Abstract
This study focused on the alterations that occur in larval molluscan cells after administration of apoptotic inducers and inhibitors used in mammalian cells in response to cold stress. This is the first report on apoptosis modulation in molluscan cells assessed by flow cytometry. Mitochondrial activity, general caspase activation, and membrane integrity of control molluscan cells were compared to those processes in frozen–thawed molluscan cells, primary mouse embryonic fibroblasts, and human colon tumor cells prior to treatment and after incubation with apoptotic inducers or inhibitors. We tested three apoptotic inducers (staurosporine, camptothecin, and mitomycin C, routinely used for the chemical induction of apoptosis in different mammalian cells) and found that only staurosporine resulted in an evident apoptotic increase in molluscan cell cultures: 9.06% early apoptotic cells in comparison with 5.63% in control frozen–thawed cells and 20.6% late apoptotic cells in comparison with 10.68% in controls. Camptothecin did not significantly induce molluscan cell apoptosis but did cause a slight increase in the number of active cells after thawing. Mitomycin C produced similar results, but its effect was less pronounced. In addition, we hypothesize that the use of the apoptotic inhibitors could reduce apoptosis, which is significant after cryopreservation in molluscan cells; however, our attempts failed. Development in this direction is important for understanding the mechanisms of marine organisms’ cold susceptibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apoptosis and necrosis have been detected in all eukaryotes and are induced by many stress factors (Arur et al. 2003). Although these major cell death pathways are highly conserved, they vary with respect to morphology, biochemistry, and physiology (Zeiss 2003; Zhivotovsky 2004; Kiss 2010). The degree of stress-induced stimulation may determine whether the cells enter the cell death pathway (Hirsch et al. 1997; Zeiss 2003), and molecular chaperones (heat shock proteins, HSPs) play a key antiapoptotic role in damage repairing (Lindquist 1986; Ellis 1987; Liu and Chen 2013). This group of evolutionarily conserved proteins has been described in almost all living organisms (Hartl 1996). In the case of several stressors, it may lead to apoptosis or necrosis (Sõti et al. 2003). The cell death programs can be activated by elevated apoptotic levels playing a crucial role in responses to stress from low and high temperatures and oxidative stress in intertidal organisms, including adult mollusks (Sokolova et al. 2004). Marine organisms’ early life stages are marked by increased susceptibility to stressors different from affected adults (Przeslawski et al. 2015). In this study, we aimed at apoptotic modulation in mussel (genus Mytilus) larval cells after ultra-low cold stress and clarification of mechanisms of apoptosis induced by cryopreservation.
There is a lack of information about the causes of apoptosis in mollusks now, although this phenomenon has been described in different mollusk classes (McFall-Ngai 1999; Mondy and Pierce 2003; Sunila and LaBanca 2003; Sokolova et al. 2004; Buckland-nicks and Tompkins 2005; Koropatnick et al. 2014; Romero-Ramírez et al. 2015).
We examined whether apoptotic inducers, such as staurosporine (STS, a natural antibiotic), camptothecin (CAM, a topoisomerase I inhibitor), and mitomycin C (MMC, a drug of the camptothecin family), which are routinely used for chemical induction of apoptosis in mammalian cells, can induce apoptosis in cultivated molluscan larval cells. We also tested three apoptotic inhibitors used for mammalian cells, such as Y-27632, cyclic pifithrin-α, and CHIR99021, in order to reduce apoptosis after cryopreservation, which can reach 24% in molluscan cell cultures (Odintsova et al. 2017). Y-27632 is a highly permeable, potent, and selective inhibitor of the Rho-associated protein kinase (ROCK) signaling pathway in mammalian cells. Human corneal endothelial cells treated with this inhibitor showed a decrease in apoptotic levels, most likely because of the inhibitor-induced effects of caspase-3’s expression and activities (Peh et al. 2015). Moreover, it was previously shown that a Rho-enzyme in oyster hemocytes may be involved in antiapoptotic mechanisms, also including P35-sensitive caspases and mitogen-activated protein kinases (Lacoste et al. 2002). In murine cell cultures, cyclic pifithrin-α reversibly prevented p53-mediated apoptosis that had developed in response to stressors, such as ultraviolet or ionizing radiation (Marión et al. 2009). Another specific apoptotic inhibitor, CHIR99021, also associated with p53-mediated apoptosis, has been shown to block the acetylation of lysine 120 in the p53 protein and thereby prevent the apoptosis initiation in human lymphoma cells exposed to ionizing radiation (Ambroise et al. 2015). p53 is a well-described mitochondrial apoptotic gene in non-model invertebrates, and its expression is considered a marker of cellular stress in mussels (Muttray et al. 2005; Böttger et al. 2008; Walker et al. 2011).
The influence of ultra-low temperatures on the inducing of apoptosis in mussel cells is understudied compared to effects of environmental factors. Mussels of the genus Mytilus are sessile organisms that inhabit highly stressful intertidal ecosystems and, therefore, must possess mechanisms to withstand the stress-induced effects (Halpin et al. 2002; Lockwood et al. 2015). Environmental pollutants and drastic temperature changes (Cheng 1988; Mičić et al. 2001; Sokolova et al. 2004; Kefaloyianni et al. 2005; Cherkasov et al. 2007; Sokolova 2009) can lead to a variety of cellular disorders in mollusks, including eventual apoptosis. Mytilus studies have shown that temperature stress induces changes in gene and protein expressions (Hofmann and Somero 1995; Chapple et al. 1998; Hofmann et al. 2002; Lockwood et al. 2010; Fields et al. 2012). There are 175 genes in the Mytilus transcriptome that show expression changes to temperature stress: 87 are induced and 88 are repressed in Mytilus californianus, M. trossulus, and M. galloprovincialis (reviewed in (Lockwood et al. 2015). The results previously reported for two species of intertidal mussels (M. galloprovincialis and M. californianus) using the hemocyte study system found that stress responses to high temperatures differed between mussel species, whereas both species appeared to be tolerant of cold extremes at least over short time intervals (Yao and Somero 2012). One of two mussel species tested, a more temperature-tolerant species, M. galloprovincialis, demonstrated a lower amount of single- and double-stranded DNA damages, faster signaling activation and transduction, and stronger repair capabilities against temperature stress (Yao and Somero 2012). Buckley et al. (2001) have measured threshold induction temperatures in the mussel Mytilus trossulus post acclimation to summer conditions in the field and post cold acclimation in the laboratory: levels of protein denaturation (the quantity of ubiquitinated proteins) and endogenous levels of Hsps from the 70 kDa family were significantly higher during warm acclimation than during cold acclimation. This data agreed with the results previously obtained by Hofmann and Somero (1995) in which the levels of ubiquitin conjugates in M. trossulus were higher in summer than in winter.
The fact of apoptosis induction in marine invertebrate cells in response to ultra-low cold stress has been previously shown by several different tests, such as fluorescent staining followed by flow cytometry, electron microscopy, and a spectroscopic analysis of the activity of some caspase types (Boroda et al. 2016; Odintsova et al. 2017).
