Abstract
Increasing bacterial resistance to common drugs is a major public health concern for the treatment of infectious diseases. Certain naturally occurring compounds of plant sources have long been reported to possess potential antimicrobial activity. This study was aimed to investigate the antibacterial activity and possible mechanism of action of andrographolide (Andro), a diterpenoid lactone from a traditional medicinal herb Andrographis paniculata. Extent of antibacterial action was assessed by minimal bactericidal concentration method. Radiolabeled N-acetyl glucosamine, leucine, thymidine, and uridine were used to determine the effect of Andro on the biosyntheses of cell wall, protein, DNA, and RNA, respectively. In addition, anti-biofilm potential of this compound was also tested. Andro showed potential antibacterial activity against most of the tested Gram-positive bacteria. Among those, Staphylococcus aureus was found to be most sensitive with a minimal inhibitory concentration value of 100 μg/mL. It was found to be bacteriostatic. Specific inhibition of intracellular DNA biosynthesis was observed in a dose-dependent manner in S. aureus. Andro mediated inhibition of biofilm formation by S. aureus was also found. Considering its antimicrobial potency, Andro might be accounted as a promising lead for new antibacterial drug development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, death account due to infectious diseases was reported to be 50–75% of all deaths in hospitals (Gowthami et al. 2012). In recent years, due to widespread and indiscriminate use of common antimicrobial drugs against infectious diseases, multiple drug resistance among the human pathogenic microorganisms has increased worldwide, thus limiting therapeutic options (Tanwar et al. 2014). In order to survive, microbes develop resistance to antimicrobials through several mechanisms. Current challenges rely on exploring different chemicals of natural sources as the model for designing and creating new functional classes of antimicrobials, and also to expand the therapeutic options against infectious diseases.
In this context, phytochemicals offer excellent antimicrobial therapeutic alternative. Lower incidences of adverse reactions as well as reduced cost make phytomedicines obvious alternatives of synthetic drugs. A lot of interest has been grown to investigate plant-derived natural products as the sources of novel antibacterial agents (Behal 2001; WHO 2007; Newman and Cragg 2012). Pharmacological potencies of most of the medicinal plants exist in their bioactive components; hence these substances provide clues to synthesize new structural types of antimicrobial agents which are relatively safe to mankind (Kalimuthu and Senthilkumar 2010; Zahra et al. 2011; Ahmad et al. 2012).
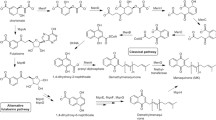
Andrographis paniculata, a herbaceous plant under the family Acanthaceae, native to Asian countries and also cultivated in Scandinavia and some parts of Europe, has a strong traditional usage from ancient time (Cáceres et al. 1997; Gabrielian et al. 2002). This plant is also known for its antibacterial, anti-inflammatory, antipyretic, antiviral, and antioxidant properties (Arifullah et al. 2013; Chen et al. 2014; Chua 2014). The primary bioactive component of A. paniculata is andrographolide (Andro), a diterpene lactone (Fig. 1), which has been reported to show anticancer (Mishra et al. 2015; Banerjee et al. 2016), anti HIV (Calabrese et al. 2000), cardioprotective (Yoopan et al. 2007), hepatoprotective (Trivedi et al. 2007), and adaptogenic (Thakur et al. 2015) potentials. Though there are few reports on antimicrobial activities of Andro (Sule et al. 2010; Sukesh et al. 2011; Malahubban et al. 2013), studies pertaining to the mechanism of Andro mediated antimicrobial activities are very scanty. Present study was undertaken to evaluate the role of Andro as a potential antimicrobial agent against a wide range of human pathogenic bacteria. The bacterial strain against which Andro was found to be most active was further investigated to determine the effect on cellular biosyntheses of macromolecules (DNA, RNA, cell wall, and protein) to find its target site of action. In addition, experiments were performed to assess its bacteriostatic or bactericidal activity and also to find its role on inhibition of biofilm formation for identifying it as a potent antivirulent agent.
