Abstract
Cell cultures of Arabidopsis thaliana (A.t.) respond to changes in the gravitational field strength with fluctuations of the amount of cytosolic calcium (Ca2+). In parabolic flight experiments, where hyper- and μg phases follow each other, μg clearly increased Ca2+, while hyper-g caused a slight reduction. Since the latter observation had not been reported before, we studied this effect in more detail. Using a special centrifuge for heavy items (ZARM, Bremen, Germany), we determined the hyper-g-dependent intracellular Ca2+ level with transgenic cell lines expressing the Ca2+ sensor, cameleon. This sensor exhibits a shift in fluorescence from 480 to 530 nm in response to Ca2+ binding. The data show a drop in the intracellular Ca2+ concentration with a threshold gravity of around 3 g. This is above hypergravity levels achieved during parabolic flights (1.8 g). The use of mutants with different sub-cellular targets of cameleon expression (nucleus, tonoplast, plasma membrane) gave the same results, i.e. Ca2+ is obviously exported from several intracellular compartments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gravity-sensing is important for plant growth and survival, directing both root and shoot orientation. Two main theories exist how plants are able to sense gravitational changes. The primary one is the statolith theory in combination with the Cholodny-Went theory or rather the auxin fountain model (Went and Thimann 1937; Haberlandt 1900; Nemec 1900; Darwin 1903). Here, specialized organs (like shoot and root) or organelles (e.g. stathocytes) act as gravity sensors. However, also non-vascular plants (e.g. Chara rhizoids) as well as unicellular organisms like Euglena, Paramecium or Loxodes are capable of perceiving alterations of the regular gravitational force (Hemmersbach et al. 1999; Hemmersbach and Häder 1999; Limbach et al. 2005; Sievers and Schröter 1971). A second theory considers the plant cytoskeleton as a major receiver as well as transducer of mechanical signals and functions as a tensegrity sensor (Wayne et al. 1992; Baluska and Hasenstein 1997; Nick 2011). In parabolic flight experiments we could show that even single cells of the higher plant Arabidopsis thaliana (A.t.) respond instantaneously to gravity changes with altered gene and protein expression, as well as protein modulation, an altered redox state, and changes in the pools sizes of the second messengers, Ca2+ and hydrogen peroxide (Maier et al. 2003; Martzivanou et al. 2006; Barjaktarović et al. 2007; Barjaktarović et al. 2009; Hausmann et al. 2014; Fengler et al. 2015). Especially the significant rise of the intracellular Ca2+ concentration during the microgravity phase is in support of the tensegrity model: the reduced gravitational force facilitates probably the opening of stretch-actived Ca2+ channels, which are directly linked to the cytoskeleton via microfilaments (Perbal and Driss-Ecole 2003). While an increase in intracellular Ca is generally linked to stress responses, a decrease under hyper-g has not been reported yet.
In the present study we analyzed therefore spatial and temporal Ca2+ transients in A.t. single cells regarding to short-term elevated g-levels (1-5g). Additionally, we used sets of transgenic A.t. cell cultures expressing the YC3.6 Ca2+ sensor in different sub-cellular compartments (cytosol, nucleus, tonoplast) to monitor the distribution of Ca2+ ions.
Material and Methods
A.t. Cell Cultures
Wildtype (WT) and transgenic YC3.6 cell cultures of Arabidopsis thaliana (c.v. Columbia) were generated as described previously (Barjaktarović et al. 2007; Neef et al. 2011; Hausmann et al. 2014). Seeds from A.t. plants expressing the Ca2+ sensor YC3.6, (NES, NLS, and TP12)(Miyawaki et al. 1999; Krebs et al. 2012; Behera et al. 2013) were kindly provided by K. Schumacher (University of Heidelberg).
Chemicals
Lanthanum chloride (LaCl 3), and ethylenglycol-bis(aminoethylether)-N,N,N’,N’-tetraacetic acid (EGTA) were obtained from SigmaAldrich (St.Louis, USA). The substances were dissolved in sterile double distilled water. Twenty μl of a 100 μM stock solution were added to the cell cultures approximately 1 h before centrifug- ation.
Hyper-G-Centrifuge
Centrifugation experiments were performed at ZARM (Centre of Applied Space Technology and Microgravity, Bremen, Germany) using the hyper-g-centrifuge (for details regarding physical basics and technical data of the hyperfuge please refer to: www.zarm.uni-bremen.de).
