Abstract
The mining wastes coming from Sidi Kamber mine stored at the surface are exposed to weathering conditions, which favour the leaching of toxic metals into the environment. This situation presents serious risks for humans and ecosystems. To better understand this problem, eight representative samples of Sidi Kamber mine tailing were taken from the surface along two vertical trenches and tested using static and dynamic leaching tests. The studied samples present a wide diversity in terms of particle size distribution, mineralogical and chemical compositions. These parameters may greatly affect the reaction rates. The mineralogical investigations show the presence of various sulphide minerals, such as pyrite, galena, sphalerite and chalcopyrite which are present in either free and/or associated with gangue minerals (i.e. quartz, albite, chlorite and muscovite). Moreover, the presence of only minerals with low neutralizing potential such as silicates, promotes the acid mine drainage generation, which is characterized by high concentrations of metals and sulphates. Leaching tests showed an acidic pH and the release of some toxic contaminants (i.e., Pb, Zn and Cu), which exceed the recommended limits (Algerian regulation law for industrial wastewater and US EPA thresholds).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
During mining and refining operations, the mining industry produces large amounts of mine wastes (tailings and waste rocks) (Förstner 1999). In the case of ore deposits with high tonnage and low grade, several million tons of mine wastes could be generated (Lottermoser 2010). In fact, a large part of the total mined materials is called gangue minerals (i.e., silicates, sulphides, oxides, carbonates, etc.), that is generally stored at the surface. Under atmospheric conditions, some iron sulphide minerals (pyrite and pyrrhotite) generate acid mine drainage (AMD), which may cause many serious environmental problems. The AMD is characterized by high acidity and concentration of metals and sulphates, which exceed the regulation limits (Benzaazoua et al. 2004; Bussière 2007). The generation of AMD is mainly controlled by: (i) type and amount of sulphide and carbonate minerals (Blowes et al. 2014), (ii) presence or absence of microorganisms, oxygen and carbon dioxide concentration, temperature, pH, and Eh (Dold 2017; Edahbi et al. 2018a). The AMD-generating potential of the mine wastes is the most important factor that determines the rehabilitation strategy regarding the mine closing and costs of the associated reclaiming. For example, the costs of the reclamation of acid-generating mine tailings in Canada are between 100,000 $ and 250,000 $ per hectare, while they are generally between 2000 and 20,000 $ per hectare for tailings that are not acid generating (Villeneuve 2004).
To predict the environmental behaviour of tailings, two type of tests are used, static and kinetic testing. The static test also called acid–base accounting (ABA) is a screening method used to determine the acid generation potential and acid-neutralization potential of mine wastes (Lawrence and Wang 1996; Bouzahzah et al. 2014). However, it does not allow to determine acid production or neutralization rates, as it requires kinetic tests like humidity cells, weathering cells, columns test, and field tests (Chotpantarat 2011; Parbhakar-Fox and Lottermoser 2015; Edahbi et al. 2018b). Furthermore, there are other tests such as leaching tests, which are used to assess the metals released into the environment. The leaching tests are used to remove soluble components from a solid matrix as defined by Kim (2003); they are classified into two categories: the batch and the dynamic leaching tests (Bassolé 2016; Washington State Department of Ecology 2003). The principal leaching tests used are as follows: the toxicity characteristic leaching procedure (TCLP), the synthetic precipitation leaching procedure (SPLP), the CTEU-9 test, and the CTEU-10 test.
In Algeria, most of the base metal mines are closed and the main mining complexes are Ouenza, Boukhadra, Rouina, Khenguet and Anini (iron mines) and Djebel Onk phosphate mines (Taib 2014). Because of the decrease in mining activity, large volumes of sulphide tailings were abandoned without any environmental management plan. The most problematic sites are: Kef Oum Teboul (Cu–Pb–Zn), Boudoukha (Pb–Zn), and Sidi Kamber (Pb–Zn). In the present study, only the Sidi Kamber Pb–Zn mine was studied. Indeed, this site is considered as one of the most problematic mine sites, because the mining effluent is close to the Essouk River (Medjram and Malika 2014), which flows into the Guenitra dam. Thus, the objective of this paper is to study the geochemical behaviour of Sidi Kamber Pb–Zn mine wastes to determine the acid-generating potential of the studied tailings and to assess the metals released into the environment. The results of this study can address knowledge gaps associated with abandoned mine wastes in Algeria and contribute to understanding their environmental behaviour.
2 Presentation of the study area
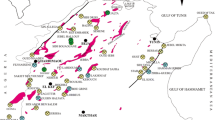
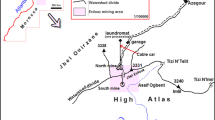
The Sidi Kamber mine (zinc–lead–barium) which covers 3 km2 is located in the Mediterranean coastline of Algeria (Fig. 1a) at approximately 36 km eastern to Skikda city (Lesser Kabylia Massif). The study area is characterized by a sub-humid Mediterranean climate (an average annual rainfall of around 650 mm) (Mebarki 2005). Furthermore, the mine site is located in the Oued Es-Essouk sub-watershed (12.49 km2), which supplies the Guenitra dam (Fig. 1b). Effluents coming from the Sidi Kamber mine could affect the water quality of Guenitra dam, which is used for drinking and irrigation in the Skikda city (Boukhalfa and Chaguer 2012).