Materials and methods
Antimicrobial agents and chemicals
Andrographolide (sc-205594, C20H30O5; MW 350.45, purity >98%) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Other antibiotics namely vancomycin, ampicillin, chloramphenicol, ciprofloxacin, and rifampicin were procured from Sigma-Aldrich (St. Louis, USA). All these antimicrobial agents were prepared in appropriate diluents as directed by the manufacturer’s recommendations, and the concentrations were calculated based on their purity. Cation adjusted Mueller-Hinton broth (CAMHB) powder was purchased from Difco (BD Diagnostic Systems, Sparks, MD). Animal blood required for culturing hemolytic strains was purchased from TCS Bioscience (UK). Haemophilus Test Medium (HTM) for culturing Haemophilus sp. and supplemented GC Broth (BD cat no. 228950, BD Diagnostic Systems, Sparks, MD) for culturing Neisseria sp. were prepared in compliance with Clinical and Laboratory Standards Institute (CLSI) guidelines (M22-A3, CLSI 2004).
Radioisotopes
The following isotopes were purchased from Perkin Elmer (USA): N-acetyl-D-[1-14C] glucosamine (specific activity, 2.15 GBq/mmol; concentration, 7.4 MBq/mL), L-[4,5-3H] leucine (specific activity, 2164.5 GBq/mmol; concentration, 37 MBq/mL), [methyl-3H] thymidine (specific activity, 740 GBq/mmol; concentration, 37 MBq/mL), and [5-3H] uridine (specific activity, 825.1 GBq/mmol; concentration, 37 MBq/mL).
Bacterial strains
Twenty-one bacterial strains were used in this study. Among these 14 were Gram-negative, namely, Escherichia coli ATCC 25922, E. coli K-12 AG100, E. coli AG100A, E. coli D22, Pseudomonas aeruginosa ATCC 27853, P. aeruginosa ATCC 15692, Salmonella typhimurium ATCC 14028, Klebsiella pneumoniae ATCC 33495, Bordetella bronchiseptica ATCC 31437, Neisseria gonorrhoea ATCC 49226, Hemophilus influenzae ATCC 49766, H. influenzae ATCC 49247, Actinobacillus baumannii ATCC 19606, Enterobactor aerogenes ATCC 13048, and seven were Gram-positive, namely, Staphylococcus aureus MTCC 96, S. aureus ATCC BAA1717, Streptococcus pneumoniae ATCC 49619, S. pneumoniae ATCC BAA255, Enterococcus faecalis ATCC 29212, E. faecalis ATCC 51575, and Bacillus subtilis ATCC 27370. They were obtained from American Type Culture Collection (ATCC) (Manassas, VA, USA), CGSC (Yale University, CT), and Microbial Type Culture Collection & Gene Bank (MTCC) (Institute of Microbial Technology, India). All the bacterial strains were stored at −70 °C and were subsequently revived in appropriate agar plates 24 h prior to experimental use.
MIC and MBC
Minimum inhibitory concentration (MIC) was determined by broth micro-dilution technique according to the guidelines of Clinical and Laboratory Standards Institute (MS100-S22, CLSI 2012). In brief, 96-well plates containing serial twofold dilutions of Andro (ranged from 0.001–1 mg/mL) were inoculated with 5 × 105 CFU/mL; the plates were incubated according to CLSI guidelines and the MIC was reported as the lowest antimicrobial concentration yielding no visible growth of bacteria. Experiments were performed twice separately for each strain, and MICs showed ≤1 dilution variance.
After MIC determination, aliquots of 50 μL from those wells where no visible bacterial growth was observed were plated on Mueller-Hinton agar (MHA) plates and were the chemical agent that either totally stops growth or results in a ≥ 99.9% decrease in the initial bacterial population. Bacteriostatic activity has been defined here as a ratio of minimal bactericidal concentration (MBC) to MIC of >4 (Pankey and Sabath 2004).
