Abstract
The aim was to determine which specific regions of the visible light spectrum were responsible for the induction or inhibition of laccase in Pycnoporus sanguineus. Cultures were exposed to various bandwidth lights: blue (460 nm), green (525 nm), white (a combination of 460 and 560 nm), red (660 nm), and darkness. The results indicate that short wavelengths strongly inhibit the production of laccase: green (3.76 ± 1.12 U/L), blue (1.94 ± 0.36 U/L), and white (1.05 ± 0.21 U/L) in proportions of 85.8, 92.6, and 96.0 %, respectively; whereas long wavelengths inhibit laccase production only partially i.e., red light (14.05 ± 4.79 U/L) in a proportion of 46.8 %. Maximum activity was induced in absence of visible light (30 °C, darkness), i.e., 30.76 ± 4.0 U/L. It is concluded that the production of laccase in P. sanguineus responds to light stimuli [measured as wavelengths and lx] and that it does so inversely. This can be explained as an ecological mechanism of environmental recognition, given that P. sanguineus develops inside lignocellulose structures in conditions of darkness. The presence of short wavelength light (460–510 nm) would indicate that the organism finds itself in an external environment, unprovided of lignin, and that it is therefore unnecessary to secrete laccase. This possible new regulation in the laccase production in P. sanguineus has important biotechnological implications, for it would be possible to control the production of laccase using light stimuli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is known that the production of some extracellular enzymes involved in the degradation of lignocellulose by certain fungi is differentially affected by the various wavelengths of the visible light spectrum (Schmoll et al. 2005; 2012). However, in spite of the fact that light has been associated to the regulatory processes in the production of exoenzymes, little is known about the mechanisms of the perception of light and the transduction of signals ( Rodríguez-Romero et al. 2010). From the adaptive viewpoint, changes in the detection of light furnishes fungi with information on orientation as well as exposition to external environments (Herrera-Estrella and Horwitz 2007; Corrochano 2011), allowing them to adjust their metabolism to the prevailing conditions for optimal use of the resources at hand. Pycnoporus sanguineus (Polyporaceae) is a basidiomycete fungus that, as a saprobe, produces extracellular enzymes that allow it to decompose organic polymers and consume its monomers. These fungi are of special interest because they produce enzymes of the laccase group that decompose mainly lignin. Laccases are enzymes with a wide specificity of substratum involved in the white decay of wood (lignin oxidation), and the biotechnological knowledge of their production mechanisms has important industrial and environmental applications. Some of their applications are the pretreatment of wood for biofuel production and the recycling or transformation of toxic waste and other pollutants such as polycyclic aromatic hydrocarbons, pesticides, and industrial dyes (Kunamneni et al. 2007; Jong-Rok and Yoon-Seok 2013; Meng-Juan et al. 2013). It is known that the production of laccases is affected by chemical signals; for example, a high abundance of simple carbonated forms (mainly glucose) leads to a genetic transcriptional inhibition of laccase. This is attributed to the carbon catabolic repression transcription factors (CreaA; Janusz et al. 2012), which respond to an epigenetic regulation via G/AMPc/proteins kinase, regulating gene clusters related to secondary metabolism. Such transcription regulators have been characterized in the laccase gene (LAC1)-promoting region of basidiomycete fungi (Janusz et al. 2013; Xiao et al. 2006).
However, in spite of the fact that light has been associated with regulation in the production of exoenzymes and with various bioprocesses in fungi such as sporulation and regulation of circadian rhythms (Saha et al. 2008), practically nothing is known of its effects on the regulation in the laccase production of P. sanguineus. This study sets out to identify a possible regulation in the production of laccase by stimuli from changes in the visible light spectrum surrounding P. sanguineus. We therefore set out to find evidence of enzymatic regulation, measured as inhibition, resulting from wavelength changes within the visible light spectrum. This may open up new avenues for a better understanding of the interaction processes between organisms and their environment, as well as those regulation mechanisms of extracellular enzymes that are mediated by visible light.
