Abstract
Heat shock protein gene (Hsp) families are thought to be important in thermal adaptation, but their expression patterns under various thermal stresses have still been poorly characterized outside of model systems. We have therefore characterized Hsp genes and their stress responses in the oriental fruit moth (OFM), Grapholita molesta, a widespread global orchard pest, and compared patterns of expression in this species to that of other insects. Genes from four Hsp families showed variable expression levels among tissues and developmental stages. Members of the Hsp40, 70, and 90 families were highly expressed under short exposures to heat and cold. Expression of Hsp40, 70, and Hsc70 family members increased in OFM undergoing diapause, while Hsp90 was downregulated. We found that there was strong sequence conservation of members of large Hsp families (Hsp40, Hsp60, Hsp70, Hsc70) across taxa, but this was not always matched by conservation of expression patterns. When the large Hsps as well as small Hsps from OFM were compared under acute and ramping heat stress, two groups of sHsps expression patterns were apparent, depending on whether expression increased or decreased immediately after stress exposure. These results highlight potential differences in conservation of function as opposed to sequence in this gene family and also point to Hsp genes potentially useful as bioindicators of diapause and thermal stress in OFM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects and other ectotherms possess various plastic responses to deal with thermal changes in the environment, including short-term acclimation and long-term diapause (Angilletta 2009; Fischer and Karl 2010; Hoffmann et al. 2012). These responses differ in their timing and duration, and their cumulative effects influence development and survival (Colinet and Hoffmann 2012; Gillespie et al. 2012; Kvist et al. 2013; Ma et al. 2015). Molecular mechanisms behind temperature stress responses are likely to be complex (Angilletta 2009), involving a number of genes/proteins activated during heat/cold treatment (Zhao and Jones 2012).
The most well-characterized molecular responses to thermal extremes in insects remains those involving heat shock proteins (Hsps), which are considered to play crucial roles in environmental stress tolerance and in thermal adaptation (Bedulina et al. 2013; Colinet et al. 2013) and often represent sensitive indicators for phenotypic adjustments (Colinet et al. 2010). Proteins like Hsp70 can respond to a range of adverse conditions and also interact with a variety of other proteins in producing phenotypic effects under stress (Bettencourt et al. 2008; Choi et al. 2014; Colinet et al. 2010). The expression of Hsp70 and other Hsps in insects can also have indirect effects on plastic responses by influencing the development of stress-resistant diapause phases, although these effects appear to be taxon specific (Gkouvitsas et al. 2008; Rinehart et al. 2007; Tungjitwitayakul et al. 2008; Yocum et al. 1998; Zhang and Denlinger 2010).
Insect Hsp genes constitute a subset of a larger group of genes coding for molecular chaperones that are involved in multiple developmental processes and that assist repair stress injuries via transportation and degradation of aggregated proteins in the organism (Choi et al. 2014; Kim et al. 2014; Shu et al. 2011). For example, Hsc70 and Hsp90 interact with ecdysone receptor activation which is vital in ecdysone response pathway (Arbeitman and Hogness 2000), while Hsp27 is known to involve in eye development (Chen et al. 2012). Hsp70 and Hsp90 as well as small heat shock proteins (Hsp22, Hsp67Ba, and Hsp67Bc) are also implicated in the maintenance of phenotypic stability under variable environmental conditions (Morrow and Tanguay 2015; Takahashi et al. 2010).
Most Hsp expression patterns under adverse conditions and during development have been characterized in Drosophila species and a few other model insects (Ashok Kumar et al. 2014; Kregel 2002; Tower 2011). Hsp expression profiles are lacking for many insect groups including Lepidoptera, which include several of the most important pest species. Where expression profiles of Hsps have been characterized, they have tended to involve a limited number of stress treatments that may not capture the complexity of organismal responses to stress in nature. In practice, plastic responses of insects to thermal extremes can be complex, depending on the speed of onset of the stress and level of stressful conditions reached. This has led to debate about relevant assays of thermal resistance in insects for simulating stressful conditions encountered under natural conditions (e.g., Choi et al. 2014; Mitchell and Hoffmann 2010; Rezende et al. 2011). This level of complexity is rarely considered in expression studies.
The oriental fruit moth (OFM), Grapholita molesta, one of the world’s most invasive orchard pest insect of stone and pome fruits, causes severe economic loss in the global fruit industry (Jones et al. 2010; Kirk et al. 2013; Russell 1986). OFM has gained recognition as a pest of peaches and other fruit crops since its introduction into the Americans in the last century (Jones 2010). The developmental threshold of OFM ranges from 4 to 11 °C at the lower end and from 30 to 35 °C at the upper end. To overwinter in cold areas, OFM enters diapause at a late larvae stage induced by low temperature and a short photoperiod (Russell 1986). These characteristics give OFM a wide thermal performance breadth (Jones 2010) and suggest that it is a useful Lepidopteran model system to investigate altered patterns of gene expression under different thermal conditions.
In this study, we considered Hsp expression in OFM under temperature extremes and diapause. We cloned four novel Hsp genes (GmHsp40, GmHsp60, GmHsp70, and GmHsc70) and examined expression patterns across development, across tissues, and in response to heat stress, cold stress, and diapause. We also considered expression profiles of 11 Hsps in response to different types of heat stresses (including ramped increases in temperature and abrupt temperature shifts), covering most of the Hsp families in OFM. We use these data to answer the following questions: (1) How do GmHsp sequences compare to those for related genes in other Lepidoptera and other insects? (2) What patterns of Hsp expression occur under thermal stresses (including ramping and acute thermal stress) and under diapause phase and how do these compare to expression patterns in other insects? (3) Can the expression patterns of Hsps be placed into different categories to assist in functional characterization? We also consider the potential of Hsps to act as bioindicators of diapause and stressed states.
Materials and methods
Insect preparation
The OFM population used in this study was sampled from Hebei Province, Northern China (N 39° 47′, E 118° 41′). Moths were reared in the laboratory over 2 years at 24 °C and 60 % humidity under a photoperiod of 15L/9D. Six developmental stages of OFM were collected to study GmHsps temporal expression, including 3-day eggs, first instar larvae, third instar larvae, fifth instar larvae, pupae, and adults. To examine the tissue distribution of Hsp expression, the fifth instar larvae were dissected on ice, and each tissue including head, thorax, abdomen, fat body, and midgut were kept in RNAlater (Qiagen, Hilden, Germany) at −80 °C before RNA extraction.
cDNA synthesis and full-length cloning of Hsp
Total RNA was obtained by RNeasy Mini kit (Qiagen, Valencia, CA, USA) following supplier’s instructions before digesting with DNase I (Invitrogen) for 15 min. The concentration of RNA was then measured by NanoDrop spectrophotometer (ND-1000, Thermo Fisher Scientific, Wilmington, DE). The single-strand complementary DNA (cDNA) from the whole body of fifth instar larvae serving as the rapid amplification of cDNA ends (RACE) template was synthesized by SMART RACE cDNA Amplification Kit (Clontech, Mountain View, CA, USA). Using 5′- and 3′-RACE degenerated primers, PCR reactions were run with the following cycles: five cycles consisting of 94 °C for 5 s, 72 °C for 3 min, then five cycles consisting of 94 °C for 5 s, 70 °C for 10 s, and 72 °C for 3 min, and then 25 cycles consisting of 94 °C for 5 s, 68 °C for 10 s, and 72 °C for 180 s. A final extension was performed under 72 °C for 10 min. The PCR products were visualized by electrophoresis in a 1.5 % agarose gel and purified using an ABgene Ultra PCR Clean-Up Kit (Thermo Scientific) before they were cloned into a pGEM-T vector (Promega, Madison, WI, USA). The products were sequenced on a 3730xl DNA Analyzer (Applied Biosystems). The final cDNA templates used for real-time PCR of gene expression were started with 2 μg total RNA in the first-strand cDNA synthesis with M-MLV reverse transcriptase (Promega, Madison, WI, USA).
Phylogenetic analysis
NCBI BLAST tools were used to detect conserved domains of each Hsp family. The open reading frames (ORFs), translations, and predicted secondary structure of sHsps were obtained online (http://www.bioinformatics.org/sms2). The molecular weights of sHsps were also estimated by an online tool (http://web.expasy.org/protparam). To construct the phylogenetic trees, the Hsp sequences derived from other insect species were downloaded from GenBank and aligned in MEGA6 (Tamura et al. 2011). All phylogenetic analyses were run using both Bayesian inference (BI) and maximum likelihood (ML) methods. BI analysis was performed using MrBayes, v 3.1.2 (Ronquist and Huelsenbeck 2003). Two sets of four chains were allowed to run simultaneously for 1,000,000 generations. Each set was sampled every 100 generations with a burn-in of 25 %. Stationarity was considered to have been reached when the average standard deviation of split frequencies was less than 0.01. Bayesian posterior probabilities (BPPs) were estimated based on a 50 % majority rule consensus tree of the remaining trees. ML analysis was conducted using an online program (http://www.atgc-montpellier.fr/phyml/) with a GTR substitution model. Bootstrap values were obtained for the nodes based on 1000 replicates. We also collected data on temperature responses of phylogenetic branch species from published references. Hsp expression patterns were characterized into three categories (upregulated, no change, or no report) under the three conditions considered (heat, cold, and diapause).
Thermal and diapause responsive tests
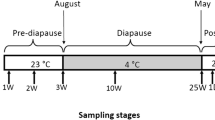
To characterize the responsive expressions of Hsps in adults under thermal extremes, high and low temperatures were initially defined based on pilot experiments as the most severe conditions to which adults could be exposed without mortality after 2-h recovery. The 12-h enclosed moths (equal numbers of males and females) were therefore kept at −5 °C (cold) or 42 °C (heat) for 1 and 2 h before recovering at 25 °C for 2 h. The 42 °C temperature is close to 41 °C which Chen et al. (2014) previously found to elicit higher expression of Hsp70 and Hsp90 in G. molesta compared to lower temperatures or 44 °C. No data have previously been collected on lower limits of adults. Controls were treated identically without being stressed. For the diapause treatment, diapause was induced by rearing moths at the same temperature of 25 °C under a 12:12 photoperiod (Dickson 1949). Each treatment consisted of five biological replicates, with 14–20 individuals (7–10 per sex with same number) per replicate. All samples were stored at −80 °C before RNA extraction. Responsive Hsps were identified based on their upregulation following high-temperature tests.
Hsp expression profiles under acute and ramping heat stress
We used two high-temperature regimes to investigate Hsp expression patterns: an acute regime (40 °C for 2 h) and a ramping regime (gradually heat from 25 to 40 °C by 0.1 °C/min before being held at 40 °C for 2 h). We chose 40 °C for two reasons: (1) It often represents the maximal temperature experienced in northern Chinese orchards in recent years; and (2) exposure to this temperature for a short period did not inhibit mating or reduce fecundity in pilot experiments. Hsp expression tests resulted in 11 responsive genes being selected, representing five families: Hsp10, sHsp, Hsp40, Hsp70, and Hsp90. Insects were collected with and without recovery for 2 h at 25 °C after acute heat stress to investigate whether Hsp chaperon expression changed with a recovery period. Insects exposed to ramping heat were all collected after a 2-h recovery period. All the treatments involved moths enclosed within the previous 12 h, equal numbers of males and females, and 7–10 individuals of a sex per treatment.
Real-time quantitative PCR
The HSP real-time PCR primers were designed based on the full-length cDNA transcripts (Appendix S1). β-actin and GAPDH were both chosen as the reference genes for normalizing the messenger RNA (mRNA) expression level (Zhang et al. 2015). The Hsp transcript standards were produced by cloning the PCR products into plasmids. Six 10-fold serial dilutions of stock plasmid served as quantitative standards to estimate the relative expression of each Hsp transcript. We also used other OFM Hsp genes for extreme temperature expression, including GmHsp11.1, 18.9, 19.8, 19.9, 21.4, 22.5, 31.8 (Zhang et al. 2015), GmHsp70 (but labeled as GmHsp70-2 in figure), and GmHsp90 (Chen et al. 2014).
Real-time quantitative PCR (RT-PCR) was conducted in a 20 μl volume comprising of 10 μl 2× SuperReal PreMix Plus (with SYBR Green I) (Tiangen, Beijing, China), 1 μl cDNA template/plasmid, 1.2 μl RT-PCR primers (10 mM working solution), and 7.8 μl dd H2O on Stratagene Mx3000P thermal cycler. The PCR cycling parameters used a two-step method: initial denaturation of 15 min at 95 °C and 40 cycles of 10 s at 95 °C and 30 s at 60 °C. Each experimental group included five biological replicates, each of which contained three technical repeats. Finally, the output data were normalized by two methods, with or without using reference genes; the latter involved the NORMA-gene normalization algorithm (Heckmann et al. 2011) which involves normalization based on the experimental data to avoid the issue of error in reference gene expression and assumption that the reference genes are not influenced by the different conditions tested.
Data statistics
To differentiate the hierarchical clustering of heat stress GmHsp genes and three heat treatments, the complete linkage clustering method was followed in Cluster v3.0 software, using the similarity metric of uncentered correlations on log-transformed data (Eisen et al. 1998). The output file was generated and read with Java TreeView software (Saldanha 2004) to indicate patterns of relationships among the GmHsp genes which were visualized using three colors.
Differences between treatments for normalized expression were compared either by t-tests (for comparison of two means) or by one-way analysis of variance (ANOVA) followed by Tukey B post hoc comparisons among means run with SPSS 20.0 software. Treatment differences were considered significant at p < 0.01 rather than p < 0.05 given that expression of several genes was compared.
Results
Cloning and characterization of GmHsps
The four full-length heat shock proteins were obtained by using 5′ and 3′ rapid amplification of cDNA ends in G. molesta whole body cDNA. Based on BLAST results, they were named as GmHsp40, GmHsp60, GmHsc70, and GmHsp70 (GenBank accession nos. from KT003961 to KT003964, respectively). The ORFs spanned between 1062 and 1968 bp, coding from 353 to 655 amino acid proteins. GmHsp90 sequence was obtained from published data (GenBank no. JX678980, Chen et al. 2014).
GmHsp40 was predicted to have a molecular weight of 40.01 kDa and isoelectric point (pI) of 5.69. It contained two conserved regions, the DnaJ domain (aa 25–79) and DnaJ-C domain (aa 130–326), from the conserved domain lcl99612 from NCBI. The predicted 60.85 kDa molecular weight of GmHsp60 has a similar pI of 5.68 with GmHsp40 as well as two conserved domains: GroEL-like equatorial domain (IPR027413) and GroEL-like apical domain (IPR027409). The conserved Hsp60 site is located between amino acid 428 and 439 (IPR018370). GmHsc70 and GmHsp70 both belonged to the Hsp70 family with a typical conserved peptide-binding domain (IPR029047) and C-terminal domain (IPR029048) but containing varying molecular weights and pIs (GmHsc70 72.62 kDa, 5.09; GmHsp70 68.45 kDa, 5.81).
Phylogenetic analysis and alignment of GmHsp proteins
To analyze the relationships of OFM Hsps with other insects, Hsps from several orders were collected to construct Bayesian phylogenetic trees (Fig. 1), which were similar to other trees obtained by different approaches (Appendix S2). The phylogenetic analysis of four Hsp genes indicated a high level of conservation among insects from different orders of insects, particularly Lepidoptera, with branches all 100 % supported through bootstrapping. The congruence suggested a conserved identity of Hsp families in insects, except in the case of Hsc70. We also collated information on stress responses to three conditions (heat, cold, and diapause) and included these on the phylogenetic trees (Fig. 1). None of the gene families showed a consistent pattern of expression changes under three conditions, with exception of Hsp60, whose expression patterns were only reported in three species. Hsp70 was the most studied Hsp family. This gene was upregulated under stress among insects except locusts. Conserved patterns were not evident for cold or diapause responses for any of the Hsp families.
Phylogenetic tree of the Hsps of G. molesta. Fourteen Hsps of insect Hsp genes and two outgroup sequences were used in these Bayesian phylogenetic trees. Percentage bootstrap values above 50 % were indicated on each cluster. The changes of expression pattern of reported genes are also summarized under heat (H), cold (C), and diapause (D) conditions. Red indicates upregulation, blue indicates no change, and green indicates downregulation. a GmHsