Abstract
The aim of the study was to screen Yarrowia lipolytica strains for keto acid production and determine optimal conditions for pyruvic acid biosynthesis from glycerol by the best producer. The analyzed parameters were thiamine concentration, medium pH, stirring speed, and substrate concentration. The screening was performed in flask cultures, whereas pyruvic acid production was carried out in 5-L stirred-tank reactor with 2 L of working volume. In total, 24 Y. lipolytica strains were compared for their abilities to produce pyruvic and α-ketoglutaric acids. The total concentration of both acids ranged from 0.1 to 15.03 g/L. Ten strains were selected for keto acid biosynthesis in bioreactor. The Y. lipolytica SKO 6 strain was identified as the best producer of pyruvic acid. In the selected conditions (thiamine concentration 1.5 μg/L, pH 4.0, stirring speed 800 rpm, 150 g/L of glycerol), the strain Y. lipolytica SKO 6 produced 99.3 g/L of pyruvic acid, with process yield of 0.63 g/g and volumetric production rate of 1.18 g/L/h. Higher titer of pyruvic acid was obtained during fed-batch culture with 200 g/L of glycerol, reaching 125.8 g/L from pure glycerol (yield 0.68 g/g) and 124.4 g/L from crude glycerol (yield 0.62 g/g). Results obtained for the strain Y. lipolytica SKO 6 proved the suitability of microbial production of pyruvic acid at industrial scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pyruvic acid (PA) is a three-carbon organic keto acid being a key intermediate in both aerobic and anaerobic cell metabolism. In eucaryotic as well as many procaryotic cells, PA is formed in cytoplasm through glycolysis (1 glucose → 2 PA). The two PA molecules can be further transformed into carbohydrates (via gluconeogenesis), fatty acids, energy (via Krebs cycle), alanine, or ethanol (via fermentation). Due to these diver functions, PA connects various metabolic pathways (Fell and Wagner 2000). Under standard conditions, PA is a clear, water miscible liquid, with an aroma similar to acetic acid. PA and its derivatives are widely used to synthesize other valuable products in pharmaceutical, cosmetic, food, and chemical industries. A set of pharmaceuticals derived from PA is presented in Table 1.
PA is also an ingredient in food supplements for athletes, improving their physical fitness and supporting body mass control. It was shown that PA calcium salts accelerate metabolism of fatty acids as well as contribute to lowering serum cholesterol level. PA has also antioxidant properties and may be used in nutraceutical support of the diabetes II treatment (Li et al. 2001a, b, Wang et al. 2007, Maleki and Eiteman 2017). In cosmetic industry, PA is used as an exfoliant and anti-comedo agent (Marczyk et al. 2012). Due to its aseptic and acidifying properties, it may be used to treat acne and many other dermatological conditions (for example, superficial scars, photo damages, and skin pigmentation disorders) (Berardesca et al. 2006).
Nowadays, the industrial production of PA is carried out through chemical synthesis, mostly:
Dehydration and decarboxylation of tartaric acid, where PA is distilled at 220 °C from a mixture of tartaric acid and potassium bisulfate (Howard and Fraser 1932, Xu et al. 2008),
Conversion of lactic acid to PA via oxidation of ethyl lactate in its gaseous phase, using a mixture of tellurium oxide and molybdenum oxide (Hayashi et al. 1993),
Oxidation of lactic acid in the presence of a catalyst (ferric sulfate and palladium), where the lactic acid is dehydrogenized, reformed into an ester, followed by its hydrolysis and PA production (Ai and Ohdan 1997).
Unfortunately, as in the case of many conventional chemical syntheses, PA production is not cost-effective due to the high costs of substrates, extreme process conditions, unsatisfactory yields, and co-production of many harmful waste materials. These issues, along with high PA price, hinder PA’s potential for industrial applications.
For over a dozen years, researchers tried to produce PA using microorganisms, including yeast. The following yeast genera are able to overproduce and secrete pyruvic acid: Blastobotrys, Debaryomyces, Torulopsis, Saccharomyces, and Yarrowia (Yanai et al. 1994, Morgunov et al. 2004, van Maris et al. 2004, Sawai et al. 2011, Kamzolova and Morgunov 2016, Cybulski et al. 2018a, b). Among the abovementioned species, the major advantage of Y. lipolytica is its auxotrophy for only a single nutrient—thiamine, which facilitates the composition of a proper medium and lowers the cultivation costs. In comparison, T. glabrata is an auxotroph for four vitamins: nicotinic acid, thiamine, pyridoxine, and biotin (Yang et al. 2014). Moreover, Y. lipolytica express a wide range of enzymes allowing for various substrates utilization including organic acids, fatty acids, hexoses, alcohols, n-alcans, and plant oils (Fickers et al. 2005, Rywińska et al. 2013). Y. lipolytica is commonly used for microbial production of many value-added products, such as lipases (Fickers et al. 2011), proteases (Ogrydziak 1988), esterases (Huang et al. 2011), biomass rich in oils and proteins (Beopoulos et al. 2009, Juszczyk et al. 2013, Blazeck et al. 2014), citric acid (Rymowicz et al. 2006), α-ketoglutaric acid (Kamzolova and Morgunov 2013), erythritol and mannitol (Tomaszewska et al. 2012), or aromas, e.g., γ-decalactone (Aguedo et al. 2004).
In the present study, wild strains of Y. lipolytica were investigated for their abilities to secrete large amount of PA and KGA from glycerol. The best keto acid producer, Y. lipolytica SKO 6, was used in studies aiming to optimize process conditions leading to high yield of PA biosynthesis.
Materials and methods
Microorganisms
Twenty-four strains of Y. lipolytica were investigated for their growth in oil-based medium. The strain Y. lipolytica CCY-29-26-5 was obtained from the Culture Collection of Yeasts. The strain ATCC 8661 was obtained from the American Type Culture Collection. The Y. lipolytica N15 strain belongs to the collection of the laboratory of Aerobic Metabolism of Microorganisms of the Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, Puschino, Russia (Kamzolova et al. 2008). The other Y. lipolytica strains belong to the yeast collection of the Department of Biotechnology and Food Microbiology at Wrocław University of Environmental and Life Sciences, Poland. The wild strains were isolated from different environments at different time: A-1, A-3, A-6, A-8, A-10, A-15, A-61, and A-311 were isolated in 1974 from soil in the vicinity of a garage in Wrocław. SKO 4, SKO 6, and SKO 12 were isolated in 2012 from soil of the land belonging to a tannery in Skoczów. 11B, 11C, 11E, and 11F were isolated in 2008 from soil. In the study, the following acetate-negative mutants of Y. lipolytica were also analyzed: Wratislavia 1.31, Wratislavia K1, Wratislavia AWG7, K1UV21, and MK1. Wratislavia 1.31 was isolated from the wild strain A-101 upon exposure to UV radiation; Wratislavia K1 strain was isolated from Wratislavia 1.31 in the course of continuous citric acid production from glucose in a nitrogen-limited chemostat culture at a dilution rate of D = 0.016 h−1. The strains K1UV21 (Rywińska et al. 2012) and MK1 (Mirończuk et al. 2015) were isolated from Wratislavia K1 upon exposure to UV radiation. The yeast strains were maintained on YM agar slants at 4 °C.
Media and culture conditions
Carbon sources
In the presented study, the following substrates were used as carbon and energy sources: pure glycerol (98% w/w) (Chempur, Poland) and crude glycerol (83% w/w) from biodiesel production (Wratislavia-Bio Poland). Among the impurities found in crude glycerol are NaCl 7.3%, metals (Mg 17.70 mg/kg, Cu 0.30 mg/kg, Fe 1.90 mg/kg, Zn 2 mg/kg, and Ca 13 mg/kg), heavy metals (Cd, Cr, Hg not detected), and water mass fraction 6.5%.
Media composition
The inoculum medium was based on YNB (Yeast Nitrogen Base) and pure glycerol. The YNB-based media 0.67 and 0.067 contained 50 g of pure glycerol (98% w/w) and 0.67 g or 0.067 g of YNB, per liter of distilled water, respectively. The screening of yeast strains was performed in a mineral medium containing 50 g of glycerol, 5 g of (NH4)2SO4, 0.7 g of MgSO4 × 7H2O, 0.5 g of NaCl, 1 g of KH2PO4, 5 g of CaCO3, and 0.25 μg of thiamine per liter of distilled water.
Keto acid biosynthesis was performed in a medium containing 100–200 g of carbon source, 10 g of (NH4)2SO4, 2 g of KH2PO4, 1.4 g of MgSO4∙7H2O, 1.0 g of NaCl, 0.8 g of Ca(NO3)2∙(4H2O), and 0–3.5 μg of thiamine per liter of tap water.
Cultivation methods
Inocula and screening of yeast strains were carried out in 300 mL flasks containing 100 mL or 30 mL of the appropriate medium, respectively, on a rotary-shaker (CERTOMAT IS, Sartorius Stedim Biotech, Germany) at 30 °C, 140 rpm for 72 h (inoculum) or 120 h (screening). The inoculum (200 mL) was introduced into a bioreactor containing 1800 mL of the production medium.
The cultures for PA biosynthesis were carried out at 30 °C in a 5-L stirred-tank reactor (Biostat B-Plus, Sartorius, Germany) with a working volume of 2 L. The pH was maintained automatically at an appropriate level, as indicated on the figures or in tables, through the addition of a 40% (w/v) NaOH solution. The agitation rate and aeration rate were 800 rpm and 0.6 vvm, respectively. Samples were collected 1–2 times per day. Production and screening cultures were performed in two and three biological replicates, respectively, and the results are presented as mean values. Error bars presented on figures are the standard deviations. Homogeneous groups (with no statistically significant differences) were selected on the basis of Duncan test (yeast screening, Table 2).
Analytical methods
For biomass determination, samples were centrifuged (10 min.; 4 °C; 3063 g) and washed two times with distilled water. Subsequently, cells were harvested by filtration on 0.45 μm pore size membranes and dried to a constant weight at 105 °C. The supernatant was centrifuged (10 min.; 4 °C; 3063 g), filtered, and diluted in de-ionized water (1:9). The concentration of pyruvic acid (PA), α-ketoglutaric acid (KGA), and glycerol (GLY) in the supernatant were determined by the HPLC method (Dionex, UltiMate 3000 LC Systems, Thermo Scientific, USA) on an HyperRez XP Carbohydrate H+ column (Thermo Scientific, USA) coupled to a UV detector (λ = 210 nm) and an RI detector (Shodex, Japan). The column was eluted at 65 °C with 20 mM trifluoroacetic acid solution at a flow rate of 0.6 mL/min.