Macromolecule biosyntheses assays
The effects of Andro on the biosyntheses of macromolecules were assayed as described earlier by Paudel et al. (2012) with slight modifications. Briefly, S. aureus MTCC 96 cells were grown to log phase in CAMHB, harvested at an absorbance of 0.8 at 600 nm, washed with 1 mL of phosphate-buffered saline (PBS, pH 7.3), resuspended (1:10) in fresh media, and allowed to incubate for 90 min with shaking at 35 °C. Cell suspension was then centrifuged, A600 was adjusted to 0.24, and 1 mL cells were added to glass tubes containing different concentrations of Andro (0-, 2-, and 4-fold MICs). [14C] N-acetyl glucosamine (FAC: 100 nmol/L), [3H] leucine (FAC: 50 nmol/L), [3H] thymidine (FAC: 2.5 nmol/L), and [3H] uridine (FAC: 2.5 nmol/L) were used as precursor macromolecules for the bacterial cell wall, protein, DNA, and RNA biosyntheses, respectively. Radioactive precursor molecules were added into the tubes and incubated for 20 min at 35 °C; reaction was then stopped with 5% trichloro acetic acid (TCA). After 30 min of incubation on ice, samples were collected through centrifugation, resuspended in TCA solution, and passed through glass fiber filters using a Cell Harvester device. Radioactivity not incorporated in the precipitate was washed away with ice cold 5% TCA and water. Finally filters were counted using a microbeta counter (Perkin Elmer, USA).
Biofilm assay and quantification
The technique to assess the inhibition of biofilm formation on polystyrene microtiter plates was adapted from a previously described method with minor modifications (Walencka et al. 2006). S. aureus MTCC 96 cells were grown overnight in Trypticase soy broth, washed in fresh media, and diluted to an A600 of 0.05. This culture was grown for 3–4 h to mid-log phase, diluted with Trypticase soy broth containing 0.2% (w/v) glucose and was inoculated into a polystyrene microtiter plate at cell density of 107 CFU/mL. A sub-MIC dilution series (0, 1/2, 1/4, 1/8, and 1/16 MIC, i.e., 0, 50, 25, 12.5, and 6.25 μg/mL, respectively) of Andro was prepared prior to the addition of cells and the plate was incubated at 37 °C for 24 h. After incubation, the wells were washed with PBS to remove the nonadherent cells. The biofilms were stained with 0.2% crystal violet for 15 min and washed again to remove the unbound stain. The plate was allowed to dry at room temperature. Then 33% acetic acid was added, incubated for 15 min and the biofilm biomass was analyzed by measuring of A595.
CLSM analysis of biofilms by DAPI staining
DAPI staining was used to study the differences in quantity and distribution of treated and untreated bacterial population on the substratum (Liu et al. 2016). The biofilms were allowed to grow on a 18 × 18 mm glass cover slip in a six-well microtiter plate in absence and presence of Andro (1/2, 1/4, 1/8, and 1/16 MIC) diluted in Trypticase soy broth containing 0.2% (w/v) glucose. After 24 h of incubation at 37 °C, the cover slips were rinsed thrice with PBS, stained with 2 μg/mL DAPI solution for 15 min in the dark, and then rinsed again with PBS. Imaging was performed with a confocal laser scanning microscope (Leica TCS SP8, Leica Microsystems, Germany) using a 40× oil-immersion lens (excitation/emission wavelength of 360 nm/455 nm). The images were analyzed and 3D imaging reconstructed by using Leica Application SuiteX software.
Statistics
Data on charts represent mean ± standard deviation of representative experiments. One-way analysis of variance (ANOVA) was employed to identify statistical differences among groups. A P value of <0.05 was considered to be statistically significant.
