophyta unicells. Bioresour Technol 38:105–110
Gaffron H, Rubin J (1942) Fermentative and photochemical production of hydrogen in algae. J Gen Physiol 26:219–240
Gao Z, Zhao H, Li Z, Tan X, Lu X (2012) Photosynthetic production of ethanol from carbon dioxide in genetically engineered cyanobacteria. Energy Environ Sci 5:9857–9865
Geider RJ, La Roche J (2002) Redfield revisited: variability of C/N/P in marine microalgae and its biochemical basis. Eur J Phycol 37:1–17
Giordano M (2001) Interactions between C and N metabolism in Dunaliella salina cells cultured at elevated CO2 and high N concentrations. J Plant Physiol 158:577–581
Giordano M, Bowes G (1997) Gas exchange and C allocation in Dunaliella salina cells in response to the N source and CO2 concentration used for growth. Plant Physiol 115:1049–1056
Grossman AR, Croft M, Gladyshev VN, Merchant SS, Posewitz MC, Prochnik S, Spalding MH (2007) Novel metabolism in Chlamydomonas through the lens of genomics. Curr Opin Plant Biol 10:190–198
Guerrini F, Cangini M, Boni L, Trost P, Pistocchi R (2000) Metabolic responses of the diatom Achnanthes brevipes (Bacillariophyceae) to nutrient limitation. J Phycol 36:882–890
Gupta PL, Lee S-M, Choi H-J (2015) A mini review: photobioreactors for large scale algal cultivation. World J Microbiol Biotechnol 31:1409–1417
Harun R, Jason W, Cherrington T, Danquah MK (2011) Exploring alkaline pre-treatment of microalgal biomass for bioethanol production. Appl Energy 88:3464–3467
Hasunuma T, Kondo A (2012) Consolidated bioprocessing and simultaneous saccharification and fermentation of lignocellulose to ethanol with thermotolerant yeast strains. Process Biochem 47:1287–1294
Hemschemeier A, Happe T (2005) The exceptional photofermentative hydrogen metabolism of the green alga Chlamydomonas reinhardtii. Portland Press Limited
Hena S, Znad H, Heong K, Judd S (2018) Dairy farm wastewater treatment and lipid accumulation by Arthrospira platensis. Water Res 128:267–277
Hernández D, Riaño B, Coca M, García-González M (2015) Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. Chem Eng J 262:939–945
Heyer H, Krumbein WE (1991) Excretion of fermentation products in dark and anaerobically incubated cyanobacteria. Arch Microbiol 155:284–287
Hirano A, Ueda R, Hirayama S, Ogushi Y (1997) CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy 22:137–142
Ho S-H et al (2017) Feasibility of CO2 mitigation and carbohydrate production by microalga Scenedesmus obliquus CNW-N used for bioethanol fermentation under outdoor conditions: effects of seasonal changes. Biotechnol Biofuels 10:27
Ho S-H, Huang S-W, Chen C-Y, Hasunuma T, Kondo A, Chang J-S (2013) Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour Technol 135:191–198
Hosono H et al (1994) Effect of culture temperature shift on the cellular sugar accumulation of Chlorella vulgaris SO-26. J Ferment Bioeng 78:235–240
Hossain MNB, Basu JK, Mamun M (2015) The production of ethanol from micro-algae Spirulina. Procedia Eng 105:733–738
Hu Q (2004) Environmental effects on cell composition vol 1. Blackwell Science Ltd., Oxford
Huang W-D, Zhang Y-HP (2011) Analysis of biofuels production from sugar based on three criteria: thermodynamics, bioenergetics, and product separation. Energy Environ Sci 4:784–792
Huo Y-X, Cho KM, Rivera JGL, Monte E, Shen CR, Yan Y, Liao JC (2011) Conversion of proteins into biofuels by engineering nitrogen flux. Nat Biotechnol 29:346
Ingram L, Conway T, Clark D, Sewell G, Preston J (1987) Genetic engineering of ethanol production in Escherichia coli. Appl Environ Microbiol 53:2420–2425