The objectives of this study were twofold: (1) to find apoptotic inducers used for chemical induction of apoptosis in mammalian cells that can operate in non-mammalian systems, particularly in cultivated molluscan larval cells, and (2) to reduce apoptosis in molluscan cells after cryopreservation using the apoptotic inhibitors.
Materials and methods
Animals
Farmed marine bivalves, M. trossulus, were collected from the Vostok Bay of the Sea of Japan (Russia) and maintained in tubs filled with running seawater (SW) for 10 to 20 days at 7–10 °C with pH between 7.3 and 8.0. The mussels were not fed during the maintenance period. The spawning of sexually mature specimens was induced by a thermal shock. The eggs from different females were artificially fertilized using pooled sperm from different males, and the zygotes were placed in two 5-L tanks filled with UV-sterilized SW. The embryos were cultivated at 17 °C and harvested at the trochophore stage (22 h post-fertilization) on nylon mesh with a pore size of 35 μm for further isolating embryonic cell cultures, as previously described (Odintsova et al. 2010).
Cell freezing and thawing
Studies concerning living human cells and embryos (Clarke et al. 2006; Riggs et al. 2010), also as theoretical modeling (Mazur 1984), assume that material cryopreserved in liquid nitrogen (LN) should not be influenced by storage time for several thousand years, because thermal energy is not sufficient for any chemical reaction at − 196 °C (McGee and Martin 1962). We previously found that storage duration in LN did not affect the number of viable cells in primary cell cultures from different bivalve species (Odintsova and Tsal 1995).
The resulting cell suspension that contained all cell types (0.6 mL, 5–10 × 106 cells/mL) was transferred into sterile 2-mL polypropylene cryotubes (TPP, Trasadingen, Switzerland), and 1.2 mL of cryoprotective solution (7.5% dimethyl sulfoxide in sterile 32‰ SW cooled to 4 °C) was gradually added over a 10-min period. The samples were then maintained in an ice-water bath for an equilibration period of 10 min prior to freezing. Cooling to − 196 °C was performed via three-step freezing: gradually cooling first to − 25 °C at a rate of 1–1.3 °C/min for 30 min, then to − 75 °C at a rate of 1.8–2.0 °C/min for 20 min; finally, the cryotubes were plunged into LN (− 196 °C) for storage. Temperature control was assessed using a TC-08 thermocouple logger (Omega Engineering, Stamford, CT, USA).
After storage in LN for 1 to 60 days, the cryotubes were placed into a 30 °C circulating water bath until the ice was completely melted (4–5 min). Immediately after thawing, the cryotube contents were transferred into cooled, sterile centrifuge tubes. The thawed samples were gradually (over a 3–5-min period) diluted tenfold in sterile SW at 0 °C with gentle shaking, centrifuged (5 min at 700g), washed once with SW, and re-suspended in 0.6 mL of SW supplemented with 2% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, USA) and antibiotics for cultivation.
Cell cultures
Control and frozen–thawed (Fr) cells were cultivated at a concentration of 120 to 150 × 103 cells/well in sterile SW supplemented with 2% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin in 6-well plates (TPP) at 17 °C for 4 to 48 h. In order to induce apoptosis and identify the concentration or exposure time dependency, the control and Fr cells were either incubated with STS (final concentration 1–5 μM), CAM (1–10 μM), or MMC (1–10 μM) during 4–48 h. The apoptotic inducers were dissolved in dimethyl sulfoxide (Me2SO) at 1 mM (stock solution) and stored at − 20 °C. Hydrogen peroxide (H2O2) was added to the cells at the final concentration of 125 μM to induce oxidative stress and apoptosis for 6 to 48 h at 17 °C, according to the previously published conditions for mammalian Jurkat T-lymphocytes (Hampton and Orrenius 1997) and mussel larval cells (Odintsova et al. 2017). In order to decrease the level of apoptosis after a freeze–thaw cycle, the Fr molluscan cells were incubated with apoptotic inhibitors: (1) Y-27632 (Y, final concentration 10–50 μM); (2) cyclic pifithrin-α (Alpha, 5 μM); or (3) CHIR99021 (CHIR, 1–5 μM); all inhibitors were purchased from Sigma-Aldrich. Specimens were examined using a CKX41 inverted microscope (Olympus, Tokyo, Japan) equipped with phase-contrast optics and imaged with an Axiocam 105 color digital camera (Carl Zeiss, Oberkochen, Germany). Cell photos from two parallel samples were obtained from 10 randomly selected microscopic fields in each experiment for each sample. Each experiment was repeated at least three times.
MEFs were obtained according to the protocol described in Peterson and Loring (2012). All of the experiments on animals were reviewed and approved by the Ethics Committee of the National Scientific Center of Marine Biology of the Far Eastern Branch of the Russian Academy of Sciences. The HCT 116 cell line was purchased from Sigma-Aldrich. Mammalian cells were cultivated in Dulbecco’s modified minimal essential medium (DMEM) supplemented with 10% FBS in 6-well plates (TPP) at 5% CO2, 37 °C. The cells were incubated with either apoptotic inducers (1–5 μM STS, 1–10 μM CAM, or 1–10 μM MMC), apoptotic inhibitors (10–50 μM Y, 5 μM Alpha, or 1–5 μM CHIR), or apoptotic inhibitors in the presence of 1 μM staurosporine for 6 to 48 h.
Flow cytometry
Flow cytometry has become a powerful tool for detecting apoptotic, live, and dead cells and is the most commonly used laboratory method for distinguishing apoptosis from necrosis in dissociated cells (Chen et al. 2000; Przeslawski et al. 2015). Our previous studies revealed that annexin V is an unreliable marker for apoptosis in molluscan primary cell cultures (Odintsova et al. 2017), since it can lead to an increase in false-positive identification of non-apoptotic annexin V-positive cells (Marión et al. 2009). We found that an analysis of cell samples conducted by two different staining combinations (FLICA®, fluorochrome-labeled inhibitors of caspases (Molecular Probes, Thermo Fisher Scientific, Eugene, OR, USA), with 4′,6′-diamidino-2-phenylindole (DAPI) (Gerbu, Biotechnik GmbH, Heidelberg, Germany) and YO-PRO™-1 stain (Molecular Probes) with DAPI) more accurately reflects apoptosis in molluscan cells and avoids confusion caused by false-positive or false-negative artifacts. Moreover, the apoptosis time window detected by this staining combination appears to be much wider than that assessed only by the annexin V binding (Morris and Geller 1996).
Flow cytometric analyses of the green fluorescent stains, H2DCFDA, FLICA®, or YO-PRO™-1 (488 nm laser), and the ultraviolet-fluorescent stain, DAPI (405 nm laser), were conducted within 20 min after staining using a CytoFLEX flow cytometer (Beckman-Coulter, Brea, CA, USA) connected to a computer running CytExpert software (version 1.2.11.0, Beckman-Coulter). At least 20,000 events were evaluated for each sample.
