Abstract
Radiotherapy accelerators have undergone continuous technological developments. We investigated the differences between Radixact™ and VMAT treatment plans. Sixty patients were included in this study. Dosimetric comparison between the Radixact™ and VMAT plans was performed for six cancer sites: whole-brain, head and neck, lymphoma, lung, prostate, and rectum. The VMAT plans were generated with two Elekta linear accelerators (Synergy® and Versa HD™). The planning target volume (PTV) coverage, organs-at-risk dose constraints, and four dosimetric indexes were considered. The deliverability of the plans was assessed using quality assurance (gamma index evaluation) measurements; clinical judgment was included in the assessment. The mean AAPM TG218 (3%–2 mm, global normalization) gamma index values were 99.4%, 97.8%, and 96.6% for Radixact™, Versa HD™, and Synergy®, respectively. Radixact™ performed better than Versa HD™ in terms of dosimetric indexes, hippocampi D100%, spinal cord Dmax, rectum V38.4 Gy, bladder V30 Gy, and V40 Gy. Versa HD™ saved more of the (lungs-PTV) V5 Gy and (lungs-PTV) Dmean, heart Dmean, breasts V4 Gy, and bowel V45 Gy. Regarding Synergy®, the head and neck Radixact™ plan saved more of the parotid gland, oral cavity, and supraglottic larynx. From a clinical point of view, for the head and neck, prostate, and rectal sites, the Radixact™ and Versa HD™ plans were similar; Radixact™ plans were preferable for the head and neck and rectum to Synergy® plans. The quality of linac plans has improved, and differences with tomotherapy have decreased. However, tomotherapy continues to be an essential add-on in multi-machine departments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Helical tomotherapy (HT) is a well-known technology for the radiotherapy treatment of multiple anatomic areas. It delivers a highly precise rotational dose while the patient is translated through the gantry bore for a highly conformal dose distribution to the target, simultaneously sparing healthy tissue. HT works in combination with megavoltage computed tomography (CT); however, kilovoltage computed tomography has recently become available for repositioning and adaptive radiotherapy. Mackie et al. [1] developed HT at the University of Wisconsin more than 20 years ago. However, the latest generation, the Radixact™ system (TOMO) (Accuray®, Sunnyvale, CA), has been designed to combine all developments that have subsequently occurred into a single platform with the Treatment Planning System (TPS) Precision™ and an integrated data management system (iDMS).
From the beginning of its development, the capabilities of HT, compared with those of traditional linear accelerators (linacs), have been explored. Several studies [2,3,4,5,6,7,8,9,10,11,12,13,14,15] have been conducted to highlight the advantages and disadvantages of HT compared with those of intensity modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT).
Radixact™ has improved the treatment time efficiency in terms of imaging and delivery more than that of previous HT releases. The time for gantry rotation for megavoltage computed tomography acquisition has been adapted to 6 s and the output to 1000 monitor units per minute (MU/min), compared to the 10 s and 850 MU/min, respectively, of previous versions.
Traditional linear accelerators (linacs) have also evolved with the introduction of more efficient collimators that improve VMAT delivery in terms of time and quality of plans. The comparison in this study was performed with two Elekta linacs, Synergy® (SYN) and Versa HD™ (VE-HD), equipped, respectively, with an MLCi2 (40 pairs of multileaf collimator [MLC] leaves; 1-cm leaf width at the isocenter) and an Agility MLC (80 pairs of MLC leaves; 0.5-cm leaf width at the isocenter) (Elekta AB, Stockholm, Sweden). The most important characteristics of the Agility MLC are the smaller and faster leaves and the many degrees of freedom in movement owing to the interdigitation property [16].
This study aimed to compare the Radixact™ and both SYN and VE-HD in different anatomical areas to identify patients (sixty were recruited) who would benefit most from the modern technology underlying TOMO treatments. The planning process involves both automatic and manual planning to minimize inter-user variability. The TOMO plan for each patient was compared to the corresponding VE-HD and SYN plans. Unlike commonly used dosimetric plan comparisons, plan comparisons in our study were also performed by five blinded clinicians. The deliverability of the plans was assessed using patient-specific dosimetric quality assurance (DQA) measurements.