Location and simplified geological map of the study area; a simplified geological map of the study area (Mahdjoub et al. 1997); b location of mine site; c photography showing an overview of the mine site, processing plant, tailings piles and tailings dam
The mined ore deposit is composed of eight quartz veins mainly hosted in gneiss and granulite formations of the metamorphic Precambrian Kabylian basement (Mahdjoub et al. 1997; Aubouin et al. 2018). The mineralization is constituted of sulphide minerals such as pyrite, pyrrhotite, marcasite, sphalerite and galena, while gangue minerals consist of magnetite and barite (Bolfa 1948).
Ore deposits were mined first in 1889 by the “Société des mines de Sidi Kamber” which continued until 1961. In 1966, the national company SONAREM restarted the mining activity (currently ORGM). During this period, the processing plant produced ore concentrate upto 60% zinc and 83% lead. The concentrations of Pb and Zn within ore minerals were 17.66% and 18.88%, respectively (ORGM 1971). The ore was processed by crushing and milling followed by gravimetric separation for galena, while sphalerite was extracted using the flotation process (36,000 t/year). In 1976, mining for lead and zinc was abandoned and barite was exploited by open-cast mining followed by gravimetric separation. In 1984, the ore-extracting activities of Sidi Kamber mine ceased and the mine was definitively closed (Oumdjbeur 1986).
During the ore enrichment process, two types of tailings were generated and disposed in two mine waste storage areas (Fig. 1c): flotation tailings, stored in the tailings dam (5 ha), across the Essouk river (Fig. 2a, b) and gravimetric tailings stored in piles (3 ha) near the flotation plant (Fig. 2c, d). During mining and refining operations, the mine wastes were deposited on the surface without environmental management strategies. Consequently, the tailings were exposed to natural weathering (such as dissolution, mineral oxidation, etc.). The oxidized tailings are of rust orange and yellowish colour.
3 Materials and methods
3.1 Sampling
A total of eight representative samples were collected from the sampling station in the mine site (Fig. 3a) in accordance with the US EPA Operating Protocol for Soil Sampling (USEPA 2014).
Trench 1 (T1) was excavated within the flotation tailings showing a wide variety of coloration with depth (Fig. 3b). The oxidized tailings were orange–brown, while the unoxidized tailings were grey–white. The sampling methodology used is as follows: the sample SK1 was collected at a depth of 10 cm in the oxidized horizon, the samples SK2 and SK3 at 20 and 30 cm, respectively, and finally SK4 was collected in the unoxidized horizon at 50 cm. In the Trench 2 (T2), where gravimetry tailings were sampled, there is no variation in terms of coloration (Fig. 3c) and that is why the sample SK5 was taken at the surface (10 cm), while other samples SK6, SK7 and SK8 were taken at different depth levels with 10 cm intervals (30, 40, and 50 cm). All samples were carefully collected and stored in double-sealed plastic bags after air evacuation. The fine tailings were dried in an oven at 40 °C. In the laboratory, samples were homogenized, quartered and then stored in plastic bags and hermetically sealed.
Field observations showed that secondary weathering products in the study area consist of hardpan crusts and efflorescent salts (Fig. 3d). The presence of these minerals is the mineralogical expression of the AMD process (Hakkou et al. 2008). Moreover, during the oxidation–neutralization reactions, significant amount of metals could be released into the drainage water (Lottermoser 2010). Once leached in the solution, metals can be sorbed and/or precipitated as secondary minerals depending on the solution composition, pH, and Eh (Dinelli and Tateo 2001). Based on the colour of the materials, various representative samples were collected for mineralogical analyses.
3.2 Analytical methods
Particle size distribution was carried out at the department of geology (USTHB) using a Horiba-Partica LA-950V2 Particle Size Distribution Analyser.
The major elements’ (Si, Al, Ca, K, Fe, Mg, Na, Ti and Mn) contents were determined at the Department of Engineering and Mineral Resources of Kyushu University (Japan) with X-ray fluorescence spectrometer, RIGAKU RIX 3100. The precision of the measurements was verified using the pulse height analysis (PHA) test and a standard reference material (Ja-3; Japanese Andesite) before starting the analysis (Imai et al. 1995).
Total sulphur contents were measured using an induction furnace analyser (ELTRA CS-2000).
Trace elements (Zn, Pb, Cu, Ba and Cd) for samples coming from T1 were analysed after HNO3/Br2/HF/HCl digestion (Potts 1987), using inductively coupled plasma with atomic emission spectroscopy (ICP-AES) Perkin Elmer Optima 3100 RL with a precision of approximately 5%.
Total sulphur content and trace element analyses were carried out in the Institute of Research in Mines and Environment (IRME) of the University of Quebec in Abitibi-Temiscamingue (UQAT).
Atomic absorption spectroscopy (ICE 3000 Series type thermo-Fisher Scientific) of the national company of mining research in Algeria (ORGM) was used to analyse trace elements (Zn, Pb, Cu, Ba and Cd) content in samples coming from T2. The accuracy is checked by analysis of each Perkin Elmer atomic spectroscopy calibration standard solution.
The elemental analysis is done in duplicate to evaluate the repeatability of the results. In the absence of a specific Algerian regulation law for industrial soils, the obtained results are compared to regulatory limits imposed by Italian environmental laws (DL 152/2006; Tlil et al. 2016) and to crustal abundances (Rankin 2009).