Materials and methods
Strain and inoculum
The strain of P. sanguineus used was isolated from a wild mushroom found in the cane fields of Jalcomulco, Veracruz, México (19°20′00″N, 96°46′00″W), registered as F. Ramírez-Guillén 932 in the Herbarium Xal (INECOL, A.C.) and identified by ITS 1, 4 amplification (GenBank accession KR013138). The strain was preserved in a potato dextrose agar medium (PDA) at 4 °C and reactivated in Petri dishes with PDA at 30 °C for 7 days prior to the experiment. As inoculum, during the experimental phase, agar squares 0.25 cm2 in size with reactivated mycelium were used.
Experimental design
A single-factor design was used to determine the effect of exposition to different types of visible light on the production of laccase in P. sanguineus. The mushroom was exposed to different light wavelengths within the visible light spectrum: blue (460 nm), green (525 nm), white (combination of 460 and 560 nm), and red (660 nm), using light-emitting diodes (LEDs). As a means of control, cultivars kept in conditions of darkness were used. Illuminance (lux = lm/m2) of each treatment, replicated four times, was measured with a lux meter (Fisher Scientific), corresponding to 22.66 lx (for blue light), 183.5 lx (red), 675 lx (green), and 750 lx (white). In order to rule out a possible adverse effect of light on the growth of P. sanguineus, which might affect the production of laccase, the glucose consumption kinetics and the final biomass production were also quantified. Samples for the determination of laccase and glucose were taken periodically over 20 days.
At the end of the experiment, the significant effects of the treatments were determined by the one-way variance analysis protocol [ANOVA; (Zar 1999)] and the Fisher LSD (Least significant difference) post hoc test. Also, in order to know if the laccase activity was affected by light wavelength (nm), and its correspondent lx values, correlations and linear regression analysis were done. The analyses were carried out on the STATISTICA 7 software (StatSoft 2004).
Cultivar medium and conditions
The experimental units consisted of glass containers with 150 mL of minimal medium (Kyi 2011), which was composed of 5 % of a salt solution base composed of 6 g of NaNO3, 0.52 g of KCl, 0.52 g of MgSO4, and 0.82 g of KH2PO4 per liter, and 0.02 % of a Hutner solution of trace elements composed of 0.22 g of ZnSO4, 0.11 g of H3BO3, 0.05 g of MnCl2, 0.05 g of FeSO4, 0.016 g of CoCl2, 0.016 g of CuSO4, and 0.011 g of (NH4) 6MO7O24 per 100 mL and 1 g/L of glucose as a source of carbon. Cultivars were kept steady and under constant temperature conditions (30 °C) in an environmental chamber (Binder, Germany).
Analytic methods
Laccase activities were estimated using syringaldazine as a substrate, according to Leonowicz and Grzywnowicz (1981) and modified by Criquet (1999). To 990 μL of cultivar sample, 10 μL of a solution of syringaldazine at 5 mM were added. The oxidation kinetics from syringaldazine to quinone was followed at 525 nm (ε = 65,000 M–1 cm–1) at 37 °C. The activity was expressed as μmol of quinone formed from syringaldazine per min (U) per one L of cultivar (U.L–1). The consumption of sugars was determined through the spectrophotometric method of dinitrosalicylic acid (DNS; Miller, 1959); 500 μL of sample was mixed with 500 μL of DNS reagent, boiled for 5 min, and cooled in an ice bath for 10 min. Subsequently, the mixture was diluted with 4 mL of distilled H2O and quantified at 540 nm in a spectrophotometer. The absorbance was transformed to glucose (g/L) through calibration according to a wave pattern. The final biomass produced at the end of the experiment was recovered by vacuum filtration, dried at 60 °C for 48 h, and gravimetrically quantified.
Results
ANOVA linear model indicated that the variable wavelength had significant effects (P = 0.027) on laccase activity.