Detection of cells with active mitochondria
2′,7′-Dichlorodihydrofluorescein diacetate (H2DCFDA) (Sigma-Aldrich), used for detection of reactive oxygen species (a marker of active mitochondria in live cells), was added to 100 μL of suspended cells at a final concentration of 10 μM and incubated at room temperature (RT) for 20 min in the dark. The cell suspension was then diluted with 150 μL of calcium- and magnesium-free artificial seawater (CMFSW for molluscan cells) or Dulbecco’s phosphate-buffered saline (DPBS for mammalian cells), centrifuged at 500×g for 5 min, and then re-suspended in 100 μL of fresh CMFSW or DPBS, respectively. The samples were stained with DAPI, used for staining the nuclei of dead cells with damaged membranes, at a final concentration of 1 μg/mL at RT for 7 min in the dark and then diluted with 150 μL of CMFSW or DPBS, respectively, followed by immediate flow cytometric analysis.
The number of apoptotic cells (general caspase detection via FLICA® binding and plasma membrane integrity detection via YO-PRO™-1 staining)
In order to estimate the number of apoptotic cells, we used two different staining combinations. First, a 50-μL cell suspension was stained at RT for 45 min in the dark with FAM-VAD-FMK FLICA®, according to the manufacturer’s recommendations. FLICA® provides an opportunity to detect general caspase activation in live cells (Peterson and Loring 2012), indicating early apoptosis. Unbound FLICA® was removed from the cells by rinsing with 150 μL CMFSW (molluscan cells) or DPBS (mammalian cells) followed by centrifugation at 500×g for 5 min and then re-suspended in 95 μL of fresh CMFSW or DPBS (depending on cell type). The samples were then stained with DAPI, as described above, and diluted with 150 μL of CMFSW or DPBS (depending on cell type) just before the flow cytometric analysis. Second, to detect the plasma membrane integrity indicating late apoptotic cells, 1 μL of YO-PRO™-1 was added to a 100-μL cell suspension in CMFSW or DPBS (depending on cell type). After a 10-min incubation with YO-PRO™-1 at RT in the dark, the samples were stained with DAPI, as described above, and diluted with 150 μL of CMFSW or DPBS (depending on cell type) just before the flow cytometric analysis.
Statistical analysis
Each experiment was performed independently at least three times. The files obtained with CytExpert Software were analyzed with Kaluza Software v.1.5a (Beckman-Coulter). All other data were subjected to a one-way analysis of variance (ANOVA) using Office Excel 2013 software (Microsoft Corporation, USA) to test whether the values of the means from each experimental group were significantly different. A p value < 0.05 was considered statistically significant in all data analyses.
Results
Effects of apoptotic inducers/inhibitors and freezing–thawing in molluscan larval cells
The larval molluscan cells were treated with apoptotic inducers or inhibitors, and their state (mitochondrial activity, general caspase activation, and membrane integrity) was compared to that of cells that had been frozen–thawed both prior to treatment and after incubation with the same compounds. Apoptotic cells become permeant to YO-PRO™-1 stain, but remained impermeant to DAPI, a dead cell stain. Live cells are not stained with YO-PRO™-1 stain. Figure 1 presents a time course of the induced alterations in mitochondrial activity of molluscan cells after treatment with apoptotic inducers or inhibitors and after a freeze–thaw cycle. Results revealed significant changes in the percentage of molluscan cells with active mitochondria experiencing oxidative stress (incubated with H2O2) at all exposure times (distinctive apoptotic changes were detectable after 6 h). The number of active cells (6 h after a freeze–thaw cycle) was slightly less than that in the intact unfrozen cells and had relatively increased after a 24- to 48-h cultivation due to the destruction of some dead cells. CAM did not cause an increase in apoptosis in molluscan cells (same results with MMC) but did slightly improve their condition after thawing, whereas only staurosporine caused a progressive increase in the percentage of apoptotic cells (Figs. 2 and 3) accompanied by a 10% decrease in cell survival. The tested apoptotic inhibitors did not increase control and Fr cell survival. The results revealed time-dependent effects of the added compounds with an increase in duration of exposure; 6 h of exposition was insufficient for the development of compound-related effects, but after 48 h, many of the cells were destroyed. Therefore, an optimal 24-h exposure period was selected for all of the following experiments.
A time course of alterations in mitochondrial activity of molluscan cells (active, non-active, and dead cells) after treatment with apoptotic inducers or inhibitors before and after a freeze–thaw cycle. Cells were cultivated for 6 h, 24 h, and 48 h and assessed by H2DCFDA and DAPI staining. Treatment key: control unfrozen cells (C); unfrozen cells undergoing staurosporine-induced apoptosis (STS); unfrozen cells undergoing camptothecin-induced apoptosis (CAM); unfrozen cells undergoing mitomycin C-induced apoptosis (MMC); unfrozen cells cultivated with apoptotic inhibitors—cyclic pifithrin-α (Alpha), CHIR99021 (CHIR), Y-27632 (Y); unfrozen cells undergoing oxidative stress (H2O2); cells frozen with 5% Me2SO (Fr). Standard deviations were less than 5.0%
Two-dimensional (2D) plots from flow cytometric analysis of frozen–thawed cells cultivated for a 24-h recovery period. The samples were analyzed with a CytoFLEX flow cytometer. At least 20,000 events were evaluated for each sample. Cells were stained with the green fluorescent stains FLICA® or YO-PRO™-1 and the ultraviolet-fluorescent stain DAPI. The combination of FLICA® and DAPI was used to identify early apoptotic and dead cells, respectively (a). The combination of YO-PRO™-1 and DAPI was used to identify late apoptotic and dead cells, respectively (b). The most typical alterations in all Fr molluscan cells are presented. Of the apoptotic inhibitors, only the evident effect of CHIR99021 is presented which reduces a number both of early apoptotic and late apoptotic cells but shows an increased number of dead molluscan cells. Treatment key: frozen–thawed cells (Fr); frozen–thawed cells cultivated with apoptotic inducers: frozen–thawed cells undergoing 5 μM STS-induced apoptosis (Fr+STS), frozen–thawed cells undergoing 10 μM CAM-induced apoptosis (Fr+CAM), frozen–thawed cells incubated with 5 μM apoptotic inhibitor CHIR99021 (Fr+CHIR)
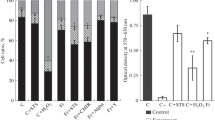
Flow cytometric analysis of apoptosis- and necrosis-associated fluorescence in molluscan cells before and after a freeze–thaw cycle. Cells were cultivated for 24 h and stained with the green fluorescent stain FLICA®, in conjunction with DAPI (a) or another green fluorescent stain, YO-PRO™-1, in conjunction with DAPI (b). Treatment key: control unfrozen cells (C); cells frozen with 5% Me2SO and then cultivated for 24 h (Fr); frozen–thawed cells undergoing STS-induced apoptosis (Fr+STS); frozen–thawed cells undergoing CAM-induced apoptosis (Fr+CAM); frozen–thawed cells undergoing MMC-induced apoptosis (Fr+MMC); frozen-thawed cells cultivated with apoptotic inhibitors: cyclic pifithrin-α (Fr+Alpha), CHIR99021 (Fr+CHIR), Y-27632 (Fr+Y). The significance levels are *P < 0.05 and **P < 0.01
Two-dimensional (2D) plots from flow cytometry of 24-h-old cultivated cells stained with apoptotic markers after a freeze–thaw cycle are illustrated in Fig. 2. STS caused an increase in a portion of both early apoptotic (9.1% in comparison with 5.6% in control frozen–thawed cells) and late apoptotic cells (20.6% in comparison with 10.5% in control frozen–thawed cells). Of the apoptotic inhibitors, only the evident CHIR99021 effect is shown, in which a number both of early apoptotic cells (with active caspases) (1.0% in comparison with 5.6% in control cells) and late apoptotic cells (with damaged plasma membranes) (16.3% in comparison with 10.5% in control cells) can be seen. There was an increase in the number of dead molluscan cells in the early apoptotic stage (13.48% in comparison with 10.57% in control frozen–thawed cells) and in the late apoptotic stage (15.81% in comparison with 6.18% in control frozen–thawed cells).