For mineralogical observations, eight polished sections from bulk samples were prepared. As the first step, the mineralogy was determined using an optical microscope (Zeiss Axio imager M2). The observations were focused on sulphide minerals. A Hitachi S-3500N scanning electron microscope (SEM), equipped with a microanalysis system (energy dispersive spectroscopy, or EDS) was used to complete the mineralogical observations and to determine the stoichiometric measurements. The semi-quantitative mineralogy was also performed, using a Bruker AXS. D8 Advance X-ray diffraction (XRD) instrument equipped with a copper anticathode (detection limit of 0.5–1 wt%). The mineral semi-quantification was determined using a Rietveld fitting of the XRD data with the EVA and TOPAS software (Young 1995). All mineralogical observations were carried out in the Institute of Research in Mines and Environment (IRME) of the University of Quebec in Abitibi-Temiscamingue (UQAT).
3.3 Static tests
Two types of static tests were carried out at the National Polytechnic School (Algiers, Algeria), to measure the acidic and neutralization potentials of the sampled tailings. The paste pH test was also used to provide an indication of the nature of the studied tailings in terms of pH (Lapakko 2002). The paste pH test (Sobek et al. 1978) consists of 20 g of samples (less than 1 mm) placed in 50 mL beakers. Water of the same mass as the sample (1:1 solid/solution ratio) is added and mixed for 10 s to make a paste. The paste pH was then measured by inserting a pH electrode into the paste. The pH value below four indicates that the sample is acid generating.
The acid–base accounting (ABA) static test was determined to predict the acid-generating potential of tailings. ABA measures the balance between the acid-producing potential (AP) and neutralizing potential (NP) of a given sample. The NP tests were run in duplicate using the Lawrence and Wang (1996) static test which is better known as the modified Sobek test static test (Sobek et al. 1978). In this method, a 250 mL flask with 90 mL of distilled water and 2.00 g of pulverized sample is placed on a shaking apparatus at room temperature. According to the fizz rating test (Bouzahzah et al. 2015), a volume of 1.0 N HCl solution is added at t = 0 h and t = 2 h to the samples. It should be noted that in Sobek et al.’s (1978) test, the sample was boiled in HCl, while the modified Sobek test as proposed by Lawrence and Wang (1996) exposed the sample to room temperature (Dold 2017). After approximately 22 h, the pH of the pulp is checked to be in agreement with the Lawrence and Wang (1996) protocol. At t = 24 h, distilled water is added to the flask to bring the volume to 125 mL. The residual acid is then back titrated to a defined end point of pH of 8.3 with certified 0.5 or 0.1 N NaOH. The NP of the sample was calculated using Eq (1); results were expressed in kg CaCO3/t.
where NP is the kg CaCO3/t, 50 is conversion factor, a is the HCl normality (mol/L), b is the NaOH normality (mol/L), x is the HCl volume (mL), y is the NaOH volume (mL), and c is the sample mass (g).
AP, also expressed in kg CaCO3/t, was calculated using the total sulphur fraction (%S) and Eq (2); results were also expressed in kg CaCO3/t.
The net neutralization potential (NNP = NP–AP) was calculated. NNP values < − 20 kg CaCO3/t indicate an acid-producing material, whereas when the NNP > 20 kg CaCO3/t, the material is considered acid consuming. Hence, an uncertainty zone exists, when NNP is between − 20 and 20 kg CaCO3/t (Miller et al. 1991; SRK 1989). NP/AP ratio can be also used to evaluate the AMD production potential of mine wastes. Typically, the material is considered non-acid generating (NP/AP > 2.5), uncertain (2.5 > NP/AP > 1), and acid generating (NP/AP < 1) (Adam et al. 1997).
3.4 Environmental leaching tests
Leaching tests, CTEU-9 (Expertise Center in Environmental Analysis of Quebec 2012) and the toxicity characteristic leaching procedure (TCLP) (USEPA 1992), were performed at the Institute of Research in Mines and Environment (IRME) of the University of Quebec in Abitibi-Temiscamingue (UQAT) to remove potentially toxic elements from their matrix under specific conditions. Four samples were used to conduct the leaching test on Sidi Kamber mine wastes; for every trench, two samples were selected: one from the weathered tailings (oxidized horizon) and a second from fresh tailings (unoxidized horizon). In trench 1, SK1 comes from the top and SK4 comes from the bottom. For trench 2, samples SK5, SK6, SK7 were mixed and homogenized, to obtain a representative composite sample, called SK567, while SK8 was used to represent potential unoxidized tailings.
The leaching behaviour of tailings in acidic environment was assessed using TCLP protocol (USEPA 1992). The samples were grinded and sieved to 9.5 mm as recommended in the TCLP protocol. These samples were placed in 1 L bottles containing 400 mL of the extraction solution with a solid/liquid ratio that is equal to 1:20. The bottles were then placed in a rotary system and agitated at a speed of 30 rpm during 18 h. The extraction solution N1, with a pH value of 4.93, was used to accelerate the extraction of metals from all the samples. The leaching solution was selected after realizing a pretest consisting of an assessment of metals’ dissolution. After filtration and measuring pH and Ec, the residual leachates were acidified with 2% HNO3 and analysed by ICP-AES (Perkin Elmer Optima 3100 RL). The elemental concentrations were compared to regulatory limits fixed by Algerian regulation law for industrial wastewater (Décret exécutif 2006) and US EPA thresholds (USEPA 2009).
The CTEU-9 leaching test was used to determine the concentration of inorganic species which may be leached upon contact with water at a neutral pH. To do this, 40 g of solid was leached with 160 mL (the solid/liquid ratio is of 1:4) of water in 1 L bottles and shaked for 7 days at room temperature. At the end of the CTEU-9 test, pH and Ec were measured after filtration using a 0.45 µm nylon filter. The leachates were acidified with 2% HNO3 and analysed by ICP-AES (Perkin Elmer Optima 3100 RL). Compositions of leachates were compared to Algerian regulation law limits for industrial wastewater (Décret exécutif 2006).
