Abstract
Collective cell migration (CCM) can be described as a large scale coordinated movement of cells that are in close proximity with each other. It is a phenomenon that is observed not only in physiological processes such as that found in embryogenesis and wound healing but also in pathophysiological processes such as cancer metastasis. Some of the factors influencing this concerted process include cell–cell adhesion, cell–substrate interaction and mechanical cues such as geometrical constraints among others. Here, we review recent research work done to investigate CCM of adherent cells. We highlight the classical example of an in vitro cell monolayer to illustrate our current understanding of the different mechanobiological mechanisms involved as these cells respond to the mechanical cues present in their environment. It is hoped that such understanding may potentially lead to better therapeutic strategies for diseases such as cancer and for tissue engineering and repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell migration is crucial in many physiological and developmental processes such as wound healing, embryogenesis, morphogenesis and organ formation.23,24 In wound healing, cells migrate towards the wound to cover up the underlying exposed tissue, while in developmental processes, cells migrate to initiate pattern and shape formation for proper morphogenesis.24,25 Cell migration is also involved in some pathophysiological processes such as cancer metastasis.25 To date, migration of a single cell has been extensively studied and fairly well understood.39 Single cells typically exhibit a persistent random walk (Fig. 1a) but can switch to a more directed movement under asymmetric environmental cues.28,38,44,60,61 On a planar substrate, single cell migration involves repeated cycles of cell front extension, cell body contraction and rear end retraction. The molecular players involved in these shape changes are also well characterized.39 Cell front extension, e.g., filopodia or lamellipodia extension, is driven by Arp2/3 and Rac mediated actin polymerization, while cell body contraction is achieved through myosin-II mediated contractility. Moreover, interaction of the cell and its substrate, e.g., extracellular matrix (ECM), is mediated by focal adhesion (FA) and is crucial for cell migration.5,8,30,46,72,79
Cells migrating singly and collectively. (a) Single cell persistent random walk. Migration involves lamellipodia extension, cell body contraction and adhesion to substrate through focal adhesions. (b) Adherent cells migrating in groups are more directed. There is additional constraint of cell–cell adhesion during migration, transmitting forces between cells
In many situations, cells are not only interacting with the ECM but also with neighboring cells as they adhere and migrate (Fig. 1b). Examples include epithelial tissue expansion,9 endothelial cells vascular sprouting59 and the invasion of cancer cells.26 For migration of epithelial cells on a 2D surface, these cells are always bound to their neighbors via cell–cell adhesion molecules such as E-cadherins.42 Also, when many cells migrate together in the tissue, typically involving tens and up to hundred thousand cells,77 this results in the swarming of cells and the formation of large scale vortices.13,50,69 Such dynamics cannot be explained by just inferring from what we know about single cell migration. The emergent migratory behavior of many cells together, explicitly due to complex cell–cell and cell–substrate interactions, is termed as collective cell migration (CCM). CCM can be seen in both migration of adherent (e.g., epithelial cells) and non-adherent (e.g., fibroblasts41) cell cohorts and is much less understood than single cell migration. Nevertheless, recent studies are now showing that much can be learned about CCM by carefully considering the mechanobiological responses of the migrating cells arising from mechanical cues present in their environment.71 Furthermore, the increasing use of modeling in CCM research also significantly contributes towards identifying the most important parameters in understanding CCM.
From recent studies, mechanical parameters that have been investigated include interaction strength between cells,32 repulsion probabilities between cells (i.e., contact inhibition of locomotion),11 cell density,15 tissue, and substrate geometry,13,69 forces between cells and substrate,63 etc. Here, we focus on adherent cells and the mechanical aspects of CCM on 2D substrates. First, we give an overview of mechanically driven responses of single cells within the tissue that are relevant to CCM. Then we introduce concepts of CCM by using the classical example of a monolayer expansion into open space. Finally, we highlight examples to demonstrate that CCM mechanics is particularly useful in studying and understanding wound healing processes and diseases triggered by gaps in the monolayer.