Izumo A, Fujiwara S, Oyama Y, Satoh A, Fujita N, Nakamura Y, Tsuzuki M (2007) Physicochemical properties of starch in Chlorella change depending on the CO2 concentration during growth: comparison of structure and properties of pyrenoid and stroma starch. Plant Sci 172:1138–1147
Jensen S, Knutsen G (1993) Influence of light and temperature on photoinhibition of photosynthesis in Spirulina platensis. J Appl Phycol 5:495–504
Jerez CG, Malapascua JR, Sergejevová M, Figueroa FL, Masojídek J (2016) Effect of nutrient starvation under high irradiance on lipid and starch accumulation in Chlorella fusca (Chlorophyta). Mar Biotechnol 18:24–36
Ji X, Cheng J, Gong D, Zhao X, Qi Y, Su Y, Ma W (2018) The effect of NaCl stress on photosynthetic efficiency and lipid production in freshwater microalga—Scenedesmus obliquus XJ002. Sci Total Environ 633:593–599
John RP, Anisha G, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour Technol 102:186–193
Jones CS, Mayfield SP (2012) Algae biofuels: versatility for the future of bioenergy. Curr Opin Biotechnol 23:346–351
Juneja A, Ceballos RM, Murthy GS (2013) Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies 6:4607–4638
Kadouche D et al (2016) Characterization of function of the GlgA2 glycogen/starch synthase in Cyanobacterium sp. Clg1 highlights convergent evolution of glycogen metabolism into starch granule aggregation. Plant Physiol:00049.02016
Kämäräinen J, Knoop H, Stanford NJ, Guerrero F, Akhtar MK, Aro EM, Steuer R, Jones PR (2012) Physiological tolerance and stoichiometric potential of cyanobacteria for hydrocarbon fuel production. J Biotechnol 162:67–74
Khalil ZI, Asker MM, El-Sayed S, Kobbia IA (2010) Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea. World J Microbiol Biotechnol 26:1225–1231
Kim HM, Wi SG, Jung S, Song Y, Bae H-J (2015) Efficient approach for bioethanol production from red seaweed Gelidium amansii. Bioresour Technol 175:128–134
Klein U, Betz A (1978) Fermentative metabolism of hydrogen-evolving Chlamydomonas moewusii. Plant Physiol 61:953–956
Kopka J et al (2017) Systems analysis of ethanol production in the genetically engineered cyanobacterium Synechococcus sp. PCC 7002. Biotechnol Biofuels 10:56
Kumar D, Murthy GS (2011) Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol Biofuels 4:27
Lakatos G, Balogh D, Farkas A, Ördög V, Nagy PT, Bíró T, Maróti G (2017) Factors influencing algal photobiohydrogen production in algal-bacterial co-cultures. Algal Res 28:161–171
Lakatos G, Deák Z, Vass I, Rétfalvi T, Rozgonyi S, Rákhely G, Ördög V, Kondorosi É, Maróti G (2014) Bacterial symbionts enhance photo-fermentative hydrogen evolution of Chlamydomonas algae. Green Chem 16:4716–4727
Lee H-J, Lim W-S, Lee J-W (2013) Improvement of ethanol fermentation from lignocellulosic hydrolysates by the removal of inhibitors. J Ind Eng Chem 19:2010–2015
Lee SY, Cho JM, Chang YK, Oh Y-K (2017) Cell disruption and lipid extraction for microalgal biorefineries: a review. Bioresour Technol
Li Y, Han D, Hu G, Sommerfeld M, Hu Q (2010) Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnol Bioeng 107:258–268
Lynn SG, Kilham SS, Kreeger DA, Interlandi SJ (2000) Effect of nutrient availability on the biochemical and elemental stoichiometry in the freshwater diatom Stephanodiscus minutulus (Bacillariophyceae). J Phycol 36:510–522
Magneschi L, Catalanotti C, Subramanian V, Dubini A, Yang W, Mus F, Posewitz MC, Seibert M, Perata P, Grossman AR (2012) A mutant in the ADH1 gene of Chlamydomonas reinhardtii elicits metabolic restructuring during anaerobiosis. Plant Physiol 158:1293–1305