A pro-survival effect of treatment with camptothecin (Fr+Cam cells) was detected in all Fr molluscan cells (Figs. 2 and 3). MMC showed similar results, but its effect was less pronounced. CAM increased a number of live Fr cells and reduced a part of apoptotic cells both detected by FLICA® and YO-PRO™-1 staining (Fig. 3a, b). STS resulted in an increased number of apoptotic and dead cells after a freeze–thaw cycle (Fig. 3a, b).
The most typical morphological alterations in all molluscan cells exposed to the compounds for 24 h (both in control intact and Fr cells) are presented in Fig. 4. These alterations include cell shape changes and spreading, which might be dependent on cell attachment. Incubation of the Fr cells with STS always led to the noticeable appearance of round-shaped cells and was accompanied by a noticeable reduction in cell density. Fr cells treated with CAM or MMC appeared healthier with no observable differences in cell morphology compared to intact or Fr control cells.
Morphologies of control non-frozen and frozen–thawed molluscan cells cultivated in different conditions for 24 h. Treatment key: control unfrozen cells (C); unfrozen cells undergoing STS-induced apoptosis (C+STS); frozen–thawed cells (Fr); frozen–thawed cells undergoing STS-induced apoptosis (Fr+STS); frozen–thawed cells undergoing CAM-induced apoptosis (Fr+CAM); frozen–thawed cells incubated with an apoptotic inhibitor, CHIR99021 (Fr+CHIR). Specimens were examined with a CKX41 inverted microscope (Olympus, Japan) equipped with phase-contrast optics and imaged with an Axiocam 105 color digital camera (Carl Zeiss, Germany). Scale bar, 25 μm
Effects of apoptotic inducers and inhibitors in mammalian cells
MEFs treated with the compounds were stained with either FLICA® or YO-PRO™-1 and DAPI followed by flow cytometric analysis (Fig. S1); significant apoptosis and many dead cells were detected after STS or CAM treatment, or after oxidative stress. In the other cell model (HCT 116 cells), apoptotic inducers caused an increase in caspase activity, wherein the most evident effects were observed only after staurosporine treatment (Fig. S2). Apoptotic inhibitors caused a decrease in apoptosis in MEFs (Fig. S1) and did so more noticeably in HCT 116 cells previously treated with staurosporine (Fig. S2).
Discussion
Apoptotic activation time has been reported to vary by type of molluscan cells (Sokolova et al. 2004). In this study, we used a culture containing all larval molluscan cell types. The apoptotic level depended mostly on the type of cryoprotectant, but the apoptotic induction occurred in a significant part of molluscan larval cells that had been subject to a freeze–thaw cycle prior to cell cultivation.
Here, we compared the effects of apoptotic inducers and inhibitors both in molluscan and mammalian cells. In vertebrates, in most cases, the apoptotic pathway is characterized by an increase in mitochondrial outer membrane permeability (Elmore 2007). However, mollusks, similar to other marine hydrobionts, always maintain increased membrane permeability due to their cell membranes’ specific lipid composition (Loomis 1996; Odintsova and Boroda 2012). Thus, interpretation of the results may be contradictory. It is difficult to obtain the true number of apoptotic cells using only flow cytometry because mechanically disrupted cells and isolated nuclei have reduced light scattering properties and may be mistakenly counted as small apoptotic cells. Nevertheless, important conclusions can be drawn from this comparative study since it includes both molluscan and mammalian cells. It is possible that the differences observed in drug activities between molluscan and mammalian cells could be due to differences in the sequences of the drug protein targets. However, this assumption requires further research.
The sensitivity of molluscan and mammalian cells to apoptosis is different among these groups. The oyster (Crassostrea gigas) genome is enriched with genes involved in stress response, including genes encoding HSPs and antioxidants, in addition to genes participating in signal transduction and stimuli responses. Only staurosporine, one of the most potent and frequently used apoptotic inducers, caused an evident increase in apoptotic processes in molluscan cells. The minimal to lack of response to other known mammalian apoptotic inducers in molluscan cells is probably connected to the existence of a powerful antiapoptotic system in bivalve mollusks. Eighty-eight genes encoding HSP70 have been detected in oysters, compared to only 17 in humans and 39 in sea urchins (Zhang et al. 2012). As the authors suggest, an antiapoptotic regulatory network in the oyster, C. gigas, is likely crucial to the oyster’s cellular defense system. The results of several Spanish studies confirm that apoptosis can be used to assess biological responses of marine organism hemocytes to stress, such as UV irradiation (Estévez-Calvar et al. 2013) or bacterial stimulation (Prado-Alvarez et al. 2012). Evidence of caspase-specific responses to pathogens and pollutants has been presented for bivalve mollusks (Romero et al. 2011; Estévez-Calvar et al. 2013). The authors described the appearance of multiple executioner caspases in molluscan cells (hemocytes of M. galloprovincialis) whereas the initiator caspases were substantially conserved. These results indicated the execution rather than the initiation phase is more important in the apoptotic process. On the other hand, no significant caspase-3-like activity (although caspase-3 is one of the key enzymes in the apoptotic destruction of cells) was detected in oyster hemocytes undergoing cadmium-induced apoptosis, which suggests that different stressors exert different effects on caspase activity in marine mollusks, even in closely related organisms (Sokolova and Pörtner 2001). Caspase-3 expression increased significantly in mussel hemocytes after heat-induced stress, suggesting that single- and double-stranded DNA damages could not be adequately repaired in some mussel species under high temperatures and that apoptosis may have been the end result of this damage (Yao and Somero 2012). However, even at 2 °C, mussel species exhibited an increase in active-caspase-3 expressions. These data indicate that cold- and heat-induced stress is sufficient to trigger apoptotic molecular mechanisms, although the mechanism of cold injury in mollusks is still largely unknown.