Single Cell Level Responses to Mechanical Cues in CCM
Cells are the basic building blocks in a tissue. They are constantly exerting forces or being exerted upon through interactions with their neighbors and the substrate. These forces, if measureable, can provide excellent read-outs and insights into the various modes of CCM. Generally, these forces result in various types of stresses in the tissue. Tensile stress arises from cells pulling on each other and depends on cell–cell adhesion.67 On the other hand, compressive stress arises when cells push against each other in a tissue and this can help to align cells in the tissue.43 At the single cell level, cells in a tissue react to mechanical cues by regulating their movement, cell stiffness, and adhesion, decision to proliferate or undergo apoptosis, etc.37
Movement
Cells can be pulled or pushed by neighboring cells to move across a substrate or be stalled by equilibrated forces from all sides. More importantly, they can actively regulate their movement in response to such forces. Single mesendoderm cells are found to polarize and move in the opposite direction to small tugging stresses (~5 Pa) on its cadherin junctions.11,75 Interestingly, this tugging stress is similar to documented rear-end stress differences for a MDCK cell in an expanding epithelial tissue sheet67—very small compared to the actual average tugging stresses (~300 Pa) in the first few rows of cells but increases steadily into the tissue (to about ~1000 Pa for a tissue size of ~2–3 mm). The same polarization machinery here is also implicated in cell clusters, reminiscent of contact inhibition of locomotion (CIL), and contributes in a non-trivial manner to CCM.7 Modeling suggests that cells can migrate together or disperse depending on the probability of two cells to move away from each other.11
Cell Stiffness and Adhesion
The migration of a cell can be influenced by its own mechanical properties and that of its neighboring cells such as cell stiffness. Indeed, the distribution of the mechanical stress or stretch in a group of mechanically linked cells will depend on the stiffness of each of these cells, as in any passive mechanical system. As stress distributions can in turn dictate the movement of a cell collective, thus cell stiffness distributions in a tissue can affect CCM. Moreover, intrinsic cell stiffness changes dynamically with stretch,31,33,65,78 rendering the dependence of CCM on cell stiffness more complex. For example, strain fluidization can happen in cells as with other soft materials, e.g., after ~4 s of transient stretch (up to 10% stretch).65 A sustained stretch however generally induces an immediate opposite response, i.e., strain stiffening like other polymeric type materials,18 before relaxation happens in the order of a few 100 s. Further, as opposed to passive materials, cells also actively remodel their cytoskeleton to increase their stiffness when stretched or sheared.21 Shear stresses as low as 7 dyn/cm2 sustained for 5 h is found to induce significant keratin fibers thickening, thus increasing the cytoskeleton stiffness of alveolar epithelial cells.
Other than cell stiffness, cell–cell adhesion is another important mechanical parameter that has to be regulated in adherent type CCM. In particular, cell–cell adhesion can be strengthened by tensile stress between cells.3,16,17 For example, α-Catenin acts as a force transducer and mechanically stabilizes the adherens junctions. Specifically, α-Catenin unfolds under forces as small as ~15 pN per molecule, thus revealing another cryptic binding site for Vinculin and doubles the adhesion forces.80,81 Cell–cell adhesion can also in turn modify cell–substrate adhesion through integrin-cadherin crosstalks.47,74
Decision to Proliferate or Undergo Apoptosis
Cells proliferate or undergo apoptosis to maintain tissue homeostasis, adding or deleting mass from the tissue and thereby influencing the movement of neighboring cells. Evidence has also shown that these fate decisions that a cell makes are dependent on mechanical cues. Some examples are (1) increase in epithelial cell proliferation rate as the cell area increases62 (proliferation rate increases from ~1/40 to ~1/20 h-1 as cell area doubled from ~200 to ~400 μm2), (2) alignment of cell proliferation axis with stress direction20,40 and (3) increase in apoptosis rate as cell area decreases.62
Experimental Techniques to Study CCM Mechanics
A plethora of in vitro techniques have been established to study the behavior of CCM. Microcontact printing and scratch and barrier assays introduce open spaces with different geometries and shapes in the tissue for migration studies.70 Also, forces involved in migration (usually tens to hundreds of nanoNewtons per cell) can be measured by techniques such as traction force microscopy,63,67 micropillars,14,56 laser ablation,58 embedding of droplets with well characterized mechanical properties,6 FRET sensors,29,45 etc. In the following sections, we will highlight some of the work that has been done using some of these assays.
Roles of CCM in Epithelial Monolayer Expansion
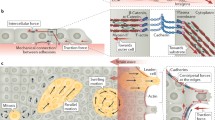
The epithelial monolayer is known to expand and invade into open space (Fig. 2). Many different underlying mechanisms contribute to this mode of migration and illustrate well the complexity of CCM. At the outset of monolayer expansion, some cells (termed “leader cells”) which are usually at the first few rows of the tissue start to polarize and extend large lamellipodia towards the empty space adjacent to them34,53 (Fig. 2a). These cells propel themselves forward by exerting large traction stresses on the substrate with the help of the lamellipodia (~2 kPa).53 Follower cells behind the leader cell are also pulled forward due to strong tensile stresses in actin cables that connect several cells through cell–cell junctions. This group of leader–follower cells (possibly up to 30 cells) can be regarded as a “super-cell” that surges forward together and forms a finger-like structure in the advancing monolayer. Strong actin cables form and align at the long edges of the finger as a mechanical constraint for these supracellular structures.53 This prevents new leader cell formation at these locations, further stabilizing the progression of long fingers. However, many fingers can emerge at the expanding tissue front.
Epithelial monolayer expansion. (a) Leader cells drag follower cells and drive finger formation at migrating front. (b) Inward moving mechanical wave (red dotted line) propagating into the tissue from front. Cells immobilize sequentially and migrate outwards to expand the monolayer. (c) Tension builds up in expanding monolayer, increasing progressively into the tissue and presenting a possible polarization cue for cells. (d) Cell division axis aligns with monolayer expansion direction. (e) Cells at the side of the hole closest to the migrating front are stretched by outward propagating neighbors but still exert forces to polarize themselves towards the hole, i.e., “Kenotaxis”. (f) Cells move in vortice-like swirls in unconfined monolayer. (g) Small enough confinement forces cells to move persistently with ordered velocity fields. (h) Leader cells preferentially emerge at sharp tissue edges indicating influence of geometry
No matter how large a force leader cells can exert, it is finite (typically up to ~100 nN)53 and insufficient to drag the whole monolayer forward. What then drives the monolayer expansion? Cells deep in the cell sheet are also known to extend lamellipodia (termed “cryptic lamellipodia”) to pull on the substrate and actively migrate.19 As such, there must be a mechanism to polarize the cells within the tissue. Although not fully understood, plausible explanations include progressive unjamming where cells consecutively acquire the space to extend lamellipodia when the neighbor before them start moving outward57 (Fig. 2b). This facilitates the cells to follow the movement of their neighbors, leading to collective movement. Regardless of the origins of polarization, evidence has shown that an active mechanical wave (termed “X-wave”) can propagate from the monolayer front and possibly communicate the information for polarization deep within the cell sheet, with speed of propagation of 1 μm/min. More interestingly, the X-wave propagation is continuously reiterated with time (~100–200 min per cycle for tissue length of ~1 mm). This can be explained by cycles of strain stiffening and softening of cells.