2 Methods and materials
Sixty patients clinically treated in our department were retrospectively included in the study; the cases involved six common cancer sites (10 patients per site): whole brain (WB), head and neck (HN), lymphoma (LYM), lung (LUNG), prostate (PRO), and rectum (REC). WB plans involved a simultaneous integrated boost on one or more metastases, with 6 of 10 patients also requiring hippocampal avoidance. HN plans included three dose levels for all patients (70 Gy/63 Gy/54.25 Gy in 35 fractions or 66 Gy/60 Gy/54 Gy in 30 fractions). All patients with LYM had a clinical target volume involving only the mediastinum and were prescribed a dose of 30 Gy. For cancers involving the LUNG site, all patients had locally advanced non-small-cell lung cancer (NSCLC) and were treated with 60 Gy in 30 fractions. A hypofractionated protocol (42.7 Gy in 7 fractions) was employed for patients with PRO cancer. For the REC site, the prescription was 50 Gy/45 Gy or 54 Gy/45 Gy in 25 or 30 fractions. Before treatment, all patients underwent a planning CT scan with a slice thickness of 3 mm. Patients with WB, HN, LUNG, and REC cancers were clinically treated using Radixact™, whereas those with PRO cancer and LYM were treated using both VE-HD and SYN.
According to the department’s organization, WB, LUNG, and PRO Radixact™ plans were compared with those of Versa HD™, whereas HN, LYM, and REC plans were compared between the two linacs. All treatment plans were performed by four expert medical physicists (more than 5 years of experience in treatment planning).
2.1 Radixact™ system plans
Radixact™ plans were performed by a medical physicist with the Precision™ v.3.1.0.0 TPS (Accuray®, Sunnyvale, CA, USA). Because the optimization module in Precision™ requires manual input, all plans were also planned by a second medical physicist to assess intra-operator variability. The plans were labeled as T1 (first operator) and T2 (second operator).
For all plans, a dynamic jaw field width of 2.5 cm was selected; the pitch had values between 0.226 and 0.303 for WB and PRO, respectively, and 0.436 for plans with a 2 Gy/1.8 Gy fraction (HN, LYM, LUNG, and REC). High-dose grid resolution was selected for the final calculation. The modulation factor and the number of iterations varied according to the complexity of the treatment plan.
2.2 Linac plans
In our clinical practice, all linac plans were generated using RayStation TPS (v. 10A; RaySearch Laboratories, Stockholm, Sweden) and the VMAT technique. Four medical physicists were involved in the planning: three for tomotherapy and all for the linacs. For the PRO plans, 6 MV flattening filter-free beams were used; all other plans used 6 MV beams. For VE-HD, the beam setup provided two coplanar 360° arcs (one arc with a 0° collimator and the other with a 90° collimator) for the HN, PRO, and REC sites. A non-coplanar 60° arc with an angle couch of 90° (or 270°) was added for the LYM and LUNG plans. In contrast, two non-coplanar arcs of 120° and couch angles of 45° and 315° were added to the two 360° arcs for WB. The same beam setup was employed for SYN, but four coplanar 360° arcs were used.
The Genetic Planning Solutions auto-planning system (GPS) implemented in RayStation was used [17] for the linac plans. For each anatomical district, the user enters the dose value for each target volume, and automatic cost functions related to the targets and organs at risk are created. Manual tuning of the optimization cost functions is almost always required to obtain the final plan. Therefore, although all the plans were manually refined, the starting point for the definition of the optimization function was the same for all the involved planners; thus, we excluded the need for double planning. The dose grid resolution was set to coincide with that of the corresponding TOMO plan. The plans were named LV for VE-HD and LS for SYN.
2.3 Dose comparison
Dose-volume histogram (DVH) plots were used to provide quantitative comparisons between the VMAT and TOMO treatment plans. To facilitate an easier comparison, the Radixact™ 3D dose volume was imported into the RayStation system so that all comparisons were performed within RayStation. The target coverage was estimated as the volume receiving 95% of the prescribed dose (Dp), while overdosage was set to the isodose 107%, according to the International Commission on Radiation Units and Measurements report 83 (ICRU 83) [18]. Several dosimetric constraints were considered for organs at risk (OARs) (Table 1).