Microplate-Fluorometer
Calcium (Ca2+) changes in transgenic plant cell cultures were monitored with a filter-based microplate fluorometer (POLARstar OPTIMA, BMG LABTECH GmbH, Offenburg, Germany) equipped with a simultaneous dual emission detection system. Wildtype (WT) cell cultures served as controls. The fluorometer was fixed with straps on top of the capsule platform, whereas the laptop control unit was fixed at the center of the centrifuge (Fig. 1). Samples were exposed in 96-well microtiter plates (Nunc, Germany) containing 200 μl solid Murashige and Skoog basal medium which contained 1.6 % Agar (SigmaAldrich, St.Louis, USA) per well. About 100 mg YC3.6 or WT calli respectively, were transferred into each well. The plate was sealed with a gas permeable foil (Dynatech microtiter system, Denkendorf, Germany) in order to prevent spilling and evaporation. Data were recorded in real-time during the entire experiment via OPTIMA—data analysis software (BMG LABTECH GmbH, Offenburg, Germany). Fluorescence ratios (535 nm / 480 nm) were calculated via MARS software (BMG LABTECH GmbH, Offenburg, Germany).
Results and Discussion
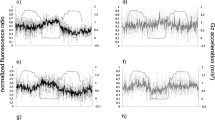
Ca2+ is an important second messenger in plants, involved in almost all physiological responses (Dodd et al. 2010; Sanders et al. 2002). Ca2+ ions specifically encode a large variety of distinct signals by using spatial and temporal Ca2+ spikes as well as by the frequency and amplitude of Ca2+ oscillations (McAinsh and Pittman 2009; Kim et al. 2009; Sanders et al. 2002). This so called Ca2+ signature operates like a characteristic fingerprint to the stimulus that provoked this reaction. Changes in the gravity vector can also induce Ca2+ transients which can be unique (Plieth and Trewavas 2002; Toyota et al. 2008). However until now, only increasing concentrations of the intracellular Ca2+ level are reported. In contrast, Fig. 2 shows a clear decrease of cytosolic Ca2+ in transgenic YC3.6 A.t. cells concurrent with elevated g-levels. Both 1 g ground control and WT cells showed no Ca2+ responses. In parabolic flight experiments, μg and hyper-g phases of up to 1.8 g follow each other, 1.8 g resulted in a slight decrease of cytosolic Ca2+ (Hausmann et al. 2014).
Cytosolic Ca2+ fluctuations: 1 g ground control (left side) in comparsion to centrifugation mode (right side). Primary y-axis: fluorescence ratio (black line: emission 530/480 nm); secondary y-axis: acceleration (grey dotted line: g); x-axis: time (minutes). WT wild type cell culture; YC3.6, Ca2+ sensor expressing cell culture
As the centrifuge experiment resulted in a clear decrease in the amount of Ca2+ at 3 and 4 g, we exposed the cell cultures within seconds to 4 g (Fig. 3). Again a strong cytosolic Ca2+ efflux resulted (Fig. 3b), while WT cells showed no specific response (Fig. 3a). In an experiment with a continuously increasing acceleration of up to 4g, a clear response started only from about 3 g (Fig. 3c). This is similar to the graph presented in Fig. 2 (YC3.6; right side) which also shows a clear response only at 3 g. We thus assume a threshold g-value of about 3 g for a hypergravity dependent decline of cytosolic Ca2+.
Another set of transgenic A.t. cell lines, which express the Ca2+ sensor YC3.6 in various subcellular compartments (Krebs et al. 2012; Behera et al. 2013), were used for localization studies (Fig. 4). NES, an improved version of the original YC3.6 construct (Allen et al. 1999; Miyawaki et al. 1999), shows a stepwise Ca2+ decline in the cytosol and the nucleus with increasing g-force (Fig. 5a). Exclusively expressed in the nucleus, NLS also exhibits a cascade of g-related Ca2+ drops (Figs. 5b + c). In this case acceleration was directly from 1 g to 3 g which makes the loss of Ca2+even more obvious. The weakest effect was observed for the TP12 construct (tonoplast). The response pattern is, however, principally the same (Fig. 5c).
Microscopic images showing the different localisation of YC3.6 expression in A.t. cells a WT (no fluorescence), b NES (YC3.6 expression in the cytosol and nucleoplasm), c NLS (expression exclusively in the nucleus), d TP12 (fluorescence detectable in the tonoplast); left side: with GFP-filter; right side: overlap GFP + bright field
Summary and Outlook
In this investigation we report for the first time a decline of the intracellular Ca2+content due to an external stimulus. To our knowledge, mainly positive stimulus-dependent Ca2+ responses are reported in the literature, even for centrifugation experiments (Toyota et al. 2007; Tatsumi et al. 2014). In the latter studies, however, whole seedlings were centrifuged for up to 300 g for extended periods. Maybe this non-physiological strain may lead to a general stress response which is different from the findings reported here. Furthermore, in these studies Ca2+ fluxes were measured by aequorin luminescence, which is not as sensitive as the recombinant YC3.6 Ca2+ sensor (Koldenkova and Nagai 2013; Whitaker 2010). Known disadvantages of the aequorin fluorophore are the low turnover rate of the luminescence reaction and a low quantum yield with difficulties in signal detection (Koldenkova and Nagai 2013).