Another proposed mechanism that explains cell polarization outwards within the tissue is based on tension buildup in the monolayer67,75 (Fig. 2c). As explained in the previous section, cells can polarize and move opposite to the direction of tugging force exerted on their cadherin junctions. Similarly, a difference in the magnitude of the tensile stresses acting on opposite sides of a cell can drive the cell towards the side with the lower stress.75 As such, tension buildup in the cell sheet due to accumulation of cell–substrate traction can give rise to a tension buildup gradient of ~600 Pa/mm. This then induces higher tensile stress at the cell side facing the inner part of the monolayer and drives CCM in the direction of monolayer expansion. The X-wave and tension buildup are interdependent, and could possibly work together to ensure the robustness of cell polarization in the cell sheet and tissue.
There are other evidence which shows that cell division can also contribute towards monolayer expansion into large empty spaces62 (Fig. 2d). With finite number of cells, the monolayer cannot expand indefinitely since cells are adhered together and will hold each other back due to buildup of elastic tension. In reality, when a cell is stretched, its area exceeds a certain threshold thus sharply increasing its division probability. Due to this addition of new cells, all the cells in the monolayer can maintain a finite stretch while still expanding the monolayer. Moreover, modeling efforts predict that cell division and apoptosis fluidizes the monolayer and should allow it to flow more easily to occupy empty spaces.51 Finally, as described before, cell division axis can align in the direction of the principal tensile stress axis (parallel to the direction of cell movement in the monolayer67) and could further increase the efficiency of the monolayer expansion.
To recap, cells can be polarized towards open space and in the direction of its neighbors’ movements. It is interesting but not clear which polarization factor will prevail or whether they will cancel out if cells are subjected to two opposing polarizing directions. In a recent experiment, a small non-adhesive zone (i.e., hole) was introduced in a large expanding monolayer, and the findings suggest that the open space has a higher polarizing power (termed “kenotaxis”)36 (Fig. 2e). At the sides of the hole nearer to the monolayer expanding front, cells clearly polarize and exert tractions forces (~10 Pa) to pull themselves towards the gap (refer Fig. 2e). Neighbors that are moving in the opposite direction (towards monolayer front) fail to induce the hole-bound cells to move with them, even though they do impart large tensile stresses (up to ~100–200 Pa through cell–cell junctions. The cells nearest to the hole are instead stretched to unusually elongated shapes.
Cell Cooperativity and Geometry
Tensile stress between cells that is mediated by cell–cell adhesion also plays a fundamental role in determining the extent of correlated cell movements or cell cooperativity. With stronger adhesions, more cells tend to move as a coherent group spanning a larger distance, i.e., larger correlation length.13,69 In an expanding monolayer, cells in the tissue migrate in the direction of minimum shear stress or maximum tensile stress imparted by their neighbors.66 They together form moving cell chains or clusters, typically of the size of few hundred micrometers (~150–200 μm for MDCK epithelial cells). In turn, these large scale cell movements emerge to form independent vortices in the cell sheet69 (Fig. 2f), and reduce the expansion efficiency. If however, the tissue moves into a confined channel with a width smaller than its correlation length, vortices disappear and the cells tend to align due to the geometrical constraints69 (Fig. 2g). This ordered polarization increases overall migration speed (velocity at tissue front doubles from ~20 to ~40 μm/h for a decreasing constraint width of ~400 to ~20 μm for MDCK cells). If the cell density increases in the channel, compressive stress also helps cells to align, as is evident from non-adhesive cells (i.e., fibroblasts) aligning in channels.15
In fact, other evidence has also shown how other geometrical cues influence CCM. For example, leader cells tend to emerge from the location of a tissue where the tissue curvature is higher52 (Fig. 2h). The collective traction force distributions of a tissue are found to be greater at places with higher curvature (~150 Pa for radius of curvature of the order of the size of a cell, i.e., ~20 μm compared to <100 Pa for radius of curvature of ~200 μm) and this can promote stress fiber formation. Large stress fibers are formed normal to the edges and polarized the cells there. Discontinuities in the actin belt surrounding the tissue are also seen at sharp concave edges, which release the constraint and further promote leader cell formation in those locations. Yet other evidence shows single cells aligning in response to topographical cues (e.g., grooves) and this applies to cells in a monolayer as well.43 The alignment effects of grooves can propagate to cells on flat surfaces by compressive stresses.