Results
Selection of the best keto acid–producing Y. lipolytica strains
The abilities of 24 Y. lipolytica strains to produce PA and KGA (pyruvic acid and α-ketoglutaric acid) from pure glycerol was investigated in shake-flasks cultures. As shown in Table 2, a sum of both acids during 120 h of cultivation was significantly different for each analyzed strain and ranged from 0.1 g/L (N15) to 15 g/L (SKO 6). Top ten strains with the highest keto acid secretion (all strains from the homogeneous group 8) were selected for further experiments.
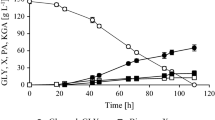
The chosen strains were compared for their PA and KGA secretion capacity during bioreactor cultures. A 120.5 g/L of crude glycerol (83%, which corresponds to 100 g/L of pure glycerol; for details see Materials and methods) was used as carbon and energy source. Results from this experiment are shown on Fig. 1. Cultivation time required for complete utilization of glycerol was strain-dependent and varied from 68 to 75 h. PA was the main acid secreted into the medium for 7 out of 10 analyzed strains. The highest titer of this acid reached 46.8 g/L for Y. lipolytica SKO 6 within 68 h. Two strains (A-101 and A-8) produced higher concentration of α-ketoglutaric acid. Y. lipolytica A-10 strain secreted equal amount of both acids.
Factors affecting pyruvic acid (PA) production by Y. lipolytica SKO 6 strain
The effect of pH
After the best PA-producing strain was chosen, the presented studies focused on the optimization of pH for the most efficient PA synthesis. The experiment was performed in bioreactor with 100 g/L of pure glycerol until complete depletion of the substrate. The obtained results are presented on Fig. 2.
The analyzed pH of the cultures ranged from 3.0 to 5.5. The acidity of the medium clearly affected yeast growth. With increasing pH, the concentration of biomass in stationary phase also increased from 4.2 g/L at pH 3.0 to 15.1 g/L at pH 5.5. The highest PA concentration was obtained at pH 4.0. The yield and volumetric production rate reached 0.57 g/g and 0.8 g/L/h, respectively. In the same conditions, the concentration of KGA, a by-product in the PA biosynthesis, was the lowest from all experiments, thus the PA to KGA ratio raised to 10.5. All the subsequent experiments were carried out at pH 4.0.
The effect of thiamine concentration
Optimization of thiamine concentration in the medium was performed. In this experiment, the culture medium was supplemented by 0–3.5 μg/L of thiamine. With increasing concentration of thiamine, the amount of biomass in the stationary phase raised from 7.4 to 15.4 g/L (Fig. 3). The lowest PA concentration (53.5 g/L) was obtained in the culture where thiamine concentration was 3.5 μg/L. The highest PA titer was reached in medium supplemented with 1.5 μg/L of thiamine. In 68 h of the culture, PA production reached 64.2 g/L with the yield of 0.6 g/g. The KGA concentration under the same conditions was relatively low (4.3 g/L).
The effect of agitation rate
The effect of medium aeration using different stirring speed on PA secretion was investigated. The experiment was carried out in the conditions established in previous stages and 100 g/L of pure glycerol. The following stirring speeds were analyzed: 600, 800, and 1000 rpm (maintained throughout the entire culture). Other strategy involving the start of the culture at 1000 rpm, followed by the stirring speed decreased to 800 rpm after 24 h, was also investigated. The obtained results are presented on Fig. 4. The time required for complete glycerol depletion ranged from 68 h at 800 rpm to 75 h at 1000 rpm. The highest biomass concentration (13.6 g/L) was measured at 1000 rpm. Nevertheless, the most efficient process was observed at 800 rpm and resulted in the highest titer of PA (64.2 g/L) and the lowest KGA (co-product) concentration (4.3 g/L). Furthermore, using both, lower or higher stirring rates, including stirring speed switch after 24 h, resulted in decreased PA secretion ranging from 53.9 to 57.9 g/L.
Batch culture with increased concentration of glycerol
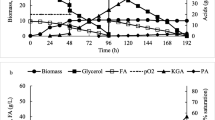
The most efficient culture conditions were applied to a culture with increased concentration of glycerol (160 g/L of pure glycerol (Fig. 5). The growing cells reached the stationary phase after 2 days of cultivation with a dry biomass concentration of 12.6 g/L. Total substrate depletion was observed at 84 h of the culture. The final PA and KGA concentrations were 99.3 g/L and 16.4 g/L, respectively (the PA:KGA ratio reached 6.05). The PA yield from glycerol was 0.63 g/g and the volumetric production rate achieved 1.18 g/L/h.
Fed-batch cultures
As the last experiment, two fed-batch cultures with pure (Fig. 6a) and crude (Fig. 6b) glycerol were conducted. The total concentration of glycerol introduced into the bioreactor was 200 g/L, divided into four equal batches (50 g/L each) supplemented every 24 h.
When pure glycerol was used, the process lasted 216.5 h and the cells consumed up to 184.5 g/L of the substrate. Yeast reached the stationary phase after 3 days of culture with a dry biomass of 13.3 g/L. PA production rate was satisfactory until 120 h of the process, when PA titer reached 110 g/L. During the following hours, the secretion of PA decreased significantly and its final concentration achieved 125.8 g/L. At the same time, the concentration of unwanted KGA obtained 34.4 g/L. The yield of PA at the end of the process was 0.68 g/g and its volumetric production rate reached 0.58 g/L/h.