Results and discussion
Antimicobial activity of andrographolide
MIC of Andro was tested in 14 Gram-negative and seven Gram-positive bacterial strains (Table 1). S. aureus MTCC 96 (MSSA) was found to be most susceptible with the MIC value of 100 μg/mL. Methicillin-resistant S. aureus (MRSA) also exhibited sensitivity towards Andro showing MIC of 1 mg/mL. MIC of Andro against Streptococcus pneumoniae R6 (uncapsulated) was also observed at 250 μg/mL whereas capsulated S. pneumoniae showed lesser sensitivity (MIC of 1 mg/mL). Similar observation was also noted in case of vancomycin susceptible Enterococcus faecalis (VSE) ATCC 29212 (MIC of 0.5 mg/mL) compared to vancomycin-resistant E. faecalis (VRE) ATCC 51575 (MIC of >1 mg/mL). Bacillus subtilis was also found to be sensitive against Andro with MIC of 250 μg/mL. Among Gram-negative bacteria, Andro showed considerable antibacterial activities against E. coli AG100A (AcrAB-TolC efflux pump system mutant) as well as E. coli D22 (EnvA1 mutant) with MIC of 125 and 250 μg/mL, respectively. This result suggests that strains with defective LPS (D22) and mutated efflux pump (AG100A) are more permeable to Andro and therefore more susceptible than the wild type. Present observation corroborates with earlier findings that Gram-positive bacteria are more susceptible to Andro in comparison to Gram-negative bacteria (Malahubban et al. 2013). The possible reasons behind the tolerance of Gram-negative bacteria to Andro might be due to the presence of outer membrane and polarity nature of the compound (Silhavy et al. 2010). The antibacterial activity of Andro is in agreement with earlier studies on other labdane type diterpenoids, like 13-epi-sclareol, 6-alpha-malonyloxymanoyl oxide, and copalic acid, which were reported to inhibit Gram-positive bacteria selectively (Mendoza et al. 2002; da Silva et al. 2008; Leandro et al. 2012). As Andro showed highest potency against S. aureus MTCC 96 in this study, further experiments were carried out using this strain.
MBC determination
The result of MBC assay showed a significant decrease in viable cell number of S. aureus MTCC 96 in the presence of Andro (Table 2). Compared to initial inoculum, Andro showed a 3-log10 reduction (99.9%) in number of colony forming units (CFU/mL) at a concentration of 0.5 mg/mL. Therefore, MBC was noted to be 0.5 mg/mL for S. aureus (MTCC 96) with Andro. Bacteriostatic activity has been defined here as a ratio of MBC to MIC of >4 (Pankey and Sabath 2004); thus, it seems Andro acted as a bacteriostatic agent for this strain.
Effect on macromolecule biosynthesis
Prior to select any natural compound having significant antibacterial potency as an attractive alternative for conventional antimicrobial therapy, it is important to find its mechanism of action. Therefore, to find the possible site of action, effects of Andro on cell wall, protein, DNA, and RNA syntheses in S. aureus MTCC 96 were determined by measuring incorporations of isotope-labeled N-acetyl glucosamine, leucine, thymidine, and uridine for the corresponding macromolecules. A summary of the effects of Andro and reference antibiotics on the incorporation of radiolabeled precursors for the syntheses of macromolecules is shown in Fig. 2. All the reference antibiotics selectively inhibited the macromolecule synthesis pathway consistent with their known mechanism of action. Following treatment with Andro (4× MIC), incorporation of thymidine into DNA was ∼31% of the control showing a strong inhibitory effect on DNA synthesis to an extent almost similar to that of reference antibiotic ciprofloxacin (25% incorporation). Inhibition of viral DNA replication by Andro was earlier reported by Chen et al. (2014). Inhibitions of RNA and protein syntheses (∼25 and 36% inhibition, respectively) were also observed in the present study. A plausible explanation of this result is that, by impairing DNA synthesis, the compound might interfere with RNA and protein syntheses resulting in the downstream biosynthetic pathway inhibition. However, cell wall biosynthesis was not hampered.
a–d The effect of Andro on cell wall, protein, DNA, and RNA synthesis measured by incorporation (%) of N-acetyl-D-[1-14C] glucosamine, L-[4,5-3H] leucine, [methyl-3H] thymidine, and [5-3H] uridine, respectively, after 20-min exposure at 2× and 4× MIC in S. aureus MTCC 96. Vancomycin (2× MIC), chloramphenicol (4× MIC), ciprofloxacin (4× MIC), and rifampicin (4× MIC) were used as control for cell wall, protein, DNA, and RNA synthesis inhibitors, respectively. Mean values ± SD for three replicates are illustrated. (*P < 0.05; **P < 0.01; ***P < 0.001)
In the past, many chemical entities showing antimicrobial activity were isolated from natural products with specific target site of action. For example, allicin acted as a specific inhibitor of RNA synthesis of Salmonella typhimurium (Feldberg et al. 1988). While quercetin, one of the most abundant natural flavonoids, was reported to inhibit DNA gyrase of E. coli (Ohemeng et al. 1993), resveratrol and bakuchiol were found to be active as a DNA polymerase inhibitor (Sun et al. 1998). In due course of time, these compounds successfully came out as novel leads for the development of new class of antimicrobial agents. Wu et al. (2008) earlier reported that Andro could recover antibiotic sensitivity in Pseudomonas aeruginosa by downregulating mexAB-oprM efflux pump expression level, suggesting another possible specific target site.