4 Results and discussion
4.1 Grain size
Figure 4 presents the grain size distributions of different samples. The grain size distribution shows a slight difference between the studied materials which may be related to ore processing changes during the lifetime of the mine. Table 1 summarizes the main grain size distribution parameters (D10, D50 and D90) of the studied tailings. For samples coming from trench T1 (SK1, SK2, SK3 and SK4), the D10 (10% passing) ranges from 0.53 µm to 19.42 µm, and the D90 is between 42.06 µm and 257.54 µm. The particle size distribution parameter D50 (50% passing) ranges from 21.17 µm to 135.58 μm. According to Wentworth (1922), the tailings from T1 can be classified as fine sand.
By contrast, the samples coming from trench T2 (SK5, SK6, SK7 and SK8) have a fine to medium sand texture. D10 is between 7.81 and 100.75 μm, D90 varies from 237.88 to 465.37 μm, and the mean particle size (D50) ranges from 122 to 250.27 μm.
4.2 Mineralogical characterization
Mineralogical characterization was performed by optical microscopy, scanning electron microscope (SEM), equipped with an energy-dispersive X-ray spectroscopy (EDS) probe (SEM-EDS) and X-ray diffraction (XRD). The mineralogical observation focuses on iron sulphides. Their oxidation produces S (as sulphates), metals and acidity, which could have an important influence as well as devastating effects on the environment (Jamieson et al. 2015). Moreover, the study also focuses on minerals that may provide acid-neutralization capacities.
The optical observations (Fig. 5) showed that the main sulphide minerals are pyrite and galena with small amounts of hematite as iron oxides. The weathering products of sulphides are observed easily during optical and SEM observations. During the weathering process, a small framboidal grain appears due to pyrite processing to hematite as oxidation products (Fig. 5a). Other secondary phases (i.e., iron oxides), resulting from pyrite oxidation throughout its boundaries and internal fractures (Fig. 5b) confirmed the weathering process. As shown in Fig. 5c and d, galena is also affected by the weathering process, which corresponds to secondary minerals coating of the weathered galena.
Optical microscopy observations using reflected light of tailings; a secondary hematite (Hem) resulting from framboidal pyrite oxidation in a gangue of quartz; b weathered pyrite (Py) coated by secondary iron oxide (FeO) and associated to quartz; c anglesite (Ang) coating weathered galena (Ga); d galena (Ga) at the early stages of weathering coated by anglesite (Ang)
SEM–EDS analyses were performed to identify new phases not observed by optical microscopy (Fig. 6). Silicate minerals (mainly quartz) (Fig. 6a, b), as well as other primary and secondary minerals such as barite (Fig. 6a, b) hematite (Fig. 6c) and sphalerite (Fig. 6d) were observed.
SEM–EDS micrographs of tailings showing: a quartz (Qtz) and barite (Ba) grains and weathering product (Fe–O–Pb–S); b barite grain; c hematite grain; d sphalerite (Sph) and pyrite (Py) coated by secondary iron oxide; e weathered galena (Ga) associated to barite and coated by anglesite (Ang); f weathered galena coated by anglesite which completely covers also barite (Ba) grains
SEM–EDS analyses confirmed the weathering of sulphide minerals and the abundance of secondary minerals as oxidation products. Furthermore, a thin layer of iron oxide along the pyrite grain boundaries was observed (Fig. 6d). Other SEM–EDS observations also showed anglesite as a secondary sulphate, covering the surface of galena and barite (Fig. 6e, f).
In summary, the mineralogical observations using optical microscope and SEM–EDS analyses showed the advanced weathering of tailings from Sidi Kamber mine and the abundance of secondary minerals as oxidation products. The precipitation of these secondary minerals at the surface of particles could affect the chemical and physical behaviour of the tailings.
Results issued from the XRD semi-quantitative analysis are summarized in Fig. 7. In all samples, aluminosilicates are abundant, which is in agreement with the gneiss nature of the host rocks. In samples coming from flotation tailings (Trench 1), the main minerals detected by XRD analysis were quartz (55.05–69.29%) muscovite (9.28–15.90%) and chlorite (3.14–13.29%). Albite was detected too (3.13–8.19%), while corundum ranged from 1.20 to 8.29%. The main sulphide mineral detected by XRD analyses is pyrite (2.05–6.62%) in addition to chalcopyrite, galena and sphalerite. Oxide minerals consist only on hematite as observed in SK1 and SK3.
The mineralogical paragenesis within gravimetric tailings (Trench 2) is dominated by quartz (39–75%), albite (2.89–12.40%), chlorite (1.92–15.55%) and muscovite (3.23–14.53%). Pyrite, which has the most important acid-generating potential ranged from 4.06 to 9.79%, in addition to chalcopyrite, galena and sphalerite. However, hematite, goethite and gypsum are the main secondary minerals. Carbonate minerals were absent in all the studied samples.
4.3 Chemical characterization
The chemical characterization (Table 1) shows that all samples contain a significant content of sulphur, ranging from 1.20 to 3.60% in T1, and from 2.76 to 5.25 wt% in T2.