Epithelium Gap Closure: Implications in Health and Disease
Understanding CCM can allow one to predict the outcome of physiological or pathophysiological events and possibly help in the development of relevant therapeutics. We present here an example on epithelium gap closure to illustrate this point. Epithelium discontinuities occur frequently in physiological systems. Some examples include wounds inflicted on the surface of organs and the skin or gaps present during morphogenetic processes such as Drosophila dorsal closure.35 Failure to restore the integrity of the monolayer (in time) can result in wound infection or morphogenetic defects. Along with the biochemical factors, many other gap closure factors are found to be mechanical in nature. This includes the geometry of the gap,1 distribution or presence of ECM in the gap,22 substrate stiffness,1,48 interaction with underlying cells,27 etc. In view of this complexity, in vitro experiments or model wounds that can control and distinguish the contribution of each of these factors are highly valued. In particular, combination of the barrier assay with the use of micro-contact printing can produce well defined epithelial gaps and ECM surface of arbitrary geometry.70 This allows systematic study of these two mechanical factors, i.e., gap geometry and the presence of ECM in the undamaged tissue, but prevents the influence of chemical factors secreted by damaged cells. The discussion below is based on this type of assay.
Experiments that probe model wound closure typically monitors epithelial movement in a tissue gap, presented over a uniform ECM surface (Fig. 3a). Studies indicate that lamellipodial extension by cells at the front1 and purse-string contraction (pluricellular actin cables)55 are important for larger and smaller gap closures, respectively. Small gaps are of a few cell sizes while large gaps can range from tens to hundreds of cells or more, e.g., in scratch wound assays. Mechanical models of the epithelium successfully integrate these components to simulate experimental data (e.g., decrease of gap area as function of time).2,10 Lamellipodial activity can be modeled as a friction force at the gap front while purse-string is described by a line tension and Laplace’s pressure. Importantly, the fact that the tissue is being pulled coherently by localized forces at the front clearly shows that CCM is an integral part of gap closure. Of therapeutic interest, experiments coupled with modeling also allows clear quantification and dissection of certain drug effects (e.g., Rho and Rac inhibition drugs) on gap closure speed10 and can help predict total wound healing time.
In vitro monolayer gap closure to study wound healing. (a) (left) Wound healing assay with uniform ECM coating and well defined gap geometry. Lamellipodia extension and purse-string contraction help closure. (a) (right) Wound healing assay involving closure of a hole with bright intensity showing actin filaments. (Image adapted from Ref. 1.) Purse-string was formed from the non-uniform accumulation of actins around the wound edge. However, there were also some protrusions in the form of lamellipodia at some parts of the edge. Scale bar is 25 μm. (b) Keratinocyte sheets are pulled over non-adhesive regions (yellow) by migrating cells in the channels, forming bridges. More migrating cells on wider channel (right) pull the sheets to larger extent. Strong stress fibers (red lines) in the bridge resist huge mechanical tensions, stretching cells horizontally. (c), (d) Images obtained from experiments conducted to study keratinocyte formed bridges. (Adapted from Ref. 68.) Cells crawling on fibronectin coated channels (red) of (c) narrow and (d) large widths pulled other cells over the non-adhesive regions (dark grey) to form epithelial bridges. Scale bar (c, d), 50 μm
In both physiological and pathophysiological processes, the underlying ECM and tissue of the epithelium can get damaged or become discontinuous, further hindering the closure event.12 Such situations can be mimicked by forming well-defined geometries of adhesive and non-adhesive patches on a substrate. In our recent work, alternating stripes of fibronectin separated far apart (>100 μm) by non-adhesive regions were fabricated for cells to migrate along68 (Figs. 3b, 3c, and 3d). It was observed that keratinocyte monolayers can be pulled over non-adhesive regions of sizes much larger than a single cell, forming multicellular bridges. Notable CCM features in this system include: (1) wider fibronectin stripes allow more migrating cells to exert forces (~1 kPa traction stresses at cell front), and collectively pull more cells over larger non-adhesive regions (Figs. 3b, 3c, and 3d), (2) large number of stress fibers permeate many cells above non-adhesive surfaces, forming a tissue-level network that resists tensile stress which pulls on those cells (Fig. 3b), and (3) cell–cell adhesion strength has to be high enough to resist the large tensile stresses. Importantly, the cell–cell adhesion strength is a crucial indicator of whether a tissue can close gaps over surfaces without ECM. Various epithelial cell lines may exhibit different behaviors based on the mechanical properties of their cell–cell junctions. For instance, MDCK cells can easily slide past each other as they exhibit more labile cell–cell adhesion than keratinocytes. Consequently, as opposed to keratinocytes, MDCK epithelial cells could not form suspended bridges over the bare surfaces under similar experimental conditions. The fact that keratinocytes can form suspended cell sheets to cover non-adhesive surfaces is hypothesized to be important for wound healing in vivo.
Understanding the fundamentals of monolayer wound healing is imperative because failure to close physiological wounds will lead to many forms of diseases in the body. One prominent example is atherosclerosis, where arterial thickening leads to blood vessel blockage and acute cardiac problems. Atherosclerosis development is triggered by excessive endothelial cell (EC) inflammation and macromolecular transport into the vessel. In late stages of atherosclerosis, EC wounds are known to aggravate disease progression by totally exposing and stimulating the underlying smooth muscle cells to proliferate uncontrollably.4 Stenting allows mechanical dilation of the blocked vessels and offers a common form of treatment. However, it is hugely complicated by in-stent restenosis, i.e., re-blocking of the vessel.64 Here again, large areas of ECs are denuded by the deployment of the stent and the vessel surface is thus devoid of EC secreted anti-thrombosis factors (e.g., nitric oxide), leading to stenosis.4,64 Evidence shows that EC wound regeneration and gap closure is crucial for atherosclerosis treatment. Other diseases that involve the breach of epithelial barrier function due to wounding include acute kidney injury,49 asthma, allergic rhinitis,73 etc.