As shown previously in the pacific oyster, an HSP-like chaperone function was also upregulated in response to temperature (Yokoyama et al. 2006). During stress, large amounts of HSC70 mRNA might be rapidly synthesized in order to protect against temperature-induced damage. Furthermore, the oyster genome includes 48 genes encoding apoptotic inhibitors, compared to eight in humans and seven in sea urchins (Zhang et al. 2012). In stressed cells, denatured proteins are either degraded via a cellular protease or refolded by molecular chaperones (Wickner et al. 1999). Yoshimi et al. (2009) have reported that HSC70 expression in Chironomidae did not increase under cold/heat stress or exposure to heavy metals. Significant differences in cold tolerance levels between some invertebrate species within the Antarctic zone have been previously found (Clarke 1991; Waller et al. 2006). This difference in resistance to thermal stress among individual invertebrate species was also reported for species in temperate seas, such as the Mediterranean, but the biological causes of such thermal tolerance remain unknown. Chaperone proteins may form an essential part of the adaptation for biochemical machinery of Antarctic mollusks to low but stable temperatures (Clark et al. 2008); however, the participation of chaperone proteins in the preservation of molluscan cells at ultra-low temperatures is still unknown. Moreover, it is possible that HSP overproduction may be cytotoxic (Feder and Hofmann 1999), and genetic factors other than HSP production might be expected to play a more important role in mollusk temperature adaptation. Compared with warm acclimation, cold acclimation of crabs induced more changes in the expression of genes encoding proteins involved in DNA and RNA binding and modification (Ronges et al. 2012). Moreover, cold acclimation could induce membrane phospholipid changes in bivalve mollusks (Pernet et al. 2007).
The lack of Y-27632-induced effects on the number of apoptotic cells in mollusks found in this study questions both its use in apoptotic inhibition after a freeze–thaw cycle and the role of the ROCK signaling pathway, associated with serine–threonine kinases, in molluscan cell apoptosis. Other mammalian apoptotic inhibitors, cyclic pifithrin-α and CHIR99021, had a slightly positive (or even negative) effect on the number of apoptotic and active cells in molluscan cultures. These results might indicate p53 mitochondrial-dependent signaling pathway non-involvement in apoptosis activation in mollusks after being subject to cold stress. However, p53 gene expression after cyclic pifithrin-α treatment was highly modulated in response to UV radiation (Estévez-Calvar et al. 2013).
Staurosporine was initially discovered by Omura et al. (1977) in some marine actinomycetes, and then it has been isolated from several taxonomically diverse marine invertebrates such as ascidians and gastropods (Kinnel and Scheuer 1992; Horton et al. 1994; Cantrell et al. 1999; Schupp et al. 2002). The presence of substances such as staurosporine in marine invertebrates, including the tissues of some mollusks, can explain the significant apoptotic effect of this apoptotic inducer on molluscan cells. However, STS did not have a significant effect on inducing hemocyte apoptosis in the oyster C. gigas (Lacoste et al. 2002). The authors suggest that mitogen-activated protein kinases and Rho, a member of the Ras GTPase family, may be involved in antiapoptotic mechanisms that modulate the apoptotic effect of noradrenaline (often referred to as one of the “stress hormones”) in these hemocytes.
Mammalian cells (MEFs and HCT 116 cells) were used as positive controls for apoptosis induction, inhibition, and detection methods since the effects of the compounds tested have been well described in the literature (Mehlen et al. 1996; Morris and Geller 1996; Pirnia et al. 2002). However, even for mammals, there are the conflicting data on the effects of apoptotic inducers: apoptotic inducers may lead to apoptotic death in some cells although other cells are unaffected or even stimulated (Schultz and Harringto 2003). The mechanism of staurosporine’s action and its analogs on mammalian cells is poorly understood. These compounds exert antiproliferative effects on certain cancer cell lines (Meyer et al. 1989), while possessing a poor or no effect on normal cell apoptosis (Chen et al. 2000). This drug not only triggers the classical mitochondrial apoptosis pathway in a variety of mammalian tumor cells but activates an additional apoptosis pathway, such as activation of caspase-9 in the absence of the apoptotic protease activating factor 1 (Manns et al. 2011). MEFs is a heterogenic model system, similar to larval molluscan cell cultures; the effects of apoptotic modulators appear to be expressed in this system, but not explicitly, in contrast to HCT 116 cells. All tested mammalian apoptosis inducers caused an increase in the number of apoptotic cells in MEFs and in the HCT 116 cell line to different extents. Significant apoptotic activation in control and Fr molluscan cells was detected only after treatment with STS. Another apoptotic inducer, CAM, did not result in a higher yield of apoptotic molluscan cells but reliably increased the number of active cells after a freeze–thaw cycle. A pro-survival effect of CAM addition to Fr molluscan cells appears to be connected with a hormetic response when a cell or organism tries to survive in an unfavorable environment and activates adaptive mechanisms (Zhang et al. 2015). CAM was shown to reliably stimulate the cell growth of rat pheochromocytoma cells by as much as 39% at low doses and even protect the cells from H2O2-induced cell death (Zhang et al. 2015). CAM activates many downstream signaling pathways by reversibly binding to and stabilizing cleavable complexes formed between DNA and topoisomerase I (Ding et al. 2009). Therefore, CAM’s protective effects may result from upregulation of both the phosphoinositide 3-kinase/Akt and nuclear factor-E2-related factor 2/heme oxygenase-1 pathways in cells under oxidative stress (Zhang et al. 2015). We cannot exclude the unknown effects of tested apoptotic inducers on molluscan cells.
Conclusions
Our findings indicate that the apoptosis in molluscan and mammalian cells is determined by the specific features of cells, and that is in agreement with previously obtained data concerning a strong dependence of apoptosis induction on the cell type (Wlodkowic et al. 2011). We are just starting to elaborate knowledge of the real signaling pathways leading to activation of apoptosis in mollusks after cold stress. In future, we plan to study the apoptotic genes and genes encoding molecular chaperones that are involved in the development of the cold stress response. At this point, apoptotic inducers and inhibitors, routinely used for chemical induction of apoptosis or its inhibition in mammalian cells, may help us to understand the mechanism of cold-induced injury in mollusks. We have found that only some apoptotic inducers (STS and cold stress) can operate in molluscan cell cultures. Unfortunately, we did not find apoptotic inhibitors (among tested mammalian inhibitors) that could significantly reduce apoptosis in larval molluscan primary cell cultures after a freeze–thaw cycle.