Inhibition of biofilm formation
Biofilms are a leading cause of chronic nosocomial infections, as bacteria grown in biofilm are often resistant to antibiotic levels 10–1000-fold higher than its planktonic form (Mah et al. 2003). Static biofilm quantification assay using different sub-MIC concentrations was performed to evaluate the effect of Andro on biofilm formation by S. aureus MTCC 96. Amount of biofilm decreased gradually with the increase of drug concentrations (Fig. 3a). After 24 h of growth, it was observed that Andro at a concentration of 50 μg/mL reduced ∼45% biofilm development on the polystyrene surface in comparison to the control set. This result clearly suggests that Andro at sub-inhibitory concentrations efficiently inhibited the biofilm formation by S. aureus MTCC 96.
a Quantification of S. aureus MTCC 96 biofilm formed in the microtiter plates in absence and presence of various concentrations (1/2, 1/4, 1/8, and 1/16 MIC, i.e., 50, 25, 12.5, and 6.25 μg/mL, respectively) of Andro after 24 h at 37 °C and stained with crystal violet. Mean values ± SD for three replicates are illustrated (*P < 0.05; **P < 0.01; ***P < 0.001). b Fluorescence images and c 3D representations of S. aureus MTCC 96 biofilm formed after incubation with different concentrations (0, 1/2, 1/4, 1/8, and 1/16 MIC) of Andro as visualized under CLSM. Data shown are from a representative of triplicate experiments. Scale bar = 25 μm
Similar dose-dependent inhibitory effects were also observed while examining the effect of Andro on the biofilm topography and architecture through CLSM studies. Sample treated with 50 μg/mL (1/2 MIC) of Andro showed significantly less viable S. aureus cells compared to control (Fig. 3b). The 3D reconstruction analysis obtained from the CLSM micrographs further supported these results (Fig. 3c). Untreated S. aureus cell mass exhibited a biofilm thickness of 17.29 μm, while biofilm treated with 1/2 MIC of Andro showed a thickness of 9.68 μm after 24 h of exposure. Overall, thickness of the biofilm gradually decreased with increasing concentration. This feature, together with the static biomass inhibition assay, seems that Andro has an important role in inhibition of staphylococcal biofilm formation.
Conclusion
In brief, this study could provide pharmacological confirmation for folklore medicinal practice of A. paniculata against microbial diseases such as wound, upper respiratory tract, and other bacterial infections. The present study showed significant antibacterial activity of Andro against Gram-positive strains including MRSA. Although several studies reported antibacterial activity of Andro (Kumar et al. 2010), however, reports on its activity against methicillin-resistant S. aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) strains are scanty. Beside these, Andro showed considerable antibacterial activities against Gram-negative E. coli AG100A (AcrAB-TolC efflux pump system mutant) as well as E. coli D22 (EnvA1 mutant). Andro at concentrations equivalent to 2× and 4× MIC could primarily target DNA synthesis in S. aureus; this finding with regard to mode of action is novel. However, a secondary effect on protein synthesis was also evident at higher dose (4× MIC). The importance of S. aureus biofilms in bacterial pathogenesis of various chronic human infections and implantation-associated infections is widely known. Therefore, the search for anti-biofilm property of Andro has become a focus of our study. Present study confirms that Andro could prevent S. aureus biofilm formation suggesting a potential strategy for the treatment of biofilm-associated problems. Taking into account the activity of Andro against bacterial strains of clinical importance, it shows a promise in the perspective of new antibacterial drug development, with the possibility of making analogs with improved pharmacological or pharmaceutical properties, and warrants further investigation.