The control treatments (darkness) showed the maximum laccase activity (30.76 ± 4.00 U/L) while the treatments exposed to different wavelengths of the visible light spectrum inhibited the laccase enzyme as follows: moderately (red light, 183.5 lx; 660 nm) and strongly (green and blue 525–460 nm; 675 and 22.66 lx, respectively) (P < 0.001; F = 17.89). Average values \( \left(\overline{x}\right) \) and standard errors (SE) showed that red, green, blue, and white light wavelengths gradually inhibited laccase activity at 46.8, 85.8, 92.6, and 96.0 %, respectively (red = 14.05 ± 4.79 U/L; green = 3.76 ± 1.12 U/L; blue = 1.94 ± 0.36 U/L; and white = 1.05 ± 0.21 U/L; Fig. 1). The treatment in darkness was used as a reference (laccase activity = 30.76 ± 4.00 U/L), taken as 100 % of enzyme activity. According to the Fisher LSD test, control and red light treatments were different to all the others (P < 0.006 and P < 0.01, respectively), while green, blue, and white treatments did not show significant differences among them (P > 0.05; see Fig. 1 and Table 1).
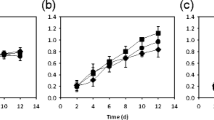
The glucose consumption (Fig. 2) and the final biomass production did not show significant differences from treatment to treatment (P = 0.97, F = 0.108; P = 0.86, F = 0.316, respectively; Table 1). Likewise, positive and significant correlations (r = 0.88; P < 0.05) were observed between laccase activity with light wavelength (Fig. 3a), while negative and significant correlations (r = −0.56; P < 0.05) were observed between laccase activity and illuminance (Fig. 3b).
Kinetics uptake of glucose dissolved in the culture medium and recorded during the experiment. The data show approximations of glucose uptake by spectrophotometer absorbance of the colorimetric reaction sugars with dinitrosalicylic acid. The points indicate the mean and bar the standard error of three replicates
Discussion
Given that glucose consumption and total biomass production were not different from treatment to treatment, the inhibition of mycelia growth or the capacity to take glucose from the environment in P. Sanguineus as a result to exposure to visible light spectrum is ruled out. The production of laccase obtained in the experiment can thus be attributed to wavelength changes (related to lux values of each treatment) within the visible light spectrum, as ANOVA’s results indicated, and not to changes in the mushroom’s growth or a decrease in the consumption of glucose.
Due to the blue light showing the second lowest laccase activity, we thought that the inhibition of laccase is ascribed mainly to the effect of wavelength recognition, as was reported for cellulase enzymes (Schmoll et al. 2012; Gyalai-Korpos et al. 2010). In plants, illuminance has important effects on the production of some enzymes, mainly those involved in the metabolism of active oxygen species derived from photosynthetic activity [i.e., superoxide dismutase, ascorbate dismutase, and monodehydroascorbate reductase (Mishra et al. 1993)], but there are not studies that report the influence of this light property on the production of extracellular enzymes of fungi.
Extracellular enzymes of fungi that modify their production at different light wavelengths are known, like the induction of glucoamylase production at blue light exposure (Aspergillus niger, Zhu and Wang 2005) and cellulase at the same visible light spectrum (Herrera-Estrella and Horwitz, 2007). In this study, we report that laccase, the main lignin-degrading enzyme in basiodiomycetes, is also light-regulated in P. sanguineus.
Our results showed a greater laccase inhibition with short wavelengths (blue light, 460 nm, 22.66 lx) than with long ones (red light, 660 nm, 183.5 lx). This suggests that extracellular laccase production could be regulated by the photosystem WC (white collar system) present in ascomycetes, zygomicetes, and basidiomycetes ( Rodríguez-Romero et al. 2010). The WC detects blue light and participates in the regulation of genetic expression in fungi (e.g., cellulases) through DNA binding proteins (zinc fingers proteins; Ballario et al. 1996; Ballario and Macino 1997). Moreover, it has been reported that blue light inhibits the production of some intracellular oxidoreductases such as isoamyl alcohol oxidase and thioredoxin peroxidase ( Rosales-Saavedra et al. 2006), which could suggest on the influence of this system in the regulation of laccase in P. sanguineus, given the strong inhibition of this enzyme (92.6 %) with blue light (460 nm, 22.66 lx) in this study.