References
Ambroise G, Portier A, Roders N, Arnoult D, Vazquez A (2015) Subcellular localization of PUMA regulates its pro-apoptotic activity in Burkitt’s lymphoma B cells. Oncotarget 6:38181–38194. https://doi.org/10.18632/oncotarget.5901
Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK (2003) Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell 4:587–598. https://doi.org/10.1016/S1534-5807(03)00090-X
Boroda AV, Kipryushina YO, Yakovlev KV, Odintsova NA (2016) The contribution of apoptosis and necrosis in freezing injury of sea urchin embryonic cells. Cryobiology 73:7–14. https://doi.org/10.1016/j.cryobiol.2016.06.007
Böttger S, Jerszyk E, Low B, Walker C (2008) Genotoxic stress–induced expression of p53 and apoptosis in leukemic clam hemocytes with cytoplasmically sequestered p53. Cancer Res 68:777–782. https://doi.org/10.1158/0008-5472.can-06-0968
Buckland-nicks J, Tompkins G (2005) Paraspermatogenesis in Ceratostoma foliatum (Neogastropoda): confirmation of programmed nuclear death. J Exp Zool A Comp Exp Biol 303A:723–741. https://doi.org/10.1002/jez.a.207
Buckley BA, Owen M-E, Hofmann GE (2001) Adjusting the thermostat: the threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J Exp Biol 204:3571–3579
Cantrell CL, Groweiss A, Gustafson KR, Boyd MR (1999) A new staurosporine analog from the prosobranch mollusk Coriocella Nigra. Nat Prod Res 14:39–46. https://doi.org/10.1080/10575639908045433
Chapple JP, Smerdon GR, Berry RJ, Hawkins AJS (1998) Seasonal changes in stress-70 protein levels reflect thermal tolerance in the marine bivalve Mytilus edulis L. J Exp Mar Biol Ecol 229:53–68. https://doi.org/10.1016/S0022-0981(98)00040-9
Chen X, Lowe M, Herliczek T, Hall MJ, Danes C, Lawrence DA, Keyomarsi K (2000) Protection of normal proliferating cells against chemotherapy by staurosporine-mediated, selective, and reversible G1 arrest. J Natl Cancer Inst 92:1999–2008. https://doi.org/10.1093/jnci/92.24.1999
Cheng TC (1988) In vivo effects of heavy metals on cellular defense mechanisms of Crassostrea virginica: total and differential cell counts. J Invertebr Pathol 51:207–214. https://doi.org/10.1016/0022-2011(88)90027-4
Cherkasov AS, Grewal S, Sokolova IM (2007) Combined effects of temperature and cadmium exposure on haemocyte apoptosis and cadmium accumulation in the eastern oyster Crassostrea virginica (Gmelin). J Therm Biol 32:162–170. https://doi.org/10.1016/j.jtherbio.2007.01.005
Clark MS, Fraser KPP, Peck LS (2008) Antarctic marine molluscs do have an HSP70 heat shock response. Cell Stress Chaperones 13:39–49. https://doi.org/10.1007/s12192-008-0014-8
Clarke A (1991) What is cold adaptation and how should we measure it? Am Zool 31:81–92. https://doi.org/10.1093/icb/31.1.81
Clarke GN, Liu DY, Baker HWG (2006) Recovery of human sperm motility and ability to interact with the human zona pellucida after more than 28 years of storage in liquid nitrogen. Fertil Steril 86:721–722. https://doi.org/10.1016/j.fertnstert.2006.01.050
Ding L, Qiu L, Zhang J, Guo B (2009) Camptothecin-induced cell proliferation inhibition and apoptosis enhanced by DNA methyltransferase inhibitor, 5-aza-2′-deoxycytidine. Chem Pharm Bull 32:1105–1108. https://doi.org/10.1248/bpb.32.1105
Ellis J (1987) Proteins as molecular chaperones. Nature 328:378–379. https://doi.org/10.1038/328378a0
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
Estévez-Calvar N, Romero A, Figueras A, Novoa B (2013) Genes of the mitochondrial apoptotic pathway in Mytilus galloprovincialis. PLoS One 8:e61502. https://doi.org/10.1371/journal.pone.0061502
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282. https://doi.org/10.1146/annurev.physiol.61.1.243
Fields PA, Zuzow MJ, Tomanek L (2012) Proteomic responses of blue mussel (Mytilus) congeners to temperature acclimation. J Exp Biol 215:1106–1116. https://doi.org/10.1242/jeb.062273
Halpin PM, Sorte CJ, Hofmann GE, Menge BA (2002) Patterns of variation in levels of Hsp70 in natural rocky shore populations from microscales to mesoscales. Integr Comp Biol 42:815–824. https://doi.org/10.1093/icb/42.4.815
Hampton MB, Orrenius S (1997) Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett 414:552–556. https://doi.org/10.1016/S0014-5793(97)01068-5
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–580. https://doi.org/10.1038/381571a0
Hirsch T, Marchetti P, Susin SA, Dallaporta B, Zamzami N, Marzo I, Geuskens M, Kroemer G (1997) The apoptosis-necrosis paradox. Apoptogenic proteases activated after mitochondrial permeability transition determine the mode of cell death. Oncogene 15:1573–1581. https://doi.org/10.1038/sj.onc.1201324
Hofmann G, Somero G (1995) Evidence for protein damage at environmental temperatures: seasonal changes in levels of ubiquitin conjugates and hsp70 in the intertidal mussel Mytilus trossulus. J Exp Biol 198:1509–1518
Hofmann GE, Buckley BA, Place SP, Zippay ML (2002) Molecular chaperones in ectothermic marine animals: biochemical function and gene expression. Integr Comp Biol 42:808–814. https://doi.org/10.1093/icb/42.4.808
Horton PA, Longley RE, McConnell OJ, Ballas LM (1994) Staurosporine aglycone (K252-c) and arcyriaflavin A from the marine ascidian, Eudistoma sp. Experientia 50:843–845. https://doi.org/10.1007/bf01956468
Kefaloyianni E, Gourgou E, Ferle V, Kotsakis E, Gaitanaki C, Beis I (2005) Acute thermal stress and various heavy metals induce tissue-specific pro-or anti-apoptotic events via the p38-MAPK signal transduction pathway in Mytilus galloprovincialis (Lam.). J Exp Biol 208:4427–4436. https://doi.org/10.1242/jeb.01924
Kinnel RB, Scheuer PJ (1992) 11-Hydroxystaurosporine: a highly cytotoxic, powerful protein kinase C inhibitor from a tunicate. J Org Chem 57:6327–6329. https://doi.org/10.1021/jo00049a049
Kiss T (2010) Apoptosis and its functional significance in molluscs. Apoptosis 15:313–321. https://doi.org/10.1007/s10495-009-0446-3
Koropatnick T, Goodson MS, Heath-Heckman EAC, McFall-Ngai M (2014) Identifying the cellular mechanisms of symbiont-induced epithelial morphogenesis in the squid-Vibrio association. Biol Bull 226:56–68. https://doi.org/10.1086/BBLv226n1p56
Lacoste A, Cueff A, Poulet SA (2002) P35-sensitive caspases, MAP kinases and Rho modulate β-adrenergic induction of apoptosis in mollusc immune cells. J Cell Sci 115:761–768
Lindquist S (1986) The heat-shock response. Annu Rev Biochem 55:1151–1191. https://doi.org/10.1146/annurev.bi.55.070186.005443
Liu D, Chen Z (2013) The expression and induction of heat shock proteins in molluscs. Protein Pept Lett 20:601–606. https://doi.org/10.2174/0929866511320050014