Four indexes were also included in the analysis to evaluate the conformity and homogeneity of the target coverage and middle/low isodoses: conformity number (CN), homogeneity index (HI), modified gradient index (mGI), and low-dose gradient index (ldGI). The CN was calculated according to van’t Riet [19]:
where TVRI is the target volume covered by 95% of the Dp, TV is the target volume, and VRI is the volume covered by 95% of the Dp. HI is defined as (D2% – D98%)/Dp [20], where D2% and D98% are the doses at 2% and 98% of the target volume, respectively. The mGI considers both the target volume coverage and dose fall-off at higher doses [21]:
where V50% is the volume irradiated by the 50% isodose, and V100% is the volume covered by the 100% isodose. A lower mGi value indicates a steeper dose fall-off.
Instead, ldGI is defined as
where V20% is the volume irradiated by the 20% isodose.
Furthermore, an analysis of beam-on time was performed.
2.4 Delivery quality accuracy
Delivery quality assurance was performed for all plans except T2 using ArcCHECK (Sun Nuclear, Melbourne, FL). A dose calibration was performed before each measurement. Gamma passing rate (GPR) analysis was conducted to compare the measured and calculated doses. The criteria of 3% dose difference, 2-mm distance to the agreement, 10% threshold with global normalization (AAPM TG218 [22]), 2% 2-mm distance to agreement, and 10% threshold with local normalization were evaluated.
2.5 Clinical evaluation
The plans were evaluated by five expert clinicians (one physician for each treatment site, except for one physician who evaluated two sites). During the blind comparisons of the plans (T1 vs. LV and T1 vs. LS), the clinician first assessed whether all plans met the dosimetric clinical goals for the target and OARs. Subsequently, the comparison was evaluated by visual inspection of the dose distribution, loading a double window for isodoses and DVHs side-by-side. For each pair of plans, the clinician used a visual analog scale to score the overall plan quality difference as follows: 1, TOMO much better; 2, TOMO better; 3, parity between the two plans (no preference); 4, SYN/VE-HD better; and 5, SYN/VE-HD much better. A short explanation of the motivation for the selected scores was reported for each patient.
2.6 Statistical analyses
Paired two-sided Wilcoxon’s signed-rank tests were performed for all dosimetric parameters with a 5% significance level for the following comparisons: T1 vs. T2, T1 vs. LV, and T1 vs. LS. All statistical analyses were performed using R software (v. 1.2.5042, www.rstudio.com).
3 Results
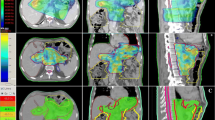
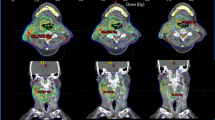
Figure 1 shows a qualitative comparison of TOMO plans and VE-HD and SYN plans for the different pathologies.
No statistically significant differences (P > 0.05) were found between the T1 and T2 plans for any of the parameters considered.
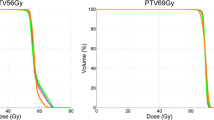
Both GPR (AAPM TG218 and 2% 2 mm local normalization) box plots are shown in Fig. 2. The mean values obtained (all patients) were 99.4% (95.8–100%), 97.8% (93.2–99.9%), and 96.6% (90.3–99.9%) for TOMO, VE-HD, and SYN plans, respectively. Those for the local 2% 2 mm GPR were 89.2% (80.2–99.9%), 86.9% (79.0–95.0%), and 84.7% (77.3–94.8%) for TOMO, VE-HD, and SYN plans, respectively.
The parameters with statistically significant differences are shown in Table 2, with the median, minimum, and maximum values for all parameters included in the comparison. TOMO achieved greater target conformity and homogeneity with respect to both linacs. Compared with that of VE-HD, the performance of TOMO was better for hippocampi D100% (P = 0.006), spinal cord maximum dose (P = 0.009 for HN, P = 0.01 for LUNG), rectum V38.4 Gy (P = 0.006), and bladder V30 Gy (P = 0.01) and V40 Gy (P = 0.02). In contrast, Versa HD™ performed better in terms of lung-PTV V5 Gy (P = 0,01; LYM, P = 0.004 for LUNG) and lung-PTV mean dose (only for LUNG plans) (P = 0.002), mean heart dose (P = 0.02), breast V4 Gy (P = 0.03), and bowel V45 Gy (P = 0.02). Regarding Synergy, the HN TOMO plans showed better sparing of the parotid glands (P = 0.02), oral cavity (P = 0.03, both V30 Gy and V35 Gy), and supraglottic larynx mean dose (P = 0.009). Regarding the REC plans, bladder saving was higher for TOMO (P = 0.009 for V30 Gy and P = 0.001 for V40 Gy). Significant boxplot comparisons are shown in Fig. 3. For a beam-on time, the TOMO plans always had statistically significantly longer delivery time (except for the head and neck district between TOMO and SYN), than that for linacs.