Abbreviations
- Andro:
-
Andrographolide
- MIC:
-
Minimum inhibitory concentration
- MBC:
-
Minimal bactericidal concentration
- CAMHB:
-
Cation-adjusted Mueller-Hinton broth
- DAPI:
-
4′, 6-Diamidino-2-phenylindole
- CLSM:
-
Confocal laser scanning microscopy
References
Ahmad N, Amir MK, Ayaz S, Ahmad S, Jan A, Ashraf JS, Zuhra FT (2012) Antimicrobial profile of the selected medicinal plants. Int J Chem Life Sci 1(2):1039–1041
Arifullah M, Namsa ND, Mandal M, Chiruvella KK, Vikrama P, Gopal GR (2013) Evaluation of anti-bacterial and anti-oxidant potential of andrographolide and echiodinin isolated from callus culture of Andrographis paniculata Nees. Asian Pac J Trop Biomed 3(8):604–610
Banerjee M, Chattopadhyay S, Choudhuri T, Bera R, Kumar S, Chakraborty B, Mukherjee SK (2016) Cytotoxicity and cell cycle arrest induced by andrographolide lead to programmed cell death of MDA-MB-231 breast cancer cell line. J Biomed Sci. doi:10.1186/s12929-016-0257-0
Behal V (2001) Nontraditional microbial bioactive metabolites. Folia Microbiol 46:363–370
Cáceres DD, Hancke JL, Burgos RA, Wikman GK (1997) Prevention of common colds with Andrographis paniculata dried extract: a pilot double blind trial. Phytomedicine 4(2):101–104
Calabrese C, Berman SH, Babish JG, Ma X, Shinto L, Dorr M, Wells K, Wenner GA, Standish LJ (2000) A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother Res 14:333–338
Chen H, Ma YB, Huang XY, Geng CA, Zhao Y, Wang LJ, Guo RH, Liang WJ, Zhang XM, Chen JJ (2014) Synthesis, structure–activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents. Bioorg Med Chem Lett 24:2353–2359
Chua LS (2014) Review on liver inflammation and anti-inflammatory activity of Andrographis paniculata for hepatoprotection. Phytother Res 28(11):1589–1598
CLSI (2004) Quality control for commercially prepared microbiological culture media; Approved Standard, 3rd edn. CLSI document M22-A3. Clinical and Laboratory Standards Institute, Wayne, PA
CLSI (2012) Performance standards for antimicrobial susceptibility testing, 22nd informational supplement. CLSI document MS100-S22. Clinical and Laboratory Standards Institute, Wayne, PA
da Silva LL, Nascimento MS, Cavalheiro AJ, Silva DH, Castro-Gamboa I, Furlan M, Bolzani Vda S (2008) Antibacterial activity of labdane diterpenoids from Stemodia foliosa. J Nat Prod 71(7):1291–1293
Feldberg RS, Chang SC, Kotik AN, Nadler M, Neuwirth Z, Sundstrom DC, Thompson NH (1988) In vitro mechanism of inhibition of bacterial cell growth by allicin. Antimicrob Agents Chemother 32(12):1763–1768
Gabrielian ES, Shukarian AK, Goukasova GI, Chandanian GL, Panossian AG (2002) A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine 9:589–597
Gowthami M, Selvi ST, Kumar GS, Panneerselvam A (2012) Phytochemical analysis and antibacterial properties of leaf extract of Azima tetracantha (Lam.). Asian J Plant Sci Res 2:110–114
Kalimuthu KSV, Senthilkumar R (2010) Antimicrobial activity of the biodiesel plant Jatropha curcas L. Int J Pharm Bio Sci 1:1–5
Kumar A, Naidu M, Rao KG (2010) In vitro antibacterial activity in the extracts of Andrographis paniculata Burm. F Int J Pharm Tech Res 2(2):1383–1385
Leandro LM, de Sousa VF, Barbosa PCS, Neves JKO, da Silva JA, da Veiga-Junior VF (2012) Chemistry and biological activities of terpenoids from copaiba (Copaifera spp.) oleoresins. Molecules 17:3866–3889
Liu R, Memarzadeh K, Chang B, Zhang Y, Ma Z, Allaker RP, Ren L, Yang K (2016) Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci Rep 6:29985
Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GA (2003) A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426(6964):306–310