The process with crude glycerol as carbon source was shorter and lasted 164 h. During that time, yeast consumed the available glycerol completely. The concentration of biomass in the stationary phase was higher, compared to the cultures with pure glycerol. Final PA and KGA concentrations in the culture with crude glycerol reached 124.4 g/L and 42.7 g/L, respectively. The yield of PA was 0.62 g/g and its volumetric production rate 0.76 g/L/h.
Discussion
In the current study, 24 Y. lipolytica strains were analyzed for their keto acid (PA and KGA) production abilities from glycerol. All of the tested strains were described earlier as being able to grow efficiently in medium containing glycerol, which was the selection criterion in the presented research (Kamzolova et al. 2011, Juszczyk et al. 2012, Tomaszewska et al. 2012, Mirończuk et al. 2015). In the available literature, small quantities of keto acids among the main metabolites were already observed during the biosynthesis of erythritol or citric acid (Tomaszewska et al. 2011, Rywińska et al. 2012). In order to evaluate the potential of yeast for the production of desired compounds, the total concentration of both acids would be compared with view to the possibility of subsequent metabolic redirection towards PA synthesis (i.a., by using proper pH and thiamine concentration). Based on the highest PA and KGA secretion during flask cultures (12.6 to 15 g/L), 10 strains were selected for further bioreactor cultures. In that experiment, in addition to pure glycerol, the crude glycerol was used as a source of carbon and energy. The time required for a complete substrate utilization ranged from 68 to 75 h. The final PA concentration reached only 12.5 g/L for A-8 strain and up to 46.8 g/L for the SKO 6 strain. During that experiment, the A-8 strain produced the highest amount of KGA (28.4 g/L). Three of the analyzed strains secreted the highest total concentration of both PA and KGA: SKO 6, Wratislavia 1.31, and A-61. SKO 6 and A-61 are natural (wild) strains, isolated from soil. The abovementioned strains were previously tested for erythritol biosynthesis from glycerol (Juszczyk et al. 2012, Tomaszewska et al. 2011). Wratislavia 1.31 strain is an acetate negative mutant (unable to grow on acetate and oils) producing high titer of citric acid from glycerol (Rywińska et al. 2010). Wratislavia 1.31 showed also great potential as a producer of pyruvic acid (Cybulski et al. 2018a). The subsequent experiments aiming to improve PA production were conducted with the SKO 6 strain, selected as the best producer during flask and bioreactor cultures.
The biotechnological potential of Y. lipolytica has been investigated by many researchers from around the world. Due to their abilities to utilize many different carbon sources, as well as the large number of secreted metabolites, these microorganisms were used to produce, i.a., erythritol (Rymowicz et al. 2009), citric acid (Rywińska and Rymowicz 2010, Kamzolova et al. 2011), or KGA (Zhou et al. 2010; Kamzolova and Morgunov 2013, Cybulski et al. 2018b). However, the available literature does not provide many examples of PA production using this yeast, especially from carbon sources other than glucose. The first evidence of PA production by Y. lipolytica strain AJ 4546 was reported in 1974 (Uchio et al. 1974a). During shake-flask cultures with acetamide as carbon source, the strain secreted 1.5 g/L of PA with a yield 0.15 g/g. Two years later, using C. lipolytica AJ 14353 (methionine-dependent phenotype) and glucose as a carbon source, researchers were able to obtain 43.6 g/L of PA with a yield 0.44 g/g (Uchio et al. 1976). Many years later, Morgunov et al. (2004) performed a screening of 12 Y. lipolytica strains and 6 Candida strains for their growth under thiamine-limited conditions in medium containing glucose and glycerol. Based on the obtained results, Y. lipolytica 374/4 strain was selected for PA production. In the glycerol-containing medium, the authors obtained 61.3 g/L of PA within 78 h, with concomitant KGA concentration reaching 10 g/L (Table 3). In a recent study, 33 natural yeast strains (belonging to 19 species) were tested for PA biosynthesis, where Y. lipolytica VKM Y-2378 strain was selected as the best producer. This strain was able to secrete 41 g/L of PA from glycerol-containing substrates, with a yield of 0.82 g/g (Kamzolova and Morgunov 2018). Besides Y. lipolytica, other yeast species were also tested for PA biosynthesis. The analyzed species were Candida maltosa (Uchio et al. 1974b), Debaryomyces coudertii (Moriguchi 1982), or Torulopsis etchellsii (Kiuchi et al. 1987), but they rarely exceeded the PA yield of 0.6 g/g. Table 3 summarizes the PA concentration presented in the available literature.