Table 3 shows the results of the clinical evaluation. For 5 of 10 WB plans, the medical preference was for the VE-HD because of its better sparing of OARs. The PRO plans were only chosen owing to the increased target coverage (Radixact™ plans are better in saving the bladder, but the dose constraint was respected for the linacs as well). The lower value of the lungs-PTV V5 Gy was the main motivation for the choice of the lung VE-HD plans (for three patients, the constraint was not respected for TOMO plans), combined with a considerable sparing of the contralateral lung for centrally located targets. The better sparing of the lungs, in addition to the lower mean heart dose (one TOMO plan did not respect the dose constraint), were also reasons for the preference for the VE-HD LYM plans. Of the 10 HN plans, in 3 plans for VE-HD and 4 for SYN, the average parotid dose exceeded the constraint value. A slightly higher dose for sparing the bowel and bladder was the motivation for the preference of VE-HD plans over the TOMO ones for REC cases. TOMO REC plans outperformed SYN for better conformity of target coverage and considerable sparing of the bladder and bowel. The preference for SYN LYM plans was due to the sparing provided by low doses, especially for large targets.
4 Discussion
We compared the latest generation of tomotherapy systems with two different linacs. Over the years, linacs have undergone constant technological development, involving both the beam (from flattening filter to flattening filter-free) and multileaf structure (smaller leaves, less leakage, increase in leaf speed, and interdigitation). Similarly, the Radixact™ system adopts several improvements that were absent in the previous version [23]. Therefore, the results reported in the literature [2,3,4,5,6,7,8,9,10,11,12,13,14,15] regarding the comparison of different accelerators need to be reviewed owing to the continuous evolution of technology.
The most recent comparative studies focused on the HN [5,6,7,8,9,10], lung [11,12,13,14], prostate [15], brain with hippocampal avoidance [4], and cervical [3] carcinomas; however, none of these studies involved the Radixact™ system.
Lu et al. [10] included 15 patients with nasopharyngeal carcinoma and performed a dosimetric comparison between HT (Tomo HD) and VMAT (Elekta Synergy with MLCi2). The performance of HT was better in terms of the mean conformity index and HI of the target volume, reduction of the maximum doses delivered to the OARs, and the volume delivered to the high-dose region, such as the V30 Gy of the parotid glands. Similar results were reported by Pigorsch et al. [9] in six patients planned for both HT with a 1-cm field width and VMAT Varian Clinac Trilogy. Compared to the HT plans, the VMAT plans with three arcs achieved the best target coverage. Wang et al. [8] enrolled 40 patients and compared tomotherapy with Hi-ART and two 360° arc Elekta Versa HD VMAT. In addition, the analysis was divided into subgroups according to tumor stage. For patients with early T-stage disease, VMAT and HT provided similar dose coverage and protection of OARs. However, the low-dose radiation volume of the HT plan was higher, especially for doses up to 20 Gy. In contrast, HT may be recommended for patients with advanced T stage tumor owing to the greater dose coverage of the high-dose target and better protection of the brain stem, spinal cord, and temporal lobes.
Regarding the thoracic site, both hypofractionated [11, 12] and conventionally fractionated NSCLC [13, 14] have been investigated. Xu et al. [13] compared 30 NSCLC HT plans with VMAT plans generated using two coplanar arc techniques. The HT plans showed a slight advantage in sparing healthy lung tissue, mainly in terms of V20 Gy and V30 Gy, at the cost of an increase in the low dose received by the normal lung and heart, especially in centrally located lesions. Klunklin et al. [14] compared HT Hi-Art and Elekta VMAT by adding a patient-specific pretreatment quality assurance assessment through a 3% 3-mm gamma index analysis for 17 patients. The VMAT plan uses a technique involving two coplanar partial arcs. No statistically significant difference was found between the two techniques in the radiation dose to the lungs, heart, spinal cord, and esophagus, and a satisfactory GPR value (more than 90%) was achieved.