Conclusions and Future Perspectives
As discussed, CCM is extremely complex where even a simple monolayer tissue expansion constitutes a plethora of different mechanisms. Studies that focus on mechanobiological aspects of the problem are fast proving to be a good approach to further our understanding of CCM. Work in this direction has elucidated mechanisms such as existence of leader–follower cell groups, polarization of cells deep within the tissue by mechanical wave propagation and tension buildup. Tissue geometry is also an important factor as well, where leader cells can emerge from sharp tissue edges while tissue flow is more persistent in confined channels.
The in vitro CCM assays, when set in an epithelial gap closure context, provide excellent models for wound healing. Geometries of the gap in the monolayer and the underlying substrate can be readily controlled and their influence on wound closure parameters easily studied. The effects of drugs on wound closure time have also been investigated. Other factors that are less studied include differences in substrate stiffness and dynamic stretching of substrates.76
Although many CCM assays used have yielded new information, they are still far from mimicking that of in vivo situations. For example, more than one cell type is usually implicated as can be seen in amnioserosa and lateral epidermis cells in drosophila wound closure35 and border and nurse cells in Drosophila oogenesis. Further co-culture assays could provide more information on this aspect. Also in vivo, cells are simultaneously presented with multiple cues such as chemical, mechanical and even electrical cues.54 Extensive work has been carried out on multi-cue directed CCM, but a comprehensive understanding of the fundamental mechanisms remains elusive. As such, research in CCM is still in its infancy stage and much still needs to be done to bridge our understanding of single cell migration to that of heterotypic and multicellular migration since CCM is so central to the development and maintenance of multicellular organizations. If we can understand why and how cells can so efficiently orchestrate their movements in specific directions to specific locations and within geometrical constraints in the human body, we may also be able to better understand why and how deviation from these processes may result in serious consequences such as tumor formation and metastasis. Hopefully, this may lead to better therapeutic strategies for diseases such as cancer and for tissue engineering and repair.
References
Anon, E., X. Serra-Picamal, P. Hersen, N. C. Gauthier, M. P. Sheetz, X. Trepat, and B. Ladoux. Cell crawling mediates collective cell migration to close undamaged epithelial gaps. Proc. Natl. Acad. Sci. USA. 109(27):10891–10896, 2012.
Arciero, J. C., Q. Mi, M. F. Branca, D. J. Hackam, and D. Swigon. Continuum model of collective cell migration in wound healing and colony expansion. Biophys. J. 100(3):535–543, 2011.
Bajpai, S., J. Correia, Y. Feng, J. Figueiredo, S. X. Sun, G. D. Longmore, G. Suriano, and D. Wirtz. α-Catenin mediates initial E-cadherin-dependent cell-cell recognition and subsequent bond strengthening. Proc. Natl. Acad. Sci. USA. 105(47):18331–18336, 2008.
Barakat, A. I. Blood flow and arterial endothelial dysfunction: mechanisms and implications. C.R. Phys. 14(6):479–496, 2013.
Burridge, K., and K. Wennerberg. Rho and Rac take center stage. Cell 116(2):167–179, 2004.
Campàs, O., T. Mammoto, S. Hasso, R. A. Sperling, D. O’Connell, A. G. Bischof, R. Maas, D. A. Weitz, L. Mahadevan, and D. E. Ingber. Quantifying cell-generated mechanical forces within living embryonic tissues. Nat. Methods 11(2):183–189, 2014.
Carmona-Fontaine, C., H. K. Matthews, S. Kuriyama, M. Moreno, G. A. Dunn, M. Parsons, C. D. Stern, and R. Mayor. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 456(7224):957–961, 2008.
Caswell, P. T., and J. C. Norman. Integrin trafficking and the control of cell migration. Traffic. 7(1):14–21, 2006.
Chung, S., and D. J. Andrew. Cadherin 99C regulates apical expansion and cell rearrangement during epithelial tube elongation. Development. 141(9):1950–1960, 2014.
Cochet-Escartin, O., J. Ranft, P. Silberzan, and P. Marcq. Border forces and friction control epithelial closure dynamics. Biophys. J. 106(1):65–73, 2014.
Desai, R. A., S. B. Gopal, S. Chen, and C. S. Chen. Contact inhibition of locomotion probabilities drive solitary versus collective cell migration. J. R. Soc. Interface 10(88):20130717, 2013.
Diegelmann, R. F., and M. C. Evans. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 9:283–289, 2004.
Doxzen, K., S. R. K. Vedula, M. C. Leong, H. Hirata, N. S. Gov, A. J. Kabla, B. Ladoux, and C. T. Lim. Guidance of collective cell migration by substrate geometry. Integr. Biol. (Camb). 5(8):1026–1035, 2013.