Ca2+ efflux is regulated by a plasma membrane type Ca2+ ATPase. Bushart et al. (2014) showed that such an ATPase is regulated by gravity. The use of ATPase inhibitors could thus help to elucidate this hypergravity effect in more detail.
References
Allen, G.J., Kwak, J.M., Chu, S.P., Llopis, J., Tsien, R.Y., Harper, J.F., Schroeder, J.I.: Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 19(6), 735–747 (1999)
Baluska, F., Hasenstein, K.H.: Root cytoskeleton: its role in perception of and response to gravity. Planta 203(Suppl), S69–78 (1997)
Barjaktarović, Z., Nordheim, A., Lamkemeyer, T., Fladerer, C., Madlung, J., Hampp, R.: Time-course of changes in amounts of specific proteins upon exposure to hyper-g, 2-D clinorotation, and 3-D random positioning of Arabidopsis cell cultures. J. Exp. Bot. 58(15-16), 4357–4363 (2007). doi:10.1093/jxb/erm302
Barjaktarović, ž., Schütz, W., Madlung, J., Fladerer, C., Nordheim, A., Hampp, R.: Changes in the effective gravitational field strength affect the state of phosphorylation of stress-related proteins in callus cultures of Arabidopsis thaliana. J. Exp. Bot. 60(3), 779–789 (2009)
Behera, S., Krebs, M., Loro, G., Schumacher, K., Costa, A., Kudla, J.: Ca2+ imaging in plants using genetically encoded Yellow Cameleon Ca2+ indicators. Cold Spring Harb. Protoc. 2013(8), 700–703 (2013). doi:10.1101/pdb.top066183
Bushart, T.J., Cannon, A., Clark, G., Roux, S.J.: Structure and function of CrACA1, the major PM-type Ca2+-ATPase, expressed at the peak of the gravity-directed trans-cell calcium current in spores of the fern Ceratopteris richardii. Plant Biol. 16, 151–157 (2014)
Darwin, F.: The Statolith Theory of Geotropism. Nature 67, 571–572 (1903)
Dodd, A.N., Kudla, J., Sanders, D.: The language of calcium signaling. Annu. Rev. Plant Biol. 61, 593–620 (2010). doi:10.1146/annurev-arplant-070109-104628
Fengler, S., Spirer, I., Neef, M., Ecke, M., Nieselt, K., Hampp, R.: A whole-genome microarray study of Arabidopsis thaliana semisolid callus cultures exposed to microgravity and nonmicrogravity related spaceflight conditions for 5 days on board of Shenzhou 8. BioMed Research International 2015, 547495 (2015). doi:10.1155/2015/547495
Haberlandt, G.: Ueber die Perception des geotropischen Reizes. Ber. Dtsch. Bot. Ges. 18(6), 261–272 (1900)
Hausmann, N., Fengler, S., Hennig, A., Franz-Wachtel, M., Hampp, R., Neef, M.: Cytosolic calcium, hydrogen peroxide and related gene expression and protein modulation in Arabidopsis thaliana cell cultures respond immediately to altered gravitation: parabolic flight data. Plant Biol. 16 Suppl 1, 120–128 (2014). doi:10.1111/plb.12051
Hemmersbach, R., Häder, D.-P.: Graviresponses of certain ciliates and flagellates. The Journal of the Federation of American Societies for Experimental Biology 13, S69–S75 (1999)
Hemmersbach, R., Volkmann, D., Häder, D.P.: Graviorientation in protists and plants. J. Plant Physiol. 154(1), 1–15 (1999)
Kim, M.C., Chung, W.S., Yun, D.J., Cho, M.J.: Calcium and calmodulin-mediated regulation of gene expression in plants. Mol. Plant 2(1), 13–21 (2009). doi:10.1093/mp/ssn091
Koldenkova, V., Nagai, T.: Genetically encoded Ca2+ indicators: Properties and evaluation. Biochim. Biophys. Acta, Mol. Cell Res. 1833(7), 1787–1797 (2013). doi:10.1016/j.bbamcr.2013.01.011
Krebs, M., Held, K., Binder, A., Hashimoto, K., Den Herder, G., Parniske, M., Kudla, J., Schumacher, K.: FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J. 69(1), 181–192 (2012). doi:10.1111/j.1365-313X.2011.04780.x
Limbach, C., Hauslage, J., Schäfer, C., Braun, M.: How to activate a plant gravireceptor. Early mechanisms of gravity sensing studied in characean rhizoids during parabolic flights. Plant Physiol. 139, 1030–1040 (2005)