Glycerol is a very good carbon source for biotechnological processes with Y. lipolytica and may be successfully used for the production of biomass, citric acid, SCO, erythritol, or KGA (Rywińska et al. 2013). Nevertheless, each of those processes required optimization of culture conditions, where pH turned out to be the most important criterion. Previous research showed that in the same medium, Y. lipolytica may produce citric acid or erythritol depending on the medium pH (Tomaszewska et al. 2014). Based on this phenomenon, the first parameter optimized in the current study was pH. The pH of the medium examined in this study ranged from 3 to 5.5 and resulted in obtaining 50.1 to 57.5 g/L of PA. The highest PA concentration was obtained at pH 4.0. Higher pH was not analyzed due to higher risk of bacterial contamination as well as literature report efficient PA secretion at pH ≤ 5.5. Recent studies performed by Kamzolova and Morgunov (2018) as well as our previous research (Cybulski et al. 2018a) indicate pH 4.0–4.5, as optimal for overproduction of PA from glycerol by Y. lipolytica. Similar studies were conducted for KGA biosynthesis and also proved the importance of culture pH for its secretion. KGA biosynthesis with a satisfactory yield was observed in pH ranging from 3.5 to 4.5 using rapeseed oil as a substrate (Kamzolova and Morgunov 2013). The studies by Holz et al. (2011) proved the pH of 5.5 as optimal for KGA production from glycerol. It is important to point out that the pH below 3.5 inhibited biomass proliferation of the SKO 6 strain and the pH optimal for yeast growth did not overlap with pH optimal for the production of keto acids. Similar conclusions were drawn by Kamzolova et al. (2012) in a study of KGA production from ethanol. The pH optimal for KGA overproduction was determined as 3.5, whereas below this level, the KGA production process slowed down by 25% (including acid secretion and yeast growth). Y. lipolytica is one of the most heterogeneous species of yeast and is characterized by various morphology and metabolism among different strains (Egermeier et al. 2017). Various strains grow totally different depending on the medium pH (Juszczyk et al. 2005). Furthermore, it was reported that during citric acid and erythritol production (in the nitrogen-limited conditions), the growth of Y. lipolytica Wratislavia 1.31 was not dependent on pH changes within the range of 3.0–6.5 (Tomaszewska et al. 2014). In the contrary, the same strain exhibit pH-related growth (pH 3.0–5.5) under thiamine-limited conditions during pyruvic acid production (Cybulski et al. 2018a). Similarly, pH-dependent growth was observed for the process of pyruvic acid production by Y. lipolytica A-10 (Cybulski et al. 2018b). Therefore, it may be concluded that the cells of Y. lipolytica under the conditions of thiamine deficiency are more sensitive to pH changes.
Y. lipolytica show a natural auxotrophy towards thiamine. Using a growth-limiting concentration of this compound allows for a metabolic shift and extracellular secretion of PA and KGA. In the presented study, Y. lipolytica SKO 6 was analyzed in a medium with thiamine concentration ranging from 0 to 3.5 μg/L. The growth of Y. lipolytica observed in medium, where thiamine was not supplemented (0 μg/L), comes from the trace amount of this vitamin introduced into the medium with the inoculum (YNB). According to YNB composition, the concentration of thiamine in the inoculum culture was 0.4 μg/L. Additionally, yeast cells probably used intracellular resources of thiamine. The most promising results were obtained for thiamine concentration of 0.5–2.5 μg/L. However, the best thiamine concentration for efficient PA production turned out to be 1.5 μg/L. Under this conditions, the yeast produced 64.2 g/L of PA with volumetric production rate of 0.94 g/L/h and the ratio of PA to KGA reaching 14.9. In comparison, Y. lipolytica Wratislavia 1.31 strain under optimal thiamine concentration (1.0 μg/L) was able to produce 57.8 g/L of PA with volumetric productivity of 0.83 g/L/h (Cybulski et al. 2018a). Similar results were recently obtained by Kamzolova and Morgunov (2018). The authors showed that optimal thiamine concentration for PA production ranges between 1 and 2 μg/L for Y. lipolytica VKM Y-2378 (Kamzolova and Morgunov 2018). The differences in thiamine concentration had, however, little effect on PA production by Y. lipolytica SKO 6 strain. The difference between the highest and the lowest concentration of the obtained PA in the investigated thiamine range reached 10.7 g/L (approx. 18% of the averaged amount of the produced PA). The effect of thiamine concentration on yeast growth and PA production from glucose and glycerol by Y. lipolytica was described in details by Morgunov et al. (2004). The authors analyzed thiamine concentration in a range from 0 to 10 μg/L. In glucose-based medium, the optimum PA production was reached when 1–2 μg/L of thiamine was added to the medium, whereas for glycerol-based medium it reached the highest PA secretion for 1–3 μg/L of thiamine. The results were obtained for Y. lipolytica 374/4 during shake-flask experiments and the maximum PA concentration achieved 5.6 g/L from glucose and 8.9 g/L from glycerol. Interestingly, comparing the results only in terms of thiamine concentration ranging from 0 to 3.5 μg/L, the differences in PA produced only in this range were as high as 90% and 160% in comparison to the averaged amount of produced PA (from glycerol and glucose respectively). One of the most recent studies by Kamzolova and Morgunov (2016) proved that the yeasts from Blastobotrys genus are able to produce PA. The B. adeninivorans VKM Y-2677 strain, also auxotrophic towards thiamine, produced 43.2 g/L of PA from glucose in a medium containing 4 μg/L of thiamine. Nevertheless, although the yield of PA production was relatively high (0.77 g/g), the culture medium contained many co-products (i.a., KGA, citric acid, isocitric acid, and succinic acid).
Stirring speed is an important parameter for processes carried out in stirred-tank reactors. It determines the availability of nutrients in the medium as well as cell distribution and the level of oxygen dissolved in the medium. In the presented study, the best results were obtained with a stirring speed of 800 rpm and oxygenation of 0.6 vvm (64.2 g/L of PA, yield = 0.6 g/g). The influence of stirring speed on PA production was also examined by Miyata and Yonehara (1996). The authors investigated the ability of Torulopsis glabrata IFO 0005 strain to produce PA from glucose under stirring speed varying from 500 to 1000 rpm. The stirring speed of 550 rpm with oxygenation of 0.5 vvm were determined as optimal and allowed for obtaining the process yield of 0.49 g/g.