Green light also significantly inhibited laccase production (85.8 %). This kind of light has been associated to important implications in fungi metabolism e.g., pigment and biomass inhibition (Velmurugan et al. 2010) and reduction in the germination of spores in phytopathogenic fungi (Stevenson and Pennypacker 1988; Buck et al. 2010). However, as far as we know, this is the first report on inhibition of extracellular enzymatic activity by green light in a basidiomycete fungus. Besides, contrary to what the literature reports (Velmurugan et al. 2010), in this study, no adverse effects were observed on biomass production or glucose consumption when P. sanguineus was exposed to this wavelength (green light, 525 nm).
Though studies on the physiological effects of green light on fungi are many, the detection mechanisms of these organisms have not been fully understood and nothing is known about the presence of a specific photoreceptor for this wavelength (525 nm). It has been suggested that opsins could act as such photoreceptors (Biezke et al. 1999; Rodríguez-Romero et al. 2010); however, this hypothesis needs testing.
Red light partially inhibited laccase production. In Aspergillus fungi, this wavelength (red to distant red) is detected by FphA phytochromes, which intervene in physiological processes such as inhibition of spore germination (Röhrig et al. 2013) and the repression of sexual development (Blumenstein et al. 2005) in Aspergillus nidulans. However, red light has not been associated to the induction/inhibition of enzymes involved in degradation of lignocellulose. Further studies will allow us to prove whether the partial inhibition of laccase observed here is carried out by the FphA phytochrome.
The results lead to a conclusion that inhibition of laccase production within the visible light spectrum responds to an illuminance gradient where low illuminance states [long wavelengths; red (660 nm; 183.5 lx)] partially inhibit enzyme production, whereas high illuminance states [short wavelengths; green (525 nm) and blue (460 nm), corresponding to 675 lx and 22.66 lx, respectively] strongly inhibit it. Likewise, white light (a combination of 460 and 560 nm, corresponding to 750 lx) almost completely inhibits laccase activity, whereas the highest activity values were obtained under conditions of darkness (0 lx).
The ecological implications of this finding contribute to the understanding of the relationship between fungi and their environment. Due to the fact that P. sanguineus is a fungus whose mycelium develops within the lignocellulose (e.g., decaying wood) and that it forms its reproductive structures externally, it can be said that the presence or absence of light is an important source of information for an energy-saving metabolic regulation, as has been proposed before (Tisch and Schmoll 2010). Following this avenue of thought, we can hypothesize that within the lignocellulose structure (in conditions of darkness), the fungus must produce laccases in order to degrade lignin and be able to obtain energy from polymers such as cellulose and hemicellulose. The presence of light would indicate that the fungus finds itself in an external environment, unprovided of lignocellulose, and therefore interprets it as a regulation signal that laccase production is unnecessary.
The inhibition of laccase production, specifically in the presence of blue light, could result from the fact that lignin (a natural laccase substrate) absorbs short light wavelengths, mainly between 200 and 230 nm and 260 to 280 nm, corresponding to ultraviolet (UV) light (Jahan and Mun 2007), and that the absorbance of products derived from the oxidation of lignocellulose (furans, pyrans) is close to the visible light spectrum (blue, 320 nm). Therefore, the absence of these wavelengths, due to their absorption by organic polymers, in combination with the known chemical inductors (phenolic), would indicate P. sanguineus’ location in a potentially lignin-rich place, and that laccase production would bring a positive trade-off, since it would mean access to energy-rich polymers (cellulose, hemicellulose). Conversely, the presence of short wavelengths would indicate that there is no need to produce laccase.