Malibari R, Sayegh F, Elazzazy AM, Baeshen MN, Dourou M, Aggelis G (2018) Reuse of shrimp farm wastewater as growth medium for marine microalgae isolated from Red Sea–Jeddah. J Clean Prod 198:160–169
Manochio C, Andrade B, Rodriguez R, Moraes B (2017) Ethanol from biomass: a comparative overview. Renew Sust Energ Rev 80:743–755
Märkl H, Bronnenmeier R, Wittek B (1991) The resistance of microorganisms to hydrodynamic stress. Int Chem Eng 31:185–196
Markou G, Angelidaki I, Georgakakis D (2012a) Microalgal carbohydrates: an overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl Microbiol Biotechnol 96:631–645
Markou G, Angelidaki I, Nerantzis E, Georgakakis D (2013) Bioethanol production by carbohydrate-enriched biomass of Arthrospira (Spirulina) platensis. Energies 6:3937–3950
Markou G, Chatzipavlidis I, Georgakakis D (2012b) Carbohydrates production and bio-flocculation characteristics in cultures of Arthrospira (Spirulina) platensis: improvements through phosphorus limitation process. BioEnergy Res 5:915–925
Matsubara K (2004) Recent advances in marine algal anticoagulants. Curr Med Chem Cardiovasc Hematol Agents 2:13–19
Melendez-Hevia E, Waddell T, Shelton E (1993) Optimization of molecular design in the evolution of metabolism: the glycogen molecule. Biochem J 295:477–483
Melis A (2007) Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae). Planta 226:1075–1086
Möllers KB, Cannella D, Jørgensen H, Frigaard N-U (2014) Cyanobacterial biomass as carbohydrate and nutrient feedstock for bioethanol production by yeast fermentation. Biotechnol Biofuels 7:64
Mühlroth A et al (2013) Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of Chromista. Mar Drugs 11:4662–4697
Mulchandani K, Kar JR, Singhal RS (2015) Extraction of lipids from Chlorella saccharophila using high-pressure homogenization followed by three phase partitioning. Appl Biochem Biotechnol 176(6):1613–1626
Mus F, Dubini A, Seibert M, Posewitz MC, Grossman AR (2007) Anaerobic acclimation in Chlamydomonas reinhardtii anoxic gene expression, hydrogenase induction, and metabolic pathways. J Biol Chem 282:25475–25486
Nakamura Y, Takahashi JI, Sakurai A, Inaba Y, Suzuki E, Nihei S, Fujiwara S, Tsuzuki M, Miyashita H, Ikemoto H, Kawachi M, Sekiguchi H, Kurano N (2005) Some cyanobacteria synthesize semi-amylopectin type α-polyglucans instead of glycogen. Plant Cell Physiol 46:539–545
Namakoshi K, Nakajima T, Yoshikawa K, Toya Y, Shimizu H (2016) Combinatorial deletions of glgC and phaCE enhance ethanol production in Synechocystis sp. PCC 6803. J Biotechnol 239:13–19
Nguyen MT, Choi SP, Lee J, Lee JH, Sim SJ (2009) Hydrothermal acid pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. J Microbiol Biotechnol 19:161–166
Nigam PS, Singh A (2011) Production of liquid biofuels from renewable resources. Prog Energy Combust Sci 37:52–68
Norsker N-H, Barbosa MJ, Vermuë MH, Wijffels RH (2011) Microalgal production—a close look at the economics. Biotechnol Adv 29:24–27
Nyvall P, Pelloux J, Davies HV, Pedersén M, Viola R (1999) Purification and characterisation of a novel starch synthase selective for uridine 5′-diphosphate glucose from the red alga Gracilaria tenuistipitata. Planta 209:143–152
Ogbonda KH, Aminigo RE, Abu GO (2007) Influence of temperature and pH on biomass production and protein biosynthesis in a putative Spirulina sp. Bioresour Technol 98:2207–2211