Seventeen patients were recruited by Serra et al. [15] to compare Hi-Art HT and Elekta Synergy with 5-mm width leaf VMAT for hypofractionated treatment (36.25 Gy in five fractions) in localized prostate cancer. The bladder, rectum, and penile bulb DVH curves were lower with VMAT, and the target coverage in terms of 95% and 98% isodoses was significantly higher for HT.
Finally, Rong et al. [4] investigated the differences between 1-cm jaw width HT and Varian RapidArc VMAT (two coplanar arcs at collimator 30° and 330° for linac TrueBeam) for hippocampal-avoidance whole-brain radiotherapy in 10 patients. HT had a significantly superior PTV HI and lower hippocampus D100%; RapidArc had a significantly lower Dmax.
In the current study, TOMO plans showed higher homogeneity and conformity indeces than both linacs. Due to binary MLC, a deeper high-dose gradient was achieved with TOMO (bladder V30 Gy and V40 Gy, rectum V38.4 Gy, and hippocampus D100%). Only VE-HD bowel V45 Gy for REC was the lowest; however, in this case, the bowel was often inside the PTV, and Precision™ gave greater priority to the target coverage. A higher dose gradient leads to an increased area at low doses in the surrounding tissue [23], with significantly higher values of lungs-PTV V5 Gy and breast V4 Gy for the LYM and LUNG. The mGi and ldGI indices were worse for TOMO plans, although not always with statistically significant differences. We found a stronger difference between the results of Xu et al. [13] and Klunklin et al. [14] because the use of a noncoplanar beam reduced the bath of low doses and increased the conformity of intermediary doses [24, 25]. The use of the non-coplanar field with the latest generation multileaf collimator allowed us to obtain a lower dose value of lungs-PTV and mean heart dose. This aspect assumes relevant importance in terms of toxicity and secondary cancer risk, particularly for patients with a long life expectancy, such as those with LYM [26].
As shown in Fig. 3, the average parotid dose was lower for the TOMO plans; a statistically significant difference was found only between TOMO and SYN, as reported by Lu et al. [10]. This is relevant from a clinical point of view, as better sparing of the parotid glands reduces the incidence of xerostomia and favors the early recovery of saliva secretion, improving the quality of life. In a multicenter comparison between HT and RapidArc, Bibault et al. [27] reported that HT induced fewer acute salivary disorders and provided better locoregional control and cancer-specific survival. Our clinicians observed more frequently acute skin reactions with TOMO plans than with VMAT plans. This may be because Precision™ is oriented toward full target coverage, even in the top skin layers, by altering the incident fluences in the non-electronic equilibrium region, and the planner cannot reduce the skin dose suitably [28]. Therefore, dose constraints are required to reduce the probability of skin toxicity.
In agreement with the literature [4, 10], the maximum dose received by OARs located close to the target was lower with TOMO, favoring the possibility of eventual retreatment.
Regarding DQA, TOMO plans showed better consistency between the planned and measured doses (greater mean values of both gamma indices). This can be explained by the performance of the multileaf and different accelerator commissioning procedures. For SYN and VE-HD, the commissioning procedure involved acquiring repeated profile curves, percentage depth-dose curves, and output factors. Subsequently, in the TPS, a virtual accelerator is created to simulate a real accelerator. In contrast, Precision™ uses the real curves and physical parameters of the TOMO system for dose calculation. During commissioning, a few curves are measured for verification and not for implementation in the TPS.
As reported in several studies [6, 10, 11, 14, 15], the TOMO plans had a longer delivery time. However, the linac beam-on time does not include the time of beam selection and preparation or the couch movement in the case of non-coplanar beams. All of these factors increase the effective treatment time. Tomotherapy machines have only one row of MLC leaves. Consequently, a smaller portion of the anatomical district is irradiated simultaneously. Although the treatment time delivery is increased, tomotherapy allows a larger dose gradient in the patient’s longitudinal direction.