Further molecular studies on the expression of the gen LAC in real time in cultivars exposed to different wavelengths of the visible light spectrum could bring suitable information that enrich the data showed here. We think it is important to experiment with the inhibition of specialized trans-membrane photoreceptors in photonic detection (rhodopsins, opsins, etc.) so as to identify those that are involved in the transduction of inhibitory signals in laccase production in P. sanguineus and other basidiomycetes.
We conclude that extracellular laccase production in P. sanguineus responds to light stimuli, whereby short wavelengths within the visible light spectrum (460–525 nm) are strongly inhibited; long wavelengths (660 nm) are inhibited only partially, and darkness activates it.
References
Ballario P, Macino G (1997) White collar proteins: PAS sing the light signal in Neurospora crassa. Trends Microbiol 5:458–462
Ballario P, Vittorioso P, Magrelli A, Talora C et al (1996) White collar-1, a central regulator of blue light responses in Neurospora, is a zinc finger protein. EMBO J 15(7):1650–1657
Biezke JA, Braun EL, Bean LE, Kang S et al (1999) The nop-1 gene of Neurospora crassa encodes a seven transmembrane helix retinal-binding protein homologous to archeal rhodopsins. Proc Natl Acad Sci U S A 96:8034–8039
Blumenstein A, Vienken K, Tasler R, Purschwitz J et al (2005) The Aspergillus nidulans phytocrome FphA represses sexual development in red light. Curr Biol 15:1833–1838
Buck JW, Dong W, Mueller DS (2010) Effect of light exposure on in vitro germination and germ tube growth of eight species of rust fungi. Mycologia 102(5):1134–1140
Corrochano LM (2011) Fungal photobiology: a synopsis. IMA Fungus 2:25–28
Criquet S (1999) La litière de chênevert (Quercus ilex L.). Aspects méthodologiques, microorganismes, enzymes et facteurs environnementaux. Paul Cézanne Aix-Marseille III University, Marseille, France
Gyalai-Korpos M, Nagy G, Mareczky Z, Schuster A, Réczey K, Schmoll M (2010) Relevance of the light signaling machinery for cellulase expression in trichoderma reesei (hypocrea jecorina). BMC Res Notes 3:330
Herrera-Estrella A, Horwitz BA (2007) Looking through the eyes of fungi: molecular genetics of photoreception. Mol Microbiol 64:5–15
Jahan MS, Mun SP (2007) Characteristics of dioxanelignins isolated at different ages of Nalita wood (Tremaorientalis). J Wood Chem Technol 27(2):83–98
Janusz G, Kucharzyk KH, Pawlik A, Staszczak M, Paszczynski AJ (2013) Fungal laccase, manganese peroxidase and lignin peroxidase: gene expression and regulation. Enzyme Microb Technol 52:1–12
Janusz G, Mazur A, Checinsks A, Malek W et al (2012) Cloning and characterization of a laccase gene from biotechnologically important basidiomycete Carrena unicolor. J Fac Agr Kyushu U 57:41–49
Jong-Rok J, Yoon-Seok C (2013) Laccase-mediated oxidation of small organics: bifunctional roles for versatile applications. Trends Biotechnol 31(6):335–341
Kunamneni A, Ballesteros A, Plou FJ, Alcalde M (2007) Fungal laccase—a versatile enzyme for biotechnological applications. In: Méndez-Vilas A (ed) Communicating current research and educational topics and trends in applied microbiology. Microbiology series vol 1. Formatex, Badajoz, Spain, pp 233–245
Kyi TS (2011) Screening of potential lignin-degrading microorganisms and evaluating their optimal enzyme producing culture conditions. Master in Science thesis, Chalmers University of Technology, Göteborg, Sweden
Leonowicz A, Grzywnowicz K (1981) Quantitative estimation of laccase forms in some white rot-fungi using syringaldazine as a substrate. Enzym Microb Technol 3(1):55–58