Ogbonna JC, Tanaka H (1996) Night biomass loss and changes in biochemical composition of cells during light/dark cyclic culture of Chlorella pyrenoidosa. J Ferment Bioeng 82:558–564
Pade N, Mikkat S, Hagemann M (2017) Ethanol, glycogen and glucosylglycerol represent competing carbon pools in ethanol-producing cells of Synechocystis sp. PCC 6803 under high-salt conditions. Microbiology 163:300–307
Perez-Garcia O, Escalante FM, de Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36
Poblete R, Cortes E, Macchiavello J, Bakit J (2018) Factors influencing solar drying performance of the red algae Gracilaria chilensis. Renew Energy 126:978–986
Rastogi M, Shrivastava S (2017) Recent advances in second generation bioethanol production: an insight to pretreatment, saccharification and fermentation processes. Renew Sust Energ Rev 80:330–340
Raven JA, Beardall J (2003) Carbohydrate metabolism and respiration in algae. In: Photosynthesis in algae. Springer, pp 205–224
Renaud SM, Thinh L-V, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211:195–214
Richmond A, Cheng-Wu Z, Zarmi Y (2003) Efficient use of strong light for high photosynthetic productivity: interrelationships between the optical path, the optimal population density and cell-growth inhibition. Biomol Eng 20:229–236
Richmond A, Hu Q (2013) Handbook of microalgal culture: applied phycology and biotechnology. John Wiley & Sons
Roopnarain A, Gray V, Sym S (2014) Phosphorus limitation and starvation effects on cell growth and lipid accumulation in Isochrysis galbana U4 for biodiesel production. Bioresour Technol 156:408–411
Salehi Jouzani G, Taherzadeh MJ (2015) Advances in consolidated bioprocessing systems for bioethanol and butanol production from biomass: a comprehensive review. Biofuel Res J 2:152–195
Sangar VK, Dugan PR (1972) Polysaccharide produced by Anacystis nidulans: its ecological implication. Appl Microbiol 24:732–734
Sassano C, Gioielli L, Ferreira L, Rodrigues M, Sato S, Converti A, Carvalho J (2010) Evaluation of the composition of continuously-cultivated Arthrospira (Spirulina) platensis using ammonium chloride as nitrogen source. Biomass Bioenergy 34:1732–1738
Scott SA, Davey MP, Dennis JS, Horst I, Howe CJ, Lea-Smith DJ, Smith AG (2010) Biodiesel from algae: challenges and prospects. Curr Opin Biotechnol 21:277–286
Shang C, Zhu S, Wang Z, Qin L, Alam MA, Xie J, Yuan Z (2017) Proteome response of Dunaliella parva induced by nitrogen limitation. Algal Res 23:196–202
Shokrkar H, Ebrahimi S, Zamani M (2017) Bioethanol production from acidic and enzymatic hydrolysates of mixed microalgae culture. Fuel 200:380–386
Show K-Y, Lee D-J, Tay J-H, Lee T-M, Chang J-S (2015) Microalgal drying and cell disruption–recent advances. Bioresour Technol 184:258–266
Siaut M, Cuiné S, Cagnon C, Fessler B, Nguyen M, Carrier P, Beyly A, Beisson F, Triantaphylidès C, Li-Beisson Y, Peltier G (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol 11:7
Sigee DC, Bahrami F, Estrada B, Webster RE, Dean AP (2007) The influence of phosphorus availability on carbon allocation and P quota in Scenedesmus subspicatus: a synchrotron-based FTIR analysis. Phycologia 46:583–592
Singh A, Nigam PS, Murphy JD (2011) Renewable fuels from algae: an answer to debatable land based fuels. Bioresour Technol 102:10–16
Skorupskaite V, Makareviciene V, Ubartas M, Karosiene J, Gumbyte M (2017) Green algae Ankistrodesmus fusiformis cell disruption using different modes. Biomass Bioenergy 107:311–316