It is worth to point out that procedures aiming to select medium pH, thiamine concentration, and bioreactor stirring speed allowed for a substantial improvement in volumetric production rate (from 0.69 to 0.94 g/L/h), which is a key parameter from an economic point of view. The PA to KGA ratio shifted from 3.4 to 14.9. As expected, an increase in glycerol concentration in the culture (from 100 to 160 g/L) resulted in higher PA concentration. In this process, the highest volumetric production rate, reaching 1.18 g/L/h, was observed. Unfortunately, in this culture, a concomitant KGA biosynthesis was relatively high and the PA to KGA ratio reached only 6.1. Additionally, an experiment of PA biosynthesis from pure and crude glycerol (from biodiesel production) was investigated. Significant differences in biomass concentration and glycerol utilization rate were observed. However, equally high concentration of PA reaching about 125 g/L was obtained in both processes. It is worth to emphasize that the obtained PA titer is one of the highest, biotechnologically obtained result reported to date. It is also important that above 110 g/L of PA in the medium, its biosynthesis rate significantly decreases. For the analyzed Y. lipolytica strain, it must be a threshold value and such high PA concentrations inhibit pyruvate kinase (ATP-specific pyruvate dehydrogenase kinase) which is directly responsible for pyruvate formation from phosphoenolpyruvate (Bhagavan and Chung-Eun 2011).
Summary
The operating conditions for the conversion of glycerol to PA by Y. lipolytica SKO 6 strain were as follows: pH 4.0, thiamine concentration 1.5 μ/L, and agitation rate of 800 rpm. During batch cultures under this conditions using 100 g/L of pure glycerol, the final concentration of PA reached 64.2 g/L. Additionally, after parameters optimization, the concentration of KGA was reduced from 13.6 to 4.3 g/L. Implementing fed-batch mode resulted in PA concentration of 125.8 and 124.4 g/L (for pure and crude glycerol, respectively). The obtained titer of PA corresponds to the yield of 0.68 and 0.62 g of PA from 1 g of glycerol (pure and crude, respectively). However, production of KGA in this mode reached 34.2 and 42.7 g/L, respectively.
Importantly, the process conditions optimized in the presented study in concert with the application of crude glycerol as a substrate are very attractive for a large-scale production of PA.
Abbreviations
- PA:
-
Pyruvic acid
- KGA:
-
α-Ketoglutaric acid
- GLY:
-
Glycerol
- Q PA :
-
Volumetric production rate of pyruvic acid (g/L/h)
- Y PA :
-
Yield of pyruvic acid production (g acid/g glycerol)
- q PA :
-
Specific production rate of pyruvic acid (g/g/h)
References
Aguedo M, Ly MH, Belo I, Teixeira JA, Belin J-M, Wache Y (2004) The use of enzymes and microorganisms to produce aroma compounds from lipids. Food Technol Biotechnol 42:327–336
Ai M, Ohdan K (1997) Oxidative dehydrogenation of lactic acid to pyruvic acid over iron phosphate catalyst. Appl Catal A Gen 150:13–20
Beopoulos A, Cescut J, Haddouche R, Uribelarrea JL, Molina-Jouve C, Nicaud JM (2009) Yarrowia lipolytica as a model for bio-oil production. Prog Lipid Res 48:375–387
Berardesca E, Cameli N, Primavera G, Carrera M (2006) Clinical and instrumental evaluation of skin improvement after treatment with a new 50% pyruvic acid peel. Dermatol Surg 32:526–531
Bhagavan NV, Chung-Eun H (2011) Essentials of medical biochemistry. Academic Press, Cambridge
Blazeck J, Hill A, Liu L, Knight R, Miller J, Pan A, Otoupal P, Alper HS (2014) Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat Commun 5:3131
Cybulski K, Tomaszewska-Hetman L, Rymowicz W, Rakicka M, Rywińska A (2018a) Yarrowia lipolytica application as a prospective approach for biosynthesis of pyruvic acid from glycerol. Chem Pap 72:3077–3083
Cybulski K, Tomaszewska-Hetman L, Rakicka M, Łaba W, Rymowicz W, Rywińska A (2018b) The bioconversion of waste products from rapeseed processing into keto acids by Yarrowia lipolytica. Industrial Crops & Products 119:102–110
Egermeier M, Russmeyer H, Sauer M, Marx H (2017) Metabolic flexibility of Yarrowia lipolytica growing on glycerol. Front Microbial 8:49
Fell DA, Wagner A (2000) The small world of metabolism. Nat Biotechnol 18:1121–1122
Fickers P, Benetti PH, Waché Y, Marty A, Mauersberger S, Smit MS, Nicaud JM (2005) Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res 5:527–543
Fickers P, Marty A, Nicaud JM (2011) The lipases from Yarrowia lipolytica: genetics, production, regulation, biochemical characterization and biotechnological applications. Biotechnol Adv 29:632–644