Meng-Juan Z, Fang D, Guo-Qing Z, He-Xiang W, Tzi-Bin N (2013) Purification a laccase exhibiting dye decolorizing ability from an edible mushroom Russula virescens. Int. Biodeterioration Biodegrad 82:33–39
Miller GL (1959) Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Mishra NP, Mishra RK, Singhal GS (1993) Changes in the activities of anti-oxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol 102:903–910
Rodríguez-Romero J, Hedtke M, Kastner C, Müller S, Fischer R (2010) Fungi, hidden in soil or up in the air: light makes a difference. Annu Rev Microbiol 64:585–610
Röhrig J, Kastner C, Fischer R (2013) Light inhibits spore germination through phytocrome in Aspergillus nidulans. Curr Genet 59:55–62
Rosales-Saavedra T, Esquivel-Naranjo EU, Casas-Flores S, Martínez-Hernández P et al (2006) Novel light-regulated genes in Trichoderma atroviridae: a dissection by cDNA microarrays. Microbiol 152:3305–3317
Saha A, Mandal P, Dasgupta S, Saha D (2008) Influence of culture media and environmental factors on mycelia growth and sporulation of Lasiodiplodia theobromae (Pat.) Griffon and Maubl. J Environ Biol 29(3):407–410
Schmoll M, Franchi L, Kubicek CP (2005) Envoy, a PAS/LOV domain protein of Hypocreaje corina (Anamorph Trichoderma reesei), modulates cellulase gene transcription in response to light. Eukaryot Cell 4(12):1998–2007
Schmoll M, Tian C, Sun J, Tisch D, Glass NL (2012) Unravelling the molecular basis for light modulated cellulase gene expression—the role of photoreceptors in Neurosporacrassa. BMC Genomics 13:127
StatSoft Inc. (2004) STATISTICA (Data analysis software system). Version 7
Stevenson RE, Pennypacker SP (1988) Effect of radiation, temperature, and moisture on conidial germination of Alternariasolanum. Phytopathology 78(7):926–930
Tisch D, Schmoll M (2010) Light regulation of metabolic pathways in fungi. Appl Microbiol Biotechnol 85:1259–1277. doi:10.1007/s00253-009-2320-1
Velmurugan P, Hoon Y, Kulandaisamy C, Lakshmanaperumalsamy P et al (2010) Effect of light on growth, intracellular and extracellular pigment production by five pigment-producing filamentous fungi in synthetic medium. J Biosci Bioeng 109(4):346–50
Xiao YZ, Hong YZ, Li JF, Hang J et al (2006) Cloning of novel laccase isozyme genes from Trametes sp. AH28-2 analysis of their differential expression. Appl Microbiol Biotechnol 71(4):493–501
Zar JH (1999) Biostatistical Analysis, Prentice Hall (Ed.), New Jersey, EE.UU.
Zhu JC, Wang XJ (2005) Effect of blue light on conidiation development and glucoamylase enhancement in Aspergillus niger. Wei Sheng Wu Xue Bao 45(2):275–8
Acknowledgments
The authors are grateful to the National Council of Science and Technology (Conacyt) of Mexico, for the doctoral scholarship (261027) of Hernández Christian, to the PROMEP program of the National Education Secretary (SEP), and to ECOS-ANUIES-CONACYT Program through the ECOS project M13A02, for financial support. The authors are grateful to Biologist Ramírez-Guillén F., from INECOL A.C., Xalapa, Mexico, for the taxonomical identification of the fungus. The authors are also grateful to the Institute of Biotechnology and Applied Ecology (INBIOTECA), and to the UVCA324 research group from Universidad Veracruzana, Mexico, for facilities support.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hernández, C.A., Perroni, Y., Pérez, J.A.G. et al. Light-induced inhibition of laccase in Pycnoporus sanguineus . Folia Microbiol 61, 137–142 (2016). https://doi.org/10.1007/s12223-015-0418-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-015-0418-7