Song Z, Chen L, Wang J, Lu Y, Jiang W, Zhang W (2014) A transcriptional regulator Sll0794 regulates tolerance to biofuel ethanol in photosynthetic Synechocystis sp. PCC 6803. Mol Cell Proteomics 13:3519–3532
Suzuki E, Suzuki R (2013) Variation of storage polysaccharides in phototrophic microorganisms. J Appl Glycosci 60:21–27
Taiz L, Zeiger E (2010) Plant physiology, 5th edn. Sinauer Associates, Sunderland
Takahashi H, Kopriva S, Giordano M, Saito K, Hell R (2011) Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol 62:157–184
Taleb A et al (2016) Screening of freshwater and seawater microalgae strains in fully controlled photobioreactors for biodiesel production. Bioresour Technol 218:480–490
Taraldsvik M, Myklestad SM (2000) The effect of pH on growth rate, biochemical composition and extracellular carbohydrate production of the marine diatom Skeletonema costatum. Eur J Phycol 35:189–194
Torzillo G, Sacchi A, Materassi R (1991) Temperature as an important factor affecting productivity and night biomass loss in Spirulina platensis grown outdoors in tubular photobioreactors. Bioresour Technol 38:95–100
Tredici MR (2010) Photobiology of microalgae mass cultures: understanding the tools for the next green revolution. Biofuels 1:143–162
Tsolcha O, Tekerlekopoulou A, Akratos C, Aggelis G, Genitsaris S, Moustaka-Gouni M, Vayenas D (2018) Agroindustrial wastewater treatment with simultaneous biodiesel production in attached growth systems using a mixed microbial culture. Water 10:1693
Tsolcha ON, Tekerlekopoulou AG, Akratos CS, Aggelis G, Genitsaris S, Moustaka-Gouni M, Vayenas DV (2017) Biotreatment of raisin and winery wastewaters and simultaneous biodiesel production using a Leptolyngbya-based microbial consortium. J Clean Prod 148:185–193
Tsolcha ON et al (2016) Treatment of second cheese whey effluents using a Choricystis-based system with simultaneous lipid production. J Chem Technol Biotechnol 91:2349–2359
Tsubaki S, Oono K, Hiraoka M, Onda A, Mitani T (2016) Microwave-assisted hydrothermal extraction of sulfated polysaccharides from Ulva spp. and Monostroma latissimum. Food Chem 210:311–316
Turpin DH (1991) Effects of inorganic N availability on algal photosynthesis and carbon metabolism. J Phycol 27:14–20
Ueno Y, Kurano N, Miyachi S (1998) Ethanol production by dark fermentation in the marine green alga, Chlorococcum littorale. J Ferment Bioeng 86:38–43
Vonshak A (1997) Spirulina platensis arthrospira: physiology, cell-biology and biotechnology. CRC Press
Wang H, Ji C, Bi S, Zhou P, Chen L, Liu T (2014) Joint production of biodiesel and bioethanol from filamentous oleaginous microalgae Tribonema sp. Bioresour Technol 172:169–173
Wang L, Li Y, Sommerfeld M, Hu Q (2013) A flexible culture process for production of the green microalga Scenedesmus dimorphus rich in protein, carbohydrate or lipid. Bioresour Technol 129:289–295
Warr S, Reed R, Stewart W (1985) Carbohydrate accumulation in osmotically stressed cyanobacteria (blue-green algae): interactions of temperature and salinity. New Phytol 100:285–292
Wirth R, Lakatos G, Böjti T, Maróti G, Bagi Z, Kis M, Kovács A, Ács N, Rákhely G, Kovács KL (2015) Metagenome changes in the mesophilic biogas-producing community during fermentation of the green alga Scenedesmus obliquus. J Biotechnol 215:52–61
Wuang SC, Khin MC, Chua PQD, Luo YD (2016) Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res 15:59–64
Xia JR, Gao KS (2005) Impacts of elevated CO2 concentration on biochemical composition, carbonic anhydrase, and nitrate reductase activity of freshwater green algae. J Integr Plant Biol 47:668–675