Du Roure, O., A. Saez, A. Buguin, R. H. Austin, P. Chavrier, P. Siberzan, and B. Ladoux. Force mapping in epithelial cell migration. Proc. Natl. Acad. Sci. USA. 102(7):2390–2395, 2005.
Duclos, G., S. Garcia, H. G. Yevick, and P. Silberzan. Perfect nematic order in confined monolayers of spindle-shaped cells. Soft Matter 10(14):2346–2353, 2014.
Dufour, S., R. M. Mege, and J. P. Thiery. alpha-catenin, vinculin, and F-actin in strengthening E-cadherin cell–cell adhesions and mechanosensing. Cell Adh. Migr. 7(4):345–350, 2013.
El Sayegh, T. Y., P. D. Arora, C. A. Laschinger, W. Lee, C. Morrison, C. M. Overall, A. Kapus, and C. A. McCulloch. Cortactin associates with N-cadherin adhesions and mediates intercellular adhesion strengthening in fibroblasts. J. Cell Sci. 117(Pt 21):5117–5131, 2004.
Erk, K. A., K. J. Henderson, and K. R. Shull. Strain stiffening in synthetic and biopolymer networks. Biomacromolecules 11(5):1358–1363, 2010.
Farooqui, R., and G. Fenteany. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J. Cell Sci. 118(Pt 1):51–63, 2005.
Fink, J., N. Carpi, T. Betz, A. Betard, M. Chebah, A. Azioune, M. Bornens, C. Sykes, L. Fetler, D. Cuvelier, and M. Piel. External forces control mitotic spindle positioning. Nat. Cell Biol. 13(7):771–778, 2011.
Flitney, E. W., E. R. Kuczmarski, S. A. Adam, and R. D. Goldman. Insights into the mechanical properties of epithelial cells: the effects of shear stress on the assembly and remodeling of keratin intermediate filaments. FASEB J. 23(7):2110–2119, 2009.
Fong, E., S. Tzlil, and D. A. Tirrell. Boundary crossing in epithelial wound healing. Proc. Natl. Acad. Sci. USA. 107(45):19302–19307, 2010.
Franz, C. M., G. E. Jones, and A. J. Ridley. Cell migration in development and disease. Dev. Cell 2(2):153–158, 2002.
Friedl, P., and D. Gilmour. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10(7):445–457, 2009.
Friedl, P., Y. Hegerfeldt, and M. Tusch. Collective cell migration in morphogenesis and cancer. Int. J. Dev. Biol. 48:441–450, 2004.
Friedl, P., J. Locker, E. Sahai, and J. E. Segall. Classifying collective cancer cell invasion. Nat. Cell Biol. 14(8):777–783, 2012.
Gabison, E. E., E. Huet, C. Baudouin, and S. Menashi. Direct epithelial-stromal interaction in corneal wound healing: role of EMMPRIN/CD147 in MMPs induction and beyond. Prog. Retin. Eye Res. 28(1):19–33, 2009.
Goguen, B. N., and B. Imperiali. Chemical tools for studying directed cell migration. ACS Chem. Biol. 6(11):1164–1174, 2011.
Grashoff, C., B. D. Hoffman, M. D. Brenner, R. Zhou, M. Parsons, M. T. Yang, M. A. McLean, S. G. Sligar, C. S. Chen, and T. Ha. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 466(7303):263–266, 2010.
Hood, J. D., and D. A. Cheresh. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2(2):91–100, 2002.
Icard-Arcizet, D., O. Cardoso, A. Richert, and S. Henon. Cell stiffening in response to external stress is correlated to actin recruitment. Biophys. J. 94(7):2906–2913, 2008.
Kabla, A. J. Collective cell migration: leadership, invasion and segregation. J. R. Soc. Interface 9(77):3268–3278, 2012.
Kasza, K. E., F. Nakamura, S. Hu, P. Kollmannsberger, N. Bonakdar, B. Fabry, T. P. Stossel, N. Wang, and D. A. Weitz. Filamin A is essential for active cell stiffening but not passive stiffening under external force. Biophys. J. 96(10):4326–4335, 2009.
Khalil, A. A., and P. Friedl. Determinants of leader cells in collective cell migration. Integr. Biol. (Camb). 2(11–12):568–574, 2010.
Kiehart, D. P., C. G. Galbraith, K. A. Edwards, W. L. Rickoll, and R. A. Montague. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell Biol. 149(2):471–490, 2000.
Kim, J. H., X. Serra-Picamal, D. T. Tambe, E. H. Zhou, C. Y. Park, M. Sadati, J. A. Park, R. Krishnan, B. Gweon, E. Millet, J. P. Butler, X. Trepat, and J. J. Fredberg. Propulsion and navigation within the advancing monolayer sheet. Nat. Mater. 12(9):856–863, 2013.
Ladoux, B., and A. Nicolas. Physically based principles of cell adhesion mechanosensitivity in tissues. Rep. Prog. Phys. 75(11):116601, 2012.
Lara Rodriguez, L., and I. C. Schneider. Directed cell migration in multi-cue environments. Integr Biol (Camb). 5(11):1306–1323, 2013.
Lauffenburger, D. A., and A. F. Horwitz. Cell migration: a physically integrated molecular process. Cell 84(3):359–369, 1996.
Legoff, L., H. Rouault, and T. Lecuit. A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development. 140(19):4051–4059, 2013.