Lockwood BL, Sanders JG, Somero GN (2010) Transcriptomic responses to heat stress in invasive and native blue mussels (genus Mytilus): molecular correlates of invasive success. J Exp Biol 213:3548–3558. https://doi.org/10.1242/jeb.046094
Lockwood BL, Connor KM, Gracey AY (2015) The environmentally tuned transcriptomes of Mytilus mussels. J Exp Biol 218:1822–1833. https://doi.org/10.1242/jeb.118190
Loomis SH (1996) Freezing tolerance of marine invertebrates. Oceanogr Lit Rev 43:337–350
Manns J, Daubrawa M, Driessen S, Paasch F, Hoffmann N, Löffler A, Lauber K, Dieterle A, Alers S, Iftner T, Schulze-Osthoff K, Stork B, Wesselborg S (2011) Triggering of a novel intrinsic apoptosis pathway by the kinase inhibitor staurosporine: activation of caspase-9 in the absence of Apaf-1. FASEB J 25:3250–3261. https://doi.org/10.1096/fj.10-177527
Marión RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA (2009) A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature 460:1149–1153. https://doi.org/10.1038/nature08287
Mazur P (1984) Freezing of living cells: mechanisms and implications. Am J Physiol Cell Physiol 247:C125–C142. https://doi.org/10.1152/ajpcell.1984.247.3.C125
McFall-Ngai MJ (1999) Consequences of evolving with bacterial symbionts: insights from the squid-Vibrio associations. Annu Rev Ecol Syst 30:235–256. https://doi.org/10.1146/annurev.ecolsys.30.1.235
McGee HA, Martin WJ (1962) Cryochemistry. Cryogenics 2:257–267. https://doi.org/10.1016/0011-2275(62)90001-2
Mehlen P, Schulze-Osthoff K, Arrigo A-P (1996) Small stress proteins as novel regulators of apoptosis: heat shock protein 27 blocks Fas/Apo-1- and staurosporine-induced cell death. J Biol Chem 271:16510–16514. https://doi.org/10.1074/jbc.271.28.16510
Meyer T, Regenass U, Fabbro D, Alteri E, Rösel J, Müller M, Caravatti G, Matter A (1989) A derivative of staurosporine (CGP 41:251) shows selectivity for PKC inhibition and in vitro antiproliferative effects as well as in vivo antitumor activity. Int J Cancer 43:851–866. https://doi.org/10.1002/(SICI)1097-0215(19990517)81:4<669::AID-IJC26>3.0.CO;2-F
Mičić M, Bihari N, Labura Ž, Müller WEG, Batel R (2001) Induction of apoptosis in the blue mussel Mytilus galloprovincialis by tri-n-butyltin chloride. Aquat Toxicol 55:61–73. https://doi.org/10.1016/S0166-445X(01)00156-4
Mondy WL, Pierce SK (2003) Apoptotic-like morphology is associated with annual synchronized death in kleptoplastic sea slugs (Elysia chlorotica). Invertebr Biol 122:126–137. https://doi.org/10.1111/j.1744-7410.2003.tb00078.x
Morris EJ, Geller HM (1996) Induction of neuronal apoptosis by camptothecin, an inhibitor of DNA topoisomerase-I: evidence for cell cycle-independent toxicity. J Cell Biol 134:757–770. https://doi.org/10.1083/jcb.134.3.757
Muttray AF, Cox RL, St-Jean S, van Poppelen P, Reinisch CL, Baldwin SA (2005) Identification and phylogenetic comparison of p53 in two distinct mussel species (Mytilus). Comp Biochem Physiol C 140:237–250. https://doi.org/10.1016/j.cca.2005.02.011
Odintsova NA, Boroda AV (2012) Cryopreservation of the cells and larvae of marine organisms. Russ J Mar Biol 38:101–111. https://doi.org/10.1134/s1063074012020083
Odintsova N, Tsal L (1995) Cryopreservation of primary-cell cultures of Bivalvia. Cryo-Lett 16:13–20
Odintsova NA, Dyachuk VA, Nezlin LP (2010) Muscle and neuronal differentiation in primary cell culture of larval Mytilus trossulus (Mollusca: Bivalvia). Cell Tissue Res 339:625–637. https://doi.org/10.1007/s00441-009-0918-3
Odintsova NA, Boroda AV, Maiorova MA, Yakovlev KV (2017) The death pathways in mussel larval cells after a freeze-thaw cycle. Cryobiology 77:41–49. https://doi.org/10.1016/j.cryobiol.2017.05.009
Omura S et al (1977) A new alkaloid AM-2282 of Streptomyces origin taxonomy, fermentation, isolation and preliminary characterization. J Antibiot 30:275–282. https://doi.org/10.7164/antibiotics.30.275
Peh GSL, Adnan K, George BL, Ang H-P, Seah X-Y, Tan DT, Mehta JS (2015) The effects of Rho-associated kinase inhibitor Y-27632 on primary human corneal endothelial cells propagated using a dual media approach. Sci Rep 5:9167. https://doi.org/10.1038/srep09167
Pernet F, Tremblay R, Comeau L, Guderley H (2007) Temperature adaptation in two bivalve species from different thermal habitats: energetics and remodelling of membrane lipids. J Exp Biol 210:2999–3014. https://doi.org/10.1242/jeb.006007
Peterson S, Loring JF (2012) Human stem cell manual: a laboratory guide. Academic Press, Cambridge
Pirnia F, Schneider E, Betticher DC, Borner MM (2002) Mitomycin C induces apoptosis and caspase-8 and -9 processing through a caspase-3 and Fas-independent pathway. Cell Death Differ 9:905–914. https://doi.org/10.1038/sj.cdd.4401062
Prado-Alvarez M, Romero A, Balseiro P, Dios S, Novoa B, Figueras A (2012) Morphological characterization and functional immune response of the carpet shell clam (Ruditapes decussatus) haemocytes after bacterial stimulation. Fish Shellfish Immunol 32:69–78. https://doi.org/10.1016/j.fsi.2011.10.019
Przeslawski R, Byrne M, Mellin C (2015) A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob Chang Biol 21:2122–2140. https://doi.org/10.1111/gcb.12833
Riggs R, Mayer J, Dowling-Lacey D, Chi T-F, Jones E, Oehninger S (2010) Does storage time influence postthaw survival and pregnancy outcome? An analysis of 11,768 cryopreserved human embryos. Fertil Steril 93:109–115. https://doi.org/10.1016/j.fertnstert.2008.09.084
Romero A, Estévez-Calvar N, Dios S, Figueras A, Novoa B (2011) New insights into the apoptotic process in mollusks: characterization of caspase genes in Mytilus galloprovincialis. PLoS One 6:e17003. https://doi.org/10.1371/journal.pone.0017003
Romero-Ramírez H, Morales-Guadarrama MT, Pelayo R, López-Santiago R, Santos-Argumedo L (2015) CD38 expression in early B-cell precursors contributes to extracellular signal-regulated kinase-mediated apoptosis. Immunology 144:271–281. https://doi.org/10.1111/imm.12370
Ronges D, Walsh JP, Sinclair BJ, Stillman JH (2012) Changes in extreme cold tolerance, membrane composition and cardiac transcriptome during the first day of thermal acclimation in the porcelain crab Petrolisthes cinctipes. J Exp Biol 215:1824–1836. https://doi.org/10.1242/jeb.069658
Schultz DR, Harringto WJ Jr (2003) Apoptosis: programmed cell death at a molecular level. Semin Arthritis Rheum 32:345–369. https://doi.org/10.1053/sarh.2003.50005