Maier, R.-M., Martzivanou, M., Magel, E., Zheng, H.Q., Cogoli-Greuter, M., Hampp, R.: Anaplerotic responses of Arabidopsis thaliana cell cultures to altered gravitational field strengths. In: Proceedings of the 16th ESA Symposium on European Rocket and Balloon Programmes and Related Research ESA SP-530, pp 253–258 (2003)
Martzivanou, M., Babbick, M., Cogoli-Greuter, M., Hampp, R.: Microgravity-related changes in gene expression after short-term exposure of Arabidopsis thaliana cell cultures. Protoplasma 229(2-4), 155–162 (2006). doi:10.1007/s00709-006-0203-1
McAinsh, M.R., Pittman, J.K.: Shaping the calcium signature. New Phytol. 181(2), 275–294 (2009). doi:10.1111/j.1469-8137.2008.02682.x
Miyawaki, A., Griesbeck, O., Heim, R., Tsien, R.Y.: Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc. Natl. Acad. Sci. U. S. A. 96(5), 2135–2140 (1999)
Neef, M., Hennig, A., Hausmann, N., Hampp, R.: A parabolic flight profile as reflected by fluctuations in cytosolic calcium and gene expression of plant cells. In: 20th Symposium on European Rocket and Balloon Programmes and Related Research, pp. 433–438 (2011)
Nemec, B.: Ueber die Art der Wahrnehmung des Schwerkraftreizes bei den Pflanzen. Ber. Dtsch. Bot. Ges. 18(6), 241–245 (1900)
Nick, P. In: Wojtaszek, P. (ed.): Mechanics of the Cytoskeleton, pp 53–90. Springer, Berlin Heidelberg (2011)
Perbal, G., Driss-Ecole, D.: Mechanotransduction in gravisensing cells. Trends Plant Sci. 8(10), 498–504 (2003). doi:10.1016/j.tplants.2003.09.005
Plieth, C., Trewavas, A.J.: Reorientation of seedlings in the Earth’s gravitational field induces cytosolic calcium transients. Plant Physiol. 129, 786–796 (2002)
Sanders, D., Pelloux, J., Brownlee, C., Harper, J.F.: Calcium at the crossroads of signaling. Plant Cell 14 Suppl, S401–417 (2002)
Sievers, A., Schröter, K.: An attempt at a causal analysis of the geotropical reaction chain in the Chara rhizoid. Planta 96, 339–353 (1971)
Tatsumi, H., Toyota, M., Furuichi, T., Sokabe, M.: Calcium mobilizations in response to changes in the gravity vector in Arabidopsis seedlings: Possible cellular mechanisms. Plant Signal. Behav. 9 (2014)
Toyota, M., Furuichi, T., Tatsumi, H., Sokabe, M.: Hypergravity stimulation induces changes in intracellular calcium concentration in Arabidopsis seedlings. Adv. Space Res. 39(7), 1190–1197 (2007)
Toyota, M., Furuichi, T., Tatsumi, H., Sokabe, M.: Cytoplasmic calcium increases in response to changes in the gravity vector in hypocotyls and petioles of Arabidopsis seedlings. Plant Physiol. 146(2), 505–514 (2008). doi:10.1104/pp.107.106450
Wayne, R., Staves, M.P., Leopold, A.C.: The contribution of the extracellular matrix to gravisensing in characean cells. J. Cell Sci. 101, 611–623 (1992)
Went, K.W., Thimann, K.V.: Phytohormones. The Macmillan Company, New York (1937)
Whitaker, M.: Genetically-encoded probes for measurement of intracellular calcium. Methods Cell Biol. 99, 153–182 (2010). doi:10.1016/B978-0-12-374841-6.00006-2
Acknowledgments
This research was made possible by a grant from the European Space Agency (ESA, Ground Based Research programme). The authors are indebted to the ZARM team for help in experiment execution (especially Holger Oelze and Henrik Kassai). We also appreciate technical and administrative help by Niklas Hausmann, as well as PD Dr. R. Hemmersbach (DLR) and J. Ngo-Anh (ESA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neef, M., Denn, T., Ecke, M. et al. Intracellular Calcium Decreases Upon Hyper Gravity-Treatment of Arabidopsis Thaliana Cell Cultures. Microgravity Sci. Technol. 28, 331–336 (2016). https://doi.org/10.1007/s12217-015-9457-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12217-015-9457-6