A key point of our comparison was the clinical evaluation of the plans by radiation oncologists. This should be a crucial consideration in all dose comparison projects; statistically significant differences are not always important from a clinical point of view and vice versa. Differences that are not statistically significant in the dose constraints of OARs can imply clinical relevance. According to clinical judgment, for HN, PRO, and REC, the TOMO and VE-HD plans were very similar, whereas the TOMO plans were judged to be superior to SYN plans for HN and REC. In four patients with PRO, linac plans were preferred for better bladder sparing at the cost of lower target coverage. In contrast, for LUNG and LYM, VE-HD plans were preferred over TOMO plans for better sparing of the lungs and heart.
Finally, technology comparisons should also evaluate the global performance of the treatment in terms of linac stability, machine downtime, annual maintenance, linac age, and technical support, among others; however, these considerations were not considered in this study.
Recently, tomotherapy treatments were implemented in the RayStation TPS; a future challenge is to explore the potential of GPS capability in Radixact™ plans.
5 Conclusions
Owing to technological improvements, the quality of linac plans has improved and the differences with HT have decreased. The possibility of non-coplanar beams allows the linac plans to decrease low doses, especially at the thoracic site. However, tomotherapy continues to be an essential add-on in a multimachine department for high-dose gradients, better delivery QA reliability, and extended-field irradiation.
References
Mackie TR, Balog J, Ruchala K, et al. Tomotherapy. Semin Radiat Oncol. 1999;9(1):108–17.
Myers PA, Mavroidis P, Papanikolaou N, et al. Comparing conformal, arc therapy and helical tomotherapy in craniospinal irradiation planning. J Appl Clin Med Phys. 2014;15(5):4724–41.
Renard-Oldrini S, Guinement L, Salleron J, et al. Dosimetric comparison between VMAT and tomotherapy with para-aortic irradiation for cervix carcinoma. Cancer Radiother. 2015;19(8):733–8.
Rong Y, Evans J, Xu-Welliver M, et al. Dosimetric evaluation of intensity-modulated radiotherapy, volumetric modulated arc therapy, and helical tomotherapy for hippocampal-avoidance whole brain radiotherapy. PLoS ONE. 2015;10(4): e0126222.
Stromberger C, Wlodarczyk W, Marnitz S, et al. Simultaneous integrated boost (SIB): RapidArc and tomotherapy plan comparison for unilateral and bilateral neck irradiation. Anticancer Res. 2015;35(5):2991–7.
Li S, Zhou Q, Shen LF, et al. Dosimetric comparisons of volumetric modulated arc therapy and tomotherapy for early T-stage nasopharyngeal carcinoma. Biomed Res Int. 2018:2653497. https://doi.org/10.1155/2018/2653497 (PMID: 29967769; PMCID: PMC6008744)
Wang YC, Li CC, Chien CR. Effectiveness of tomotherapy vs linear accelerator image-guided intensity-modulated radiotherapy for localized pharyngeal cancer treated with definitive concurrent chemoradiotherapy: a Taiwanese population-based propensity score-matched analysis. Br J Radiol. 2018;91(1086):20170947.
Wang Q, Qin J, Cao R, et al. Comparison of dosimetric benefits of three precise radiotherapy techniques in nasopharyngeal carcinoma patients using a priority-classified plan optimization model. Front Oncol. 2021;11: 646584.
Pigorsch SU, Kampfer S, Oechsner M, et al. Report on planning comparison of VMAT, IMRT and helical tomotherapy for the ESCALOX-trial pre-study. Radiat Oncol. 2020;15(1):253–63.
Lu S, Fan H, Hu X, et al. Dosimetric comparison of helical tomotherapy, volume-modulated arc therapy, and fixed-field intensity-modulated radiation therapy in locally advanced nasopharyngeal carcinoma. Front Oncol. 2021;11: 764946.
Xhaferllari I, El-Sherif O, Gaede S. Comprehensive dosimetric planning comparison for early-stage, non-small cell lung cancer with SABR: fixed-beam IMRT versus VMAT versus TomoTherapy. J Appl Clin Med Phys. 2016;17(5):329–40.
Li S, Yang J, Liu J, et al. Dosimetric comparison of helical tomotherapy and conventional Linac-based X-knife stereotactic body radiation therapy for primary lung cancer or pulmonary metastases. J Thorac Dis. 2018;10(2):999–1006.