Leong, M. C., S. R. K. Vedula, C. T. Lim, and B. Ladoux. Geometrical constraints and physical crowding direct collective migration of fibroblasts. Commun. Integr. Biol. 6:e23197, 2013.
Li, L., R. Hartley, B. Reiss, Y. Sun, J. Pu, D. Wu, F. Lin, T. Hoang, S. Yamada, J. Jiang, and M. Zhao. E-cadherin plays an essential role in collective directional migration of large epithelial sheets. Cell. Mol. Life Sci. 69(16):2779–2789, 2012.
Londono, C., M. J. Loureiro, B. Slater, P. B. Lucker, J. Soleas, S. Sathananthan, J. S. Aitchison, A. J. Kabla, and A. P. McGuigan. Nonautonomous contact guidance signaling during collective cell migration. Proc. Natl. Acad. Sci. USA. 111(5):1807–1812, 2014.
Majumdar, R., M. Sixt, and C. A. Parent. New paradigms in the establishment and maintenance of gradients during directed cell migration. Curr. Opin. Cell Biol. 30C:33–40, 2014.
Meng, F., and F. Sachs. Visualizing dynamic cytoplasmic forces with a compliance-matched FRET sensor. J. Cell Sci. 124(2):261–269, 2011.
Mercurio, A. M., I. Rabinovitz, and L. M. Shaw. The α6β4 integrin and epithelial cell migration. Curr. Opin. Cell Biol. 13(5):541–545, 2001.
Mertz, A. F., Y. Che, S. Banerjee, J. M. Goldstein, K. A. Rosowski, S. F. Revilla, C. M. Niessen, M. C. Marchetti, E. R. Dufresne, and V. Horsley. Cadherin-based intercellular adhesions organize epithelial cell-matrix traction forces. Proc. Natl. Acad. Sci. USA. 110(3):842–847, 2013.
Ng, M. R., A. Besser, G. Danuser, and J. S. Brugge. Substrate stiffness regulates cadherin-dependent collective migration through myosin-II contractility. J. Cell Biol. 199(3):545–563, 2012.
Palmyre, A., J. Lee, G. Ryklin, T. Camarata, M. K. Selig, A.-L. Duchemin, P. Nowak, M. A. Arnaout, I. A. Drummond, and A. Vasilyev. Collective epithelial migration drives kidney repair after acute injury. PLoS ONE 9(7):e101304, 2014.
Petitjean, L., M. Reffay, E. Grasland-Mongrain, M. Poujade, B. Ladoux, A. Buguin, and P. Silberzan. Velocity fields in a collectively migrating epithelium. Biophys. J. 98(9):1790–1800, 2010.
Ranft, J., M. Basan, J. Elgeti, J. F. Joanny, J. Prost, and F. Julicher. Fluidization of tissues by cell division and apoptosis. Proc. Natl. Acad. Sci. USA. 107(49):20863–20868, 2010.
Rausch, S., T. Das, J. R. Soine, T. W. Hofmann, C. H. Boehm, U. S. Schwarz, H. Boehm, and J. P. Spatz. Polarizing cytoskeletal tension to induce leader cell formation during collective cell migration. Biointerphases. 8(1):36, 2013.
Reffay, M., M. C. Parrini, O. Cochet-Escartin, B. Ladoux, A. Buguin, S. Coscoy, F. Amblard, J. Camonis, and P. Silberzan. Interplay of RhoA and mechanical forces in collective cell migration driven by leader cells. Nat. Cell Biol. 16(3):217–223, 2014.
Rodriguez, L. L., and I. C. Schneider. Directed cell migration in multi-cue environments. Integr. Biol. 5(11):1306–1323, 2013.
Russo, J. M., P. Florian, L. Shen, W. V. Graham, M. S. Tretiakova, A. H. Gitter, R. J. Mrsny, and J. R. Turner. Distinct temporal-spatial roles for rho kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterology 128(4):987–1001, 2005.
Saez, A., A. Buguin, P. Silberzan, and B. Ladoux. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys. J. 89(6):L52–L54, 2005.
Serra-Picamal, X., V. Conte, R. Vincent, E. Anon, D. T. Tambe, E. Bazellieres, J. P. Butler, J. J. Fredberg, and X. Trepat. Mechanical waves during tissue expansion. Nat. Phys. 8:2012, 2012.
Shen, N., D. Datta, C. B. Schaffer, P. LeDuc, D. E. Ingber, and E. Mazur. Ablation of cytoskeletal filaments and mitochondria in live cells using a femtosecond laser nanoscissor. Mech. Chem. Biosyst. 2(1):17–25, 2005.
Shin, Y., J. S. Jeon, S. Han, G. S. Jung, S. Shin, S. H. Lee, R. Sudo, R. D. Kamm, and S. Chung. In vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. Lab Chip 11(13):2175–2181, 2011.
Smith, J. T., J. T. Elkin, and W. M. Reichert. Directed cell migration on fibronectin gradients: effect of gradient slope. Exp. Cell Res. 312(13):2424–2432, 2006.
Smith, J. T., D. H. Kim, and W. M. Reichert. Haptotactic gradients for directed cell migration: stimulation and inhibition using soluble factors. Comb. Chem. High Throughput Screen. 12(6):598–603, 2009.