The contents of major elements show the same orders of magnitude in all samples. In accordance with the mineralogical composition of samples from T1 to T2, chemical analyses showed that Si (20.45–30.04 wt% and 21.97–24.69 wt%, respectively), Al (4.59–8.82wt% and 4.27–7.89 wt%, respectively) and K (1.42–4.43 wt% and 0.89–4.39 wt%, respectively) were significant in most of the tailings samples. Mg was also present (0.18–0.76 wt% and 0.11–0.89 wt%, respectively). Ca concentrations are relatively low and reached 1.17% which confirms the absence and/or low content of calcium carbonate minerals within the studied tailings. Iron is present in high quantities in all samples (1.99–3.20 wt% and 1.73–8.92 wt%, respectively) due to the presence of iron sulphide (pyrite) or secondary iron-bearing minerals (oxides and sulphates) in a high amount.
The abundance of Zn (640–1640 mg/kg and 277–13,340 mg/kg, respectively) and Pb (210–3080 mg/kg and 177–3172 mg/kg, respectively) is due to the presence of galena and sphalerite in the bulk ore. The tailings contain also significant Cu amounts (60–140 mg/kg and 33–263 mg/kg, respectively). Barium mainly issued from barite is below the detection limits in T1 and ranged from 491 to 2308 mg/kg in T2.
Chemical analyses of tailings also showed that all the trace elements detected (Zn, Pb, Cu, Ba and Cd) are higher than crustal abundance (Rankin 2009) and may exceed the recommended limits for industrial soils (Table 1 reported by the Italian limits for industrial soils as defined by DL 152/2006).
4.4 Static tests
Static test results (Pastes pH and Acid–base accounting) are highlighted in Table 2. The paste pH results of the studied tailings (T1 and T2) are between 2.49 and 3.70 and correspond to acid-generating tailings. As shown in Fig. 2b and d, the pore water issued from tailings showed an acidic pH (between 3 and 4 as showed in), which is in accordance with these findings. Low paste pH can be related to iron sulphide oxidation and also to soluble minerals dissolution (most probably as secondary phases) (Yucel and Baba 2016).
Acid–base accounting results according to the modified static test proposed by Lawrence and Wang (1996) are shown in Table 2 and Fig. 8. All samples have low NP values with maximum value in samples SK1 (18.56 kg CaCO3/t) and SK5 (14.56 kg CaCO3/t). SK4 (6.36 kg CaCO3/t) and SK6 (5.26 kg CaCO3/t) have the lowest NP values compared to other samples. The AP values were high in all samples (37.49 kg CaCO3/t to 163.99 kg CaCO3/t). NNP values vary between − 31.13 and − 152.38 kg CaCO3/t. The criteria of Miller et al. (1991) or the NP/AP ratio criteria of Adam et al. (1997) show that all Sidi Kamber mine wastes are acid generating. Tailings coming from T2 (average value of NNP = − 133.57 kg CaCO3/t) is the most problematic compared to the flotation tailings (average value of NNP = − 69.22 kg CaCO3/t). According to mineralogical observations, the AP values result from sulphide minerals oxidation, while the NP values are provided by dissolution of silicate minerals (albite, chlorite and muscovite) under acidic conditions (Jambor et al. 2002).
Environmental characterization of the studied tailings according to Adam et al. (1997)
4.5 Environmental leaching tests
As shown is Fig. 9, the leachate of the studied tailings provided by TCLP and CTEU-9 leaching tests is in ‘‘acid-high metal’’ and ‘‘acid-extreme metal’’ domain, except SK8 leachate (CTEU-9) which corresponds to the acid-ultra metal domain (Plumlee et al. 1999).
Ficklin’s diagram showing the sum of dissolved Pb, Zn, Cu in leachates issued from TCLP test (red square) and the CTEU-9 (blue diamond) in the base metal environments (Plumlee et al. 1999)
The TCLP test results are highlighted in Fig. 10 and show an acidic pH (4.50–4.62) with high electrical conductivity (3.05–4.90 mS/cm). This finding is in agreement with the high net acid-generating potential of the Sidi Kamber tailings. The high concentrations of Si (9.51–31.10 mg/L), Al (7.89–98.50 mg/L) and Ca (19.70–220 mg/L) indicate the dissolution reactions of gangue minerals such as silicate minerals and secondary phase’s minerals.
The high concentrations of Fe (55.40–205 mg/L) and S (24.80–393 mg/L) are mainly associated with the iron sulphide mineral oxidation (i.e., pyrite) and secondary iron oxide dissolution (i.e., goethite and hematite).
In the pH conditions encountered in this study, Fe, S, Al and K could precipitate as various possible secondary phases like iron oxides, jarosite, gypsum and anhydrite (Fig. 11), as demonstrated by geochemical simulations with VMinteq and confirmed by XRD and SEM–EDS analyses.
The concentrations of Pb and Zn, resulting from galena and sphalerite dissolution, reached, respectively, 21.60 and 32.80 mg/L, which exceed the Algerian regulation law (0.5 mg/L and 3 mg/L) and US EPA limits (5 mg/L and 2 mg/L, respectively). The Cu concentrations (0.00–0.28 mg/L) were under the Algerian limits (0.5 ml/L).
The results of CTEU-9 indicate an acidic pH value range of 4.70–4.82. The electrical conductivity is between 3.95 and 4.29 mS/cm. This test shows that the Sidi Kamber mine wastes are acid generating with high release of Si (45.90–70.60 mg/L), Ca (65.90–516.00 mg/L) and Al (1.20–378 mg/L) in leachate, which results from the acidic dissolution of silicates.