First EA (2011) L-DOPA ropes in tRNAPhe. Chem Biol 18:1201–1202
Gunawan C, Satianegara G, Chen AK, Breuer M, Hauer B, Rogers PL, Rosche B (2007) Yeast pyruvate decarboxylases: variation in biocatalytic characteristics for (R)-phenylacetylcarbinol production. FEMS Yeast Res 7:33–39
Hayashi H, Shigemoto N, Sugiyama S, Masaoka N, Saitoh K (1993) X-ray photoelectron spectra for the oxidation state of TeO2-MoO3 catalyst in the vapor-phase selective oxidation of ethyl lactate to pyruvate. Catal Lett 19:273–277
Holz M, Otto C, Kretzschmar A, Yovkova Y, Aurich A, Poetter M et al (2011) Overexpression of α-ketoglutarate dehydrogenase in Yarrowia lipolytica and its effect on organic acids. Appl Microbiol Biotechnol 89:1519–1526
Howard JW, Fraser WA (1932) Preparation of pyruvic acid. Org Synth Coll 1:475–480
Huang YC, Chen YF, Chen CY, Chen WI, Ciou YP, Liu WH et al (2011) Production of ferulic acid from lignocellulolytic agricultural biomass by Thermobifida fusca thermostable esterase produced in Yarrowia lipolytica transformant. Bioresour Technol 102:8117–8122
Juszczyk P, Musiał I, Rymowicz W (2005) Selection of yeast strains for biomass production from raw glicerol (in polish). Acta Sci Pol Biotechnologia 4:65–67
Juszczyk P, Marcinkiewicz M, Rywińska A, Rymowicz W (2012) Biosynthesis of erythritol from glycerol by Yarrowia lipolytica yeasts in a batch culture (in polish). Inż Ap Chem 51:133–134
Juszczyk P, Tomaszewska L, Kita A, Rymowicz W (2013) Biomass production by novel strains of Yarrowia lipolytica using raw glycerol, derived from biodiesel production. Bioresour Technol 137:124–131
Kamzolova SV, Morgunov IG (2013) α-Ketoglutaric acid production from rapeseed oil by Yarrowia lipolytica yeast. Appl Microbiol Biotechnol 97:5517–5525
Kamzolova SV, Morgunov IG (2016) Biosynthesis of pyruvic acid from glucose by Blastobotrys adeninivorans. Appl Microbiol Biotechnol 100:7689–7697
Kamzolova SV, Morgunov IG (2018) Biosynthesis of pyruvic acid from glycerol-containing substrates and its regulation in the yeast Yarrowia lipolytica. Bioresour Technol 266:125–133. https://doi.org/10.1016/j.biortech.2018.06.071
Kamzolova SV, Finogenova TV, Morgunov IG (2008) Microbiological production of citric and isocitric acids from sunflower oil. Food Technol Biotechnol 46:51–59
Kamzolova SV, Fatykhova AR, Dedyukhina EG, Anastassiadis SG, Morgunov IG (2011) Citric acid production by yeast grown on glycerol-containing waste from biodiesel industry. Food Technol Biotechnol 46:51–59
Kamzolova SV, Chinglintseva MN, Yusupova AI, Vinokurova NG, Lysanskaya VY, Morgunov IG (2012) Biotechnological potential of Yarrowia lipolytica grown under thiamine limitation. Food Technol Biotechnol 50:412–419
Kim E (1999) Effect of thiamine on the by-products formation by Yarrowia lipolytica. Biotechnol Bioprocess Eng 4:185–188
Kiuchi M, Mori T, Takami I, Monma M, Tabei H (1987) Pyruvate production by a halophilic yeast Torulopsis etchellsii. J Jpn Soc Food Sci Technol 33:579–584
Lee YC, Chien HC, Hsu WH (2007) Production of N-acetyl-D-neuraminic acid by recombinant whole cells expressing Anabaena sp. CH1 N-acetyl-D-glucosamine 2-epimerase and Escherichia coli N-acetyl-D-neuraminic acid lyase. J Biotechnol 129:453–460
Li Y, Chen J, Lun SY (2001a) Biotechnological production of pyruvic acid. Appl Microbiol Biotechnol 57:451–459
Li Y, Chen J, Lun SY, Rui XS (2001b) Efficient pyruvate production by a multi-vitamin auxotroph of Torulopsis glabrata: key role and optimization of vitamin levels. Appl Microbiol Biotechnol 55:680–685
Liu LM, Li Y, Li HZ, Chen J (2004) Manipulating the pyruvate dehydrogenase bypass of a multi-vitamin auxotrophic yeast Torulopsis glabrata enhanced pyruvate production. Lett Appl Microbiol 39:199–206
Liu L, Xu Q, Li Y, Shi Z, Zhu Y, Du G, Chen J (2007) Enhancement of pyruvate production by osmotic-tolerant mutant of Torulopsis glabrata. Biotechnol Bioeng 97:825–832
Lütke-Eversloh T, Santos CN, Stephanopoulos G (2007) Perspectives of biotechnological production of L-tyrosine and its applications. Appl Microbiol Biotechnol 77:751–762
Maleki N, Eiteman MA (2017) Recent progress in the microbial production of pyruvic acid. Fermentation 3:8
Marczyk B, Mucha P, Rotsztejn H (2012) Effect of chemical peelings the most often used in acne vulgaris (in polish). Dermatologia Kliniczna 14:183–187
Mirończuk AM, Dobrowolski A, Rakicka M, Rywińska A, Rymowicz W (2015) Newly isolated mutant of Yarrowia lipolytica MK1 as a proper host for efficient erythritol biosynthesis from glycerol. Process Biochem 50:61–68
Miyata R, Yonehara T (1996) Improvement of fermentative production of pyruvate from glucose by Torulopsis glabrata IFO 0005. J Ferment Bioeng 82:475–479