Malahubban M, Alimon AR, Sazili AQ, Fakurazi S, Zakry FA (2013) Phytochemical analysis of Andrographis paniculata and Orthosiphon stamineus leaf extracts for their antibacterial and antioxidant potential. Trop Biomed 30:467–480
Mendoza L, Tapia L, Wilkens M, Urzúa A (2002) Antibacterial activity of 13-epi-sclareol, a labdane type diterpene isolated from Pseudognaphalium heterotrichium and P. cheiranthifolium (Asteraceae). Bol Soc Chil Quím 47:91–98
Mishra SK, Tripathi S, Shukla A, Oh SH, Kim HM (2015) Andrographolide and analogues in cancer prevention. Front Biosci (Elite Ed) 7:255–266
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335
Ohemeng KA, Schwender CF, Fu KP, Barrett JF (1993) DNA gyrase inhibitory and antibacterial activity of some flavones. Bioorg Med Chem Lett 3:225–230
Pankey GA, Sabath LD (2004) Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis 38:864–870
Paudel A, Hamamoto H, Kobayashi Y, Yokoshima S, Fukuyama T, Sekimizu K (2012) Identification of novel deoxyribofuranosyl indole antimicrobial agents. J Antibiot (Tokyo) 65(2):53–57
Silhavy TJ, Kahne D, Walker S (2010) The bacterial cell envelope. Cold Spring Harb Perspect Biol 2:a000414
Sukesh K, Shafi ST, Densingh J (2011) Phytochemical investigation and antibacterial activity of Gymnema sylvestre and Andrographis paniculata from western ghats. Int J Phytomedicine 3:254–260
Sule A, Ahmed QU, Samah OA, Omar MN (2010) Screening for antibacterial activity of Andrographis paniculata used in Malaysian folkloric medicine: a possible alternative for the treatment of skin infections. Ethnobot Leaflets 14:445–456
Sun NJ, Woo SH, Cassady JM, Snapka RM (1998) DNA polymerase and topoisomerase II inhibitors from Psoralea corylifolia. J Nat Prod 61(3):362–366
Tanwar J, Das S, Fatima Z, Hameed S (2014) Multidrug resistance: an emerging crisis. Interdiscip Perspect Infect Dis 2014:541340. doi:10.1155/2014/541340
Thakur AK, Chatterjee SS, Kumar V (2015) Adaptogenic potential of andrographolide: an active principle of the king of bitters (Andrographis paniculata). J Tradit Complement Med 5:42–50
Trivedi NP, Rawal UM, Patel BP (2007) Hepatoprotective effect of andrographolide against hexachlorocyclohexane-induced oxidative injury. Integr Cancer Ther 6:271–280
Walencka E, Sadowska B, Rózalska S, Hryniewicz W, Rózalska B (2006) Staphylococcus aureus biofilm as a target for single or repeated doses of oxacillin, vancomycin, linezolid and/or lysostaphin. Folia Microbiol 51(5):381–386
WHO (2007) WHO monographs on selected medicinal plants. Volume 3, ISBN 978 92 4 154702 4
Wu CM, Cao JL, Zheng MH, Ou Y, Zhang L, Zhu XQ, Song JX (2008) Effect and mechanism of andrographolide on the recovery of Pseudomonas aeruginosa susceptibility to several antibiotics. J Int Med Res 36:178–186
Yoopan N, Thisoda P, Rangkadilok N, Sahasitiwat S, Pholphana N, Ruchirawat S, Satayavivad J (2007) Cardiovascular effects of 14-deoxy-11,12 didehydroandrographolide and Andrographis paniculata extracts. Planta Med 73:503–511
Zahra N, Jahan N, Nosheen S, Rehman K (2011) Antimicrobial activity of aqueous, ethanolic extracts and crude extracted phytoconstituents of Nigella sativa seeds. Biosci Res 8:19–25
Acknowledgements
Authors acknowledge the supports received from University of Kalyani, Kalyani, India, and TCG Life Science Ltd., Kolkata, India. We also acknowledge Mr. Kunal Bhattacharyya for his technical assistance during the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Banerjee, M., Parai, D., Chattopadhyay, S. et al. Andrographolide: antibacterial activity against common bacteria of human health concern and possible mechanism of action. Folia Microbiol 62, 237–244 (2017). https://doi.org/10.1007/s12223-017-0496-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-017-0496-9