The main conclusions that can be drawn from this test are that the neutralization potential was mainly provided by albite or Ca-rich plagioclase and the chlorite and/or muscovite dissolution under acidic conditions was provided by iron sulphide oxidation. Furthermore, the hydrolysis of iron and aluminium hydroxides could also generate acidity.
Fe concentrations in leachates reached 863 mg/L; this highlights how iron sulphide dissolution is important. Moreover, the incongruent dissolution of silicates at acidic pH (e.g., chlorite) may release a small amount of iron in the leachate.
As expected, S concentrations were high (134–1570 mg/L) in all leachates and this is related to sulphides (i.e., pyrite) oxidation and secondary sulphates (i.e., gypsum) dissolution.
Except for leachate from sample SK567 (1.5 mg/L), Pb concentrations were under the considered limits (0.5 mg/L) because of the passivation of galena grains. Indeed, as observed by optical microscopy and SEM–EDS analyses, the formation of low solubility minerals, as anglesite, under weathering conditions, may encapsulate sulphides like galena preventing further oxidation (Lin 1997; Plumlee et al. 1999; Acero et al. 2007; Lottermoser 2010).
Cu concentrations are relatively low in all the leachates due to both the low amount of chalcopyrite within samples and its low degree of liberation (encapsulation). Contrary to Pb and Cu, leachates show high concentrations of Zn (2.31–121 mg/L) due to its high mobility at acidic and near-neutral conditions (Smith 1999; Pope and Trumm 2015).
The environmental characterization of mine wastes from Sidi Kamber mine demonstrate the acid-generating nature of the studied tailings, which correspond to an acidic pH associated to high metal release. A similar pattern of results was obtained in the case of Moroccan abandoned mine sites, as described by Hakkou et al. (2008), Michard et al. (2011), Goumih et al. (2013) and El Adnani et al. (2016). Contrary to Algeria and Morocco, in Tunisia, most of problematic mine sites correspond to carbonate-rich tailings which implies mainly the neutral mine drainage as observed by Souissi et al. (2013), Othmani et al. (2013) and Tlil et al. (2016). However, the environmental behaviour of the studied samples is more problematic, because of the low reactivity of neutralizing minerals (e.g., plagioclase and other silicates minerals) compared to carbonates.
5 Conclusions
Tailings from the former Pb–Zn mine of Sidi Kamber in north-eastern Algeria showed fine-grained to medium-grained textures. Mineralogical studies identified as acid generator minerals, pyrite, in addition to galena, sphalerite and chalcopyrite which could produce acidity under oxidation conditions. On the other hand, quartz, albite, muscovite, chlorite and corundum may only contribute slightly to neutralization reactions, because of their low neutralization potential. Hematite, goethite, jarosite, anglesite and gypsum occurred as a result of oxidation of primary sulphides. The results of geochemical analyses of tailings show abundance of Si, Al and Ca which is in accordance with the mineralogical observations. In term of metal release, leaching tests have shown that Zn and Pb concentrations in leachates reached values which may exceed the recommended limits as fixed by US EPA and Algerian regulation law. Acid base accounting static test showed a high AP associated with low NP and confirmed the acid-generating nature of tailings. Leachates from leaching tests (TCLP and CTEU-9) showed an acidic pH and high metal concentrations (Zn, Pb, Al, Si, Ca) due to sulphide oxidation and dissolution of silicate minerals during water–rock interactions. Concentrations of Fe and S in leachates were controlled by secondary mineral precipitation, such as jarosite and gypsum. However, mobility of potential toxic elements seems to be controlled both by passivation phenomena associated with secondary phase’s precipitations and the degree of liberation of sulphide minerals from the silicate matrix.
References
Acero P, Cama J, Ayora C (2007) Rate law for galena dissolution in acidic environment. Chem Geol 245:219–229
Adam K, Kourtis A, Gazea B, Kontopoulos A (1997) Evaluation of static tests used to predict the potential for acid generation at sulphide mine sites. Transactions of the Institution of Mining and Metallurgy, section A, mining industry, 106, A1A8
Aubouin J, Gaussen H, Harant H (2018) Méditerranéenne aire. Encyclopædia Universalis. Accessed 12 Nov 2018
Bassolé MR (2016) Pertinence des essais de lixiviation en batch dans la prédiction du comportement hydrogéochimique des rejets miniers. Mémoire. Université du Québec en Abitibi-Témiscamingue, Science appliquées, Rouyn-Noranda, p 238
Benzaazoua M, Bussière B, Dagenais AM, Archambault M (2004) Kinetic tests comparison and interpretation for prediction of the Joutel tailings acid generation potential. Environ Geol 46:10861101. https://doi.org/10.1007/s00254-004-1113-1
Blowes DW, Ptacek CJ, Jambor JL, Weisener CG, Paktunc D, Gould WD, Johnson DB (2014) The geochemistry of acid mine drainage. In: Treatise on geochemistry. Elsevier, pp 131–190. https://doi.org/10.1016/B978-0-08-095975-7.00905-0