Schupp P, Proksch P, Wray V (2002) Further new staurosporine derivatives from the ascidian Eudistoma toealensis and its predatory flatworm Pseudoceros sp. J Nat Prod 65:295–298. https://doi.org/10.1021/np010259a
Sokolova IM (2009) Apoptosis in molluscan immune defense. Invertebr Surviv J 6:49–58
Sokolova IM, Pörtner HO (2001) Physiological adaptations to high intertidal life involve improved water conservation abilities and metabolic rate depression in Littorina saxatilis. Mar Ecol Prog Ser 224:171–186. https://doi.org/10.3354/meps224171
Sokolova IM, Evans S, Hughes FM (2004) Cadmium-induced apoptosis in oyster hemocytes involves disturbance of cellular energy balance but no mitochondrial permeability transition. J Exp Biol 207:3369–3380. https://doi.org/10.1242/jeb.01152
Sõti C, Sreedhar AS, Csermely P (2003) Apoptosis, necrosis and cellular senescence: chaperone occupancy as a potential switch. Aging Cell 2:39–45. https://doi.org/10.1046/j.1474-9728.2003.00031.x
Sunila I, LaBanca J (2003) Apoptosis in the pathogenesis of infectious diseases of the eastern oyster Crassostrea virginica. Dis Aquat Org 56:163–170. https://doi.org/10.3354/dao056163
Walker CW, Van Beneden RJ, Muttray AF, Böttger SA, Kelley ML, Tucker AE, Thomas WK (2011) Chapter one - p53 superfamily proteins in marine bivalve cancer and stress biology. In: Lesser M (ed) Advances in marine biology, vol 59. Academic Press, pp 1–36. https://doi.org/10.1016/B978-0-12-385536-7.00001-7
Waller CL, Worland MR, Convey P, Barnes DKA (2006) Ecophysiological strategies of Antarctic intertidal invertebrates faced with freezing stress. Polar Biol 29:1077–1083. https://doi.org/10.1007/s00300-006-0152-3
Wickner S, Maurizi MR, Gottesman S (1999) Posttranslational quality control: folding, refolding, and degrading proteins. Science 286:1888–1893. https://doi.org/10.1126/science.286.5446.1888
Wlodkowic D, Telford W, Skommer J, Darzynkiewicz Z (2011) Chapter 4 - apoptosis and beyond: cytometry in studies of programmed cell death. In: Darzynkiewicz Z, Holden E, Orfao A, Telford W, Wlodkowic D (eds) Methods in cell biology, vol 103. Academic Press, pp 55–98. https://doi.org/10.1016/B978-0-12-385493-3.00004-8
Yao C-L, Somero GN (2012) The impact of acute temperature stress on hemocytes of invasive and native mussels (Mytilus galloprovincialis and M. californianus): DNA damage, membrane integrity, apoptosis and signalling pathways. J Exp Biol 215:4267–2477. https://doi.org/10.1242/jeb.073577
Yokoyama Y et al (2006) cDNA cloning of Japanese oyster stress protein homologous to the mammalian 78-kDa glucose regulated protein and its induction by heatshock. Fish Sci 72:402–409. https://doi.org/10.1111/j.1444-2906.2006.01163.x
Yoshimi T, Odagiri K, Hiroshige Y, S-i Y, Takahashi Y, Sugaya Y, Miura T (2009) Induction profile of HSP70-cognate genes by environmental pollutants in Chironomidae. Environ Toxicol Pharmacol 28:294–301. https://doi.org/10.1016/j.etap.2009.05.008
Zeiss CJ (2003) The apoptosis-necrosis continuum: insights from genetically altered mice. Vet Pathol 40:481–495. https://doi.org/10.1354/vp.40-5-481
Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, Yang P, Zhang L, Wang X, Qi H, Xiong Z, Que H, Xie Y, Holland PWH, Paps J, Zhu Y, Wu F, Chen Y, Wang J, Peng C, Meng J, Yang L, Liu J, Wen B, Zhang N, Huang Z, Zhu Q, Feng Y, Mount A, Hedgecock D, Xu Z, Liu Y, Domazet-Lošo T, du Y, Sun X, Zhang S, Liu B, Cheng P, Jiang X, Li J, Fan D, Wang W, Fu W, Wang T, Wang B, Zhang J, Peng Z, Li Y, Li N, Wang J, Chen M, He Y, Tan F, Song X, Zheng Q, Huang R, Yang H, du X, Chen L, Yang M, Gaffney PM, Wang S, Luo L, She Z, Ming Y, Huang W, Zhang S, Huang B, Zhang Y, Qu T, Ni P, Miao G, Wang J, Wang Q, Steinberg CEW, Wang H, Li N, Qian L, Zhang G, Li Y, Yang H, Liu X, Wang J, Yin Y, Wang J (2012) The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490:49–54. https://doi.org/10.1038/nature11413
Zhang C, Chen S, Bao J, Zhang Y, Huang B, Jia X, Chen M, Wan JB, Su H, Wang Y, He C (2015) Low doses of camptothecin induced hormetic and neuroprotective effects in PC12 cells. Dose-Response 13:1–7. https://doi.org/10.1177/1559325815592606
Zhivotovsky B (2004) Apoptosis, necrosis and between. Cell Cycle 3:63–65. https://doi.org/10.4161/cc.3.1.606
Acknowledgments
This study was supported in part by the Russian Science Foundation of Far Eastern Federal University (grant No 14-50-00034). Flow cytometric analysis was performed in the National Scientific Center of Marine Biology, FEB RAS (Vladivostok), and in the Laboratory of Marine Invertebrate Biology of the Far Eastern Federal University (Vladivostok, Russia). We would like to express special thanks to Dr. I.V. Kudryavtsev for his help in interpreting flow cytometric data and Dr. Mariia Miorova for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
Results of flow cytometric detection of apoptotic and dead MEFs after a 24 h-treatment with inducers or inhibitors of apoptosis. Cells were stained with the green fluorescent stain, FLICA®, in conjunction with DAPI (a) or another green fluorescent stain, YO-PRO™-1, in conjunction with DAPI (b). The samples were analyzed with a CytoFLEX flow cytometer. Treatment key: control cells (C); cells undergoing STS-induced apoptosis (STS), cells undergoing CAM-induced apoptosis (CAM), cells undergoing MMC-induced apoptosis (MMC); cells cultivated with apoptotic inhibitors: cyclic pifithrin-α (Alpha), CHIR99021 (CHIR), Y-27632 (Y); cells undergoing oxidative stress (H2O2). The significance levels are *P < 0.05 and **P < 0.01 (PNG 89 kb)
Fig. S2
Results of flow cytometric detection of apoptotic and dead HCT 116 cells after a 24 h-treatment with inducers or inhibitors of apoptosis. Cells were stained with FLICA® in conjunction with DAPI. The samples were analyzed in a CytoFLEX flow cytometer. Treatment key: control cells (C); cells cultivated with apoptotic inducers: cells undergoing STS-induced apoptosis (STS); cells undergoing CAM-induced apoptosis (CAM); cells undergoing MMC-induced apoptosis (MMC); cells cultivated with apoptotic inhibitors: cyclic pifithrin-α (Alpha), CHIR99021 (CHIR), Y-27632 (Y); STS-treated cells incubated with apoptotic inhibitors: STS + Alpha, STS + CHIR, STS + Y; cells undergoing oxidative stress (H2O2). The significance levels are *P < 0.05 and **P < 0.01 (PNG 55 kb)
Rights and permissions
About this article
Cite this article
Boroda, A.V., Kipryushina, Y.O. & Odintsova, N.A. Chemical modulation of apoptosis in molluscan cell cultures. Cell Stress and Chaperones 24, 905–916 (2019). https://doi.org/10.1007/s12192-019-01014-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-019-01014-x