Streichan, S. J., C. R. Hoerner, T. Schneidt, D. Holzer, and L. Hufnagel. Spatial constraints control cell proliferation in tissues. Proc. Natl. Acad. Sci. USA. 111(15):5586–5591, 2014.
Style, R. W., R. Boltyanskiy, G. K. German, C. Hyland, C. W. MacMinn, A. F. Mertz, L. A. Wilen, Y. Xu, and E. R. Dufresne. Traction force microscopy in physics and biology. Soft Matter 10(23):4047–4055, 2014.
Tahir, H., C. Bona-Casas, and A. G. Hoekstra. Modelling the effect of a functional endothelium on the development of in-stent restenosis. PLoS ONE 8(6):e66138, 2013.
Trepat, X., L. Deng, S. S. An, D. Navajas, D. J. Tschumperlin, W. T. Gerthoffer, J. P. Butler, and J. J. Fredberg. Universal physical responses to stretch in the living cell. Nature 447(7144):592–595, 2007.
Trepat, X., and J. J. Fredberg. Plithotaxis and emergent dynamics in collective cellular migration. Trends Cell Biol. 21(11):638–646, 2011.
Trepat, X., M. R. Wasserman, T. E. Angelini, E. Millet, D. A. Weitz, J. P. Butler, and J. J. Fredberg. Physical forces during collective cell migration. Nat. Phys. 5:2009, 2009.
Vedula, S. R. K., H. Hirata, M. H. Nai, A. Brugues, Y. Toyama, X. Trepat, C. T. Lim, and B. Ladoux. Epithelial bridges maintain tissue integrity during collective cell migration. Nat. Mater. 13(1):87–96, 2014.
Vedula, S. R. K., M. C. Leong, T. L. Lai, P. Hersen, A. J. Kabla, C. T. Lim, and B. Ladoux. Emerging modes of collective cell migration induced by geometrical constraints. Proc. Natl. Acad. Sci. USA. 109(32):12974–12979, 2012.
Vedula, S. R. K., A. Ravasio, E. Anon, T. Chen, G. Peyret, M. Ashraf, and B. Ladoux. Microfabricated environments to study collective cell behaviors. Methods Cell Biol. 120:235–252, 2014.
Vedula, S. R. K., A. Ravasio, C. T. Lim, and B. Ladoux. Collective cell migration: a mechanistic perspective. Physiology (Bethesda). 28(6):370–379, 2013.
Vega, F. M., A. Colomba, N. Reymond, M. Thomas, and A. J. Ridley. RhoB regulates cell migration through altered focal adhesion dynamics. Open Biol. 2(5):120076, 2012.
Wang, Y., C. Bai, K. Li, K. B. Adler, and X. Wang. Role of airway epithelial cells in development of asthma and allergic rhinitis. Respir. Med. 102(7):949–955, 2008.
Weber, G. F., M. A. Bjerke, and D. W. DeSimone. Integrins and cadherins join forces to form adhesive networks. J. Cell Sci. 124(Pt 8):1183–1193, 2011.
Weber, G. F., M. A. Bjerke, and D. W. DeSimone. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev. Cell 22(1):104–115, 2012.
Wegener, J., and J. Seebach. Experimental tools to monitor the dynamics of endothelial barrier function: a survey of in vitro approaches. Cell Tissue Res. 355(3):485–514, 2014.
Weijer, C. J. Collective cell migration in development. J. Cell Sci. 122(18):3215–3223, 2009.
Wolff, L., P. Fernandez, and K. Kroy. Resolving the stiffening-softening paradox in cell mechanics. PLoS ONE 7(7):e40063, 2012.
Xu, Y., T. A. Bismar, J. Su, B. Xu, G. Kristiansen, Z. Varga, L. Teng, D. E. Ingber, A. Mammoto, R. Kumar, and M. A. Alaoui-Jamali. Filamin A regulates focal adhesion disassembly and suppresses breast cancer cell migration and invasion. J. Exp. Med. 207(11):2421–2437, 2010.
Yao, M., B. T. Goult, H. Chen, P. Cong, M. P. Sheetz, and J. Yan, Mechanical activation of vinculin binding to talin locks talin in an unfolded conformation. Scientific Rep. 4, 2014.
Yonemura, S., Y. Wada, T. Watanabe, A. Nagafuchi, and M. Shibata. α-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 12(6):533–542, 2010.
Acknowledgments
The authors wish to thank Manon Prost, Chun Xi Wong and Larisa Bulavina at the Mechanobiology Institute for preparing the illustrations for the figures. Financial supports from the Human Frontier Science Program (Grant RGP0040/2012), and the Mechanobiology Institute (team project funding) are gratefully acknowledged. B. L. acknowledges the Institut Universitaire de France for its support.
Conflict of interest
T. B. Saw, S. Jain, B. Ladoux and C. T. Lim declare that they have no conflicts of interest.
Ethical Standards
No human and animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Cheng Dong oversaw the review of this article.
Thuan Beng Saw and Shreyansh Jain contributed equally to this work.
Rights and permissions
About this article
Cite this article
Saw, T.B., Jain, S., Ladoux, B. et al. Mechanobiology of Collective Cell Migration. Cel. Mol. Bioeng. 8, 3–13 (2015). https://doi.org/10.1007/s12195-014-0366-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-014-0366-3