Bolfa JA (1948) Contribution à l’étude des gîtes métallifères de la Kabylie de Collo et de la région de Bône. impr. Berger-Levrault. Nancy, Paris, Strasbourg
Boukhalfa C, Chaguer M (2012) Characterisation of sediments polluted by acid mine drainage in the northeast of Algeria. Int J Sedim Res 27(402):407
Bouzahzah H, Benzaazoua M, Bussière B, Plante B (2014) Prediction of acid mine drainage: importance of mineralogy and the test protocols for static and kinetic tests. Mine Water Environ 33:5465. https://doi.org/10.1007/s10230-013-0249-1
Bouzahzah H, Benzaazoua M, Plante B, Bussière B (2015) A quantitative approach for the estimation of the “fizz rating” parameter in the acid-base accounting tests: a new adaptations of the Sobek test. J Geochem Explor 153:5365. https://doi.org/10.1016/j.gexplo.2015.03.003
Bussière B (2007) Colloquium 2004: hydrogeotechnical properties of hard rock tailings from metal mines and emerging geoenvironmental disposal approaches. Can Geotech J 44:10191052. https://doi.org/10.1139/T07-040
Chotpantarat S (2011) A review of static tests and recent studies. Am J Appl Sci 8:400406. https://doi.org/10.3844/ajassp.2011.400.406
Décret exécutif (2006) no. 06-141 du 20 Rabie El Aouel 1427 correspondant au 19 avril 2006 définissant les valeurs limites des rejets d’effluents liquides industriels
Dinelli E, Tateo F (2001) Sheet silicates as effective carriers of heavy metals in the ophiolitic mine area of Vigonzano (northern Italy). Mineral Mag 65:121–132
DL 152/2006. Decreto Legislativo 3 aprile 2006, n. 152 ‘‘Norme in materia ambientale’’. Gazzetta Ufficiale n. 88 del 14-4-2006, Supplemento Ordinario no. 96. http://www.camera.it/parlam/leggi/deleghe/06152dl.htm. Accessed 13 March 2019
Dold B (2017) Acid rock drainage prediction: a critical review. J Geochem Explor 172:120132. https://doi.org/10.1016/j.gexplo.2016.09.014
Edahbi M, Plante B, Benzaazoua M, Pelletier M (2018a) Geochemistry of rare earth elements within waste rocks from the Montvielcarbonatite deposit, Québec, Canada. Environ Sci Pollut Res 25:1099711010. https://doi.org/10.1007/s11356-018-1309-7
Edahbi M, Plante B, Benzaazoua M, Ward M, Pelletier M (2018b) Mobility of rare earth elements in mine drainage: influence of iron oxides, carbonates, and phosphates. Chemosphere 199:647654
El Adnani M, Plante B, Benzaazoua M, Hakkou R, Bouzahzah H (2016) Tailings weathering and arsenic mobility at the abandoned Zgounder silver mine, Morocco. Mine Water Environ 35:508–524. https://doi.org/10.1007/s10230-015-0370-4
Expertise Center in Environmental Analysis of Quebec (2012) Protocole de lixiviation pour les espèces inorganiques, MA. 100 Lix.com.1.1, Rév. 1, Ministère du Développement durable, de l’Environnement, de la Faune et des Parcs du Québec, Canada
Förstner U (1999) Introduction. In: Azcue JM (ed) Environmental impacts of mining activities: emphasis on mitigation and remedial measures. Springer, Heidelberg, p 13
Goumih A, El Adnani M, Hakkou R, Benzaazoua M (2013) Geochemical behavior of mine tailings and waste rock at the abandoned Cu–Mo–W Azegour mine (occidental high atlas, Morocco). Mine Water Environ 32:121–132. https://doi.org/10.1007/s10230-013-0221-0
Hakkou R, Benzaazoua M, Bussière B (2008) Acid mine drainage at the abandoned Kettara mine (Morocco): 1. environmental characterization. Mine Water Environ 27:145–159. https://doi.org/10.1007/s10230-008-0036-6
Imai N, Terashima S, Itoh S, Ando A (1995) 1994 Compilation of analytical data for minor and trace elements in seventeen GSJ geochemical reference samples, “igneous rock series”. Geostand Geoanal Res 19:135213. https://doi.org/10.1111/j.1751-908X.1995.tb00158.x
Jambor JL, Dutrizac JE, Groat LA, Raudsepp M (2002) Static tests of neutralization potentials of silicate and alumina silicate minerals. Environ Geol 43:117. https://doi.org/10.1007/s00254-002-0615-y
Jamieson HE, Walker SR, Parsons MB (2015) Mineralogical characterization of mine waste. Appl Geochem 57:85105. https://doi.org/10.1016/j.apgeochem.2014.12.014
Kim AG (2003) Leaching methods applied to the characterization of coal utilization by-products. (Kim 2002), 89–96
Lapakko K (2002) Metal mine rock and waste characterization tools: an overview. Min Miner Sustain Dev 67:130
Lawrence RW, Wang Y (1996) Determination of neutralizing potential for acid rock drainage prediction. MEND/NEDEM report 1.16.3. Canadian Centre for Mineral and Energy Technology, Ottawa
Lin Z (1997) Mineralogical and chemical characterization of wastes from the sulfuric acid industry in Falun, Sweden. Environ Geol 30:152–162
Lottermoser B (2010) Mine wastes. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-12419-8
Mahdjoub Y, Choukroune P, Kienast JR (1997) Kinematics of a complex Alpine segment; superimposed tectonic and metamorphic events in the Petite Kabylie Massif (northern Algeria). Bull Soc Géol Fr 168:649–661
Mebarki A (2005) Hydrologie des bassins de l’est algérien: ressources en eau, aménagement et environnement. Université Mentouri de Constantine, Thèsed’Etat
Medjram S, Malika K (2014) Study and evaluation of risk related to waters contamination of dam of Guenitra, by heavy metals, from mine of Sidi Kamber. J Selcuk Univ Nat Appl Sci, pp 268–274