Morgunov IG, Kamzolova SV, Perevoznikova OA, Shishkanova NV, Finogenova TV (2004) Pyruvic acid production by a thiamine auxotroph of Yarrowia lipolytica. Process Biochem 39:1469–1474
Moriguchi M (1982) Fermentative production of pyruvic acid from citrus peel extract by Debaryomyces coudertii. Agric Biol Chem 46:955–961
Nakazawa H, Enei H, Okamura S, Yamada H (1972) Synthesis of L-tryptophan from pyruvate. Agric Biol Chem 32:2528–2532
Ogrydziak DM (1988) Production of alkaline extracellular protease produced by Yarrowia lipolytica. Crit Rev Biotechnol 8:177–187
Rymowicz W, Rywińska A, Żarowska B, Juszczyk P (2006) Citric acid production from raw glycerol by acetate mutants of Yarrowia lipolytica. Chem Pap 60:391–394
Rymowicz W, Rywińska A, Marcinkiewicz M (2009) High-yield production of erythritol from raw glycerol in fed-batch cultures of Yarrowia lipolytica. Biotechnol Lett 31:377–380
Rywińska A, Rymowicz W (2010) High-yield production of citric acid by Yarrowia lipolytica on glycerol in repeated-batch bioreactors. J Ind Microbiol Biotechnol 37:431–435
Rywińska A, Rymowicz W, Żarowska B, Skrzypiński Adam (2010) Comparison of citric acid production from glycerol and glucose by different strains of Yarrowia lipolytica. World J Microbiol Biotechnol 26 (7):1217–1224
Rywińska A, Bąk M, Rakicka M, Tomaszewska L, Boruczkowski T, Lazar Z, Rymowicz W (2012) Selection of the UV mutants of Yarrowia lipolytica yeast for erythritol biosynthesis from glycerol (in polish). Acta Sci Pol Biotechnologia 11:23–38
Rywińska A, Juszczyk P, Wojtatowicz M, Robak M, Lazar Y, Tomaszewska L, Rymowicz W (2013) Glycerol as a promising substrate for Yarrowia lipolytica biotechnological applications. Biomass Bioenergy 48:148–166
Sawai H, Mimitsuka T, Minegishi SI, Henmi M, Yamada K, Shimizu S, Yonehara T (2011) A novel membrane-integrated fermentation reactor system: application to pyruvic acid production in continuous culture by Torulopsis glabrata. Bioprocess Biosyst Eng 34:721–725
Tomaszewska L, Rywińska A, Musiał I, Utecht M, Juszczyk P, Rymowicz W, Połomska X (2011) Screening of Yarrowia lipolytica yeast strains for erythritol production from glycerol (in polish). Acta Sci Pol, Biotechnologia 10:15–28
Tomaszewska L, Rywińska A, Gładkowski W (2012) Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J Ind Microbiol Biotechnol 39:1333–1343
Tomaszewska L, Rakicka M, Rymowicz W, Rywińska A (2014) A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Res 14:966–976
Uchio R, Hirose Y, Node I (1974a) Fermentative production of pyruvic acid. JP patent 74132291
Uchio R, Maeyashiki I, Okada H (1974b) Fermentative production of puruvic acid. JP patent 74102894
Uchio R, Kikuchi K, Enei H, Hirose Y (1976) Process for producing pyruvic acid by fermentation. US Patent 3993534
van Maris AJ, Geertman J-MA, Vermeulen A, Groothuizen MK, Winkler AA, Piper MD, van Dijken JP, Pronk JT (2004) Directed evolution of pyruvate decarboxylase-negative Saccharomyces cerevisiae, yielding a C2-independent, glucose-tolerant, and pyruvate-hyperproducing yeast. Appl Environ Microbiol 70:159–166
Wang Q, He P, Lu D, Shen A, Jiang N (2002) Screening of pyruvate-producing yeast and effect of nutritional conditions on pyruvate production. Lett Appl Microbiol 35:338–342
Wang X, Perez E, Liu R, Yan L-J, Mallet RT, Yang S-H (2007) Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res 1132:1–9
Xu P, Qui J, Gao C, Ma C (2008) Biotechnological routes to pyruvate production. J Biosci Bioeng 105:169–175
Yanai T, Tsunekawa H, Okamura K, Okamoto R (1994) Manufacture of pyruvic acid with Debaryomyces. JP patent 0600091
Yang S, Chen X, Xu N, Liu L, Chen J (2014) Urea enhances cell growth and pyruvate production in Torulopsis glabrata. Biotechnol Prog 30:19–27
Zhang J, Gao NF (2007) Application of response surface methodology in medium optimization for pyruvic acid production of Torulopsis glabrata TP19 in batch fermentation. J Zhejiang Univ Sci B 8:98–104
Zhou J, Zhou H, Du G, Liu L, Chen J (2010) Screening of a thiamine-auxotrophic yeast for α-ketoglutaric acid overproduction. Lett Appl Microbiol 51:264–271
Funding
Publication was supported by Wrocław Centre of Biotechnology, program The Leading National Research Centre (KNOW) for years 2014–2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cybulski, K., Tomaszewska-Hetman, L., Rakicka, M. et al. Production of pyruvic acid from glycerol by Yarrowia lipolytica. Folia Microbiol 64, 809–820 (2019). https://doi.org/10.1007/s12223-019-00695-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-019-00695-2