Xu Y, Deng W, Yang S, et al. Dosimetric comparison of the helical tomotherapy, volumetric- modulated arc therapy and fixed-field intensity-modulated radiotherapy for stage IIB-IIIB non-small cell lung cancer. Sci Rep. 2017;17(1):14863.
Klunklin P, Manoharn T, Wanwilairat S, et al. Analysis of the planned, delivered dose distributions and quality assurance for helical tomotherapy and volumetric modulated arc therapy in locally advanced non-small cell lung cancer. Rep Pract Oncol Radiother. 2021;26(6):939–47.
Serra M, Ametrano G, Borzillo V, et al. Dosimetric comparison among cyberknife, helical tomotherapy and VMAT for hypofractionated treatment in localized prostate cancer. Medicine (Baltimore). 2020;99(50): e23574.
Bedford J, Thomas MDR, Smyth G. Beam modeling and VMAT performance with the Agility 160-leaf multileaf collimator. J Appl Clin Med Phys. 2013;14(2):172–85.
Fiandra C, Rossi L, Alparone A, et al. Automatic genetic planning for volumetric modulated arc therapy: a large multi-centre validation for prostate cancer. Radiother Oncol. 2020;148:126–32.
Hodapp N. The ICRU-Report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT). Strahlenther Onkol. 2012;188(1):97–106.
Riet van’t A, Mak AC, Moerland MA, et al. A conformal number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys. 1997;37:731–6.
Wu Q, Mohan R, Morris M, et al. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. I: dosimetric results. Int J Radiat Oncol Biol Phys. 2003;56(2):573–85.
Ohtakara K, Hayashi S, Hoshi H. Dose Gradient analyses in linac-based intracranial stereotactic radiosurgery using Paddick’s gradient index: consideration of the optimal method for plan evaluation. J Radiat Res. 2011;52(5):592–9.
Miften M, Olch A, Mihailidis D, et al. Tolerance limits and methodologies for IMRT measurement-based verification QA: Recommendations of AAPM Task Group No 218. Med Phys. 2018;45(4):e53–83.
Kraus KM, Kampfer S, Wilkens JJ, et al. Helical tomotherapy: comparison of Hi-ART and Radixact clinical patient treatments at the Technical University of Munich. Sci Rep. 2020;10(5):4928–38.
Fiandra C, Filippi AR, Catuzzo P, et al. Different IMRT solutions vs. 3D-conformal radiotherapy in early stage Hodgkin’s lymphoma: dosimetric comparison and clinical considerations. Radiat Oncol. 2012;7:186–94.
Levis M, Filippi AR, Fiandra C, et al. Inclusion of heart substructures in the optimization process of volumetric modulated arc therapy techniques may reduce the risk of heart disease in Hodgkin’s lymphoma patients. Radiother Oncol. 2019;138:52–8.
Filippi AR, Vanoni V, Meduri B, et al. Intensity modulated radiation therapy and second cancer risk in adults. Int J Radiat Oncol Biol Phys. 2018;100(1):17–20.
Bibault JE, Dussart S, Pommier P, et al. Clinical outcomes of several imrt techniques for patients with head and neck cancer: a propensity score-weighted analysis. Int J Radiat Oncol Biol Phys. 2017;99(4):929–37.
Mori M, Cattaneo GM, Dell’Oca I, et al. Skin DVHs predict cutaneous toxicity in Head and Neck Cancer patients treated with Tomotherapy. Phys Med. 2019;59:133–41.
Funding
No funds, grants, or other support was received for conducting this study. The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have all participated in the design, execution, and analysis of the paper and that they have approved the final version. Also, they declare that they have no conflict of interest in connection with the paper and that material described is not under publication or consideration for publication elsewhere.
Ethics approval
This research study was conducted retrospectively from data obtained for clinical purposes. No approval of research ethics committees was required.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Patients signed informed consent regarding publishing their data and photographs. No identifying information is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Gallio, E., Sardo, A., Badellino, S. et al. Helical tomotherapy and two types of volumetric modulated arc therapy: dosimetric and clinical comparison for several cancer sites. Radiol Phys Technol 16, 272–283 (2023). https://doi.org/10.1007/s12194-023-00716-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12194-023-00716-3