Yamada R, Tanaka T, Ogino C, Fukuda H, Kondo A (2010) Novel strategy for yeast construction using δ-integration and cell fusion to efficiently produce ethanol from raw starch. Appl Microbiol Biotechnol 85:1491–1498
Yang S, Pan C, Tschaplinski TJ, Hurst GB, Engle NL, Zhou W, Dam PA, Xu Y, Rodriguez M, Dice L, Johnson CM, Davison BH, Brown SD (2013) Systems biology analysis of Zymomonas mobilis ZM4 ethanol stress responses. PLoS One 8:e68886
Yao C-H, Ai J-N, Cao X-P, Xue S (2013) Characterization of cell growth and starch production in the marine green microalga Tetraselmis subcordiformis under extracellular phosphorus-deprived and sequentially phosphorus-replete conditions. Appl Microbiol Biotechnol 97:6099–6110
Yao C, Ai J, Cao X, Xue S, Zhang W (2012) Enhancing starch production of a marine green microalga Tetraselmis subcordiformis through nutrient limitation. Bioresour Technol 118:438–444
Yoshikawa K, Toya Y, Shimizu H (2017) Metabolic engineering of Synechocystis sp. PCC 6803 for enhanced ethanol production based on flux balance analysis. Bioprocess Biosyst Eng 40:791–796
Zabed H, Sahu J, Suely A, Boyce A, Faruq G (2017) Bioethanol production from renewable sources: current perspectives and technological progress. Renew Sust Energ Rev 71:475–501
Zachleder V, Kawano S, Kuroiwa T (1996) Uncoupling of chloroplast reproductive events from cell cycle division processes by 5-fluorodeoxyuridine in the alga Scenedesmus quadricauda. Protoplasma 192:228–234
Zhang Z, Shrager J, Jain M, Chang C-W, Vallon O, Grossman AR (2004) Insights into the survival of Chlamydomonas reinhardtii during sulfur starvation based on microarray analysis of gene expression. Eukaryot Cell 3:1331–1348
Zhao G, Chen X, Wang L, Zhou S, Feng H, Chen WN, Lau R (2013) Ultrasound assisted extraction of carbohydrates from microalgae as feedstock for yeast fermentation. Bioresour Technol 128:337–344
Zhou N, Zhang Y, Wu X, Gong X, Wang Q (2011) Hydrolysis of Chlorella biomass for fermentable sugars in the presence of HCl and MgCl2. Bioresour Technol 102:10158–10161
Zhu L, Hiltunen E, Antila E, Zhong J, Yuan Z, Wang Z (2014) Microalgal biofuels: flexible bioenergies for sustainable development. Renew Sust Energ Rev 30:1035–1046
Zhu Z, Luan G, Tan X, Zhang H, Lu X (2017) Rescuing ethanol photosynthetic production of cyanobacteria in non-sterilized outdoor cultivations with a bicarbonate-based pH-rising strategy. Biotechnol Biofuels 10:93
Acknowledgments
The authors thank Ms. Soňa Pekařová for the technical assistance and Dr. Kateřina Bišová for the discussion.
Author contribution statement
Gergely Lakatos took the leading role in the writing of the manuscript. Karolína Ranglová, Jiří Kopecký, Tomáš Grivalský and João Câmara Manoel contributed during the manuscript preparation. Jiří Masojídek revised and finalized the manuscript.
Funding
This study was funded partly by the National Sustainability Program of the Czech Ministry of Education, Youth and Sports (project Algatech Plus LO1416), and by cross-border InterReg projects between Austria and the Czech Republic (Algenetics No. ATCZ15) and Bavaria and the Czech Republic (CZ-BAV 41).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Dedicated to the memory of Prof. Ivan Šetlík.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lakatos, G.E., Ranglová, K., Manoel, J.C. et al. Bioethanol production from microalgae polysaccharides. Folia Microbiol 64, 627–644 (2019). https://doi.org/10.1007/s12223-019-00732-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-019-00732-0