Michard A, Saddiqi O, Chalouan A, Rjimati EC, Moutaqi A (2011) Les principales mines du Maroc/Main Mines of Morocco. Notes et mémoires du service géologique, n° 564, vol 9. Editions du Service Géologique du Maroc, Rabat
Miller SD, Jeffery JJ, Wong JWC (1991) Use and misuse of the acid base account for AMD prediction. In: Proceedings of the 2nd international conference on acid rock drainage (ICARD), Montréal, Canada, vol 3, pp 489–506
ORGM (1971) Rapport des travaux de recherche de prospection géologique effectués sur le gisement polymétalique de Sidi Kamber. Internal technical report: unpublished
Othmani MA, Souissi F, Benzaazoua M, Bouzahzah H, Bussiere B, Mansouri A (2013) The geochemical behaviour of mine tailings from the Touiref Pb–Zn district in Tunisia in weathering cells leaching tests. Mine Water Environ 32:28–41
Oumdjbeur A (1986) Evaluation de la qualité physico-chimique des eaux du bassin versant du barrage de Guenitra (Wilaya de Skikda, Algérie). Université de Savoie, France
Parbhakar-Fox A, Lottermoser BG (2015) A critical review of acid rock drainage prediction methods and practices. Miner Eng 82:107–124. https://doi.org/10.1016/j.mineng.2015.03.015
Plumlee GS, Smith KS, Montour MR, Ficklin WH, Mosier EL (1999) Geologic controls on the composition of natural waters and mine waters draining diverse mineral-deposit types. The environmental geochemistry of mineral deposits. Part B: case studies and research topics 6, pp 373–432
Pope J, Trumm D (2015) Controls on Zn concentrations in acidic and neutral mine drainage from New Zealand’s bituminous coal and epithermal mineral deposits. Mine Water Environ 34:455–463. https://doi.org/10.1007/s10230-015-0372-2
Potts PJ (1987) A handbook of silicate rock analysis. Blakie, London
Rankin DWH (2009) CRC handbook of chemistry and physics, 89th edition, edited by David R. Lide. Crystallogr Rev 15:223–224. https://doi.org/10.1080/08893110902764125
Smith KS (1999) Metal sorption on mineral surfaces: an overview with examples relating to mineral deposits. Rev Econ Geol 6A:161–182
Sobek AA, Schuller WA, Freeman JR, Smith RM (1978) Field and laboratory methods applicable to overburdens and mine soils. EPA, Washington, pp 47–50
Souissi R, Souissi F, Chakroun HK, Bouchardon JL (2013) Mineralogical and geochemical characterization of mine tailings and Pb, Zn, and Cd mobility in a carbonate setting (Northern Tunisia). Mine Water Environ 32:16–27
SRK (1989) Acid rock drainage technical guide. BCAMD task force, vol 1. BiTech, Richmond
Taib M (2014) The Mineral Industry of Algeria. U.S. Geological Survey, Minerals Yearbook Algeria, p 13
Tlil H, Souissi R, Souissi F, Lattanzi P, Podda F, Concas S, Ardau C, Cidu R (2016) Environmental mineralogy and geochemistry of Pb–Zn mine wastes, Northern Tunisia. Rend Fis Acc Lincei 28:133141. https://doi.org/10.1007/s12210-016-0585-8
USEPA (1992) SW-846 Test method 1311: toxicity characteristic leaching procedure. Washington, DC
USEPA (2009) Hazardous waste characteristics. A User-friendly reference document. October 2009
USEPA (2014) Operating protocol for soil sampling. Science and Ecosystem Support Division operating procedure. Athens, Georgia
Villeneuve M (2004) Évaluation du comportement géochimique à long terme de rejets miniers à faible potentiel de génération d’acide à l’aide d’essais cinétiques. Mémoire de maîtrise en génie minéral, École Polytechnique de Montréal (2), pp 81–87
Washington State Department of Ecology (2003) An assessment of laboratory leaching tests for predicting the impacts of fill material on ground water and surface water quality. A report to the legislature (03)
Wentworth CK (1922) A scale of grade and class terms for clastic sediments. J Geol 30:377–392
Young RA (1995) The Rietveld method. Oxford University Press, Oxford, p 312
Yucel DS, Baba A (2016) Prediction of acid mine drainage generation potential of various lithologies using static tests: Etili coal mine (NW Turkey) as a case study. In: Environmental monitoring and assessment 188. https://doi.org/10.1007/s10661-016-5462-5
Acknowledgements
This study was partly carried out during scientific stay of the first author in the Institute of Research in Mines and Environment (IRME) of the University of Quebec in Abitibi-Temiscamingue. We thank Dr Kotaro Yonezu from Department of Engineering and Mineral Resources of Kyushu University (Japan) for assistance with X-ray fluorescence and X-ray Powder Diffraction. We would like to show our gratitude to the Pr Mameri Nabil (Ecole Nationale Polytechnique, Algiers, Algeria) for assistance and comments during static tests. We are also immensely grateful to ANESI’s Student Exchange Programme (UNESCO) for providing short period grants to Cadi Ayyad University (Morocco). We acknowledged the constructive criticism of two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Issaad, M., Boutaleb, A., Kolli, O. et al. Environmental characterization of mine waste at the Pb–Zn Sidi Kamber abandoned mine (NE Algeria). Rend. Fis. Acc. Lincei 30, 427–441 (2019). https://doi.org/10.1007/s12210-019-00806-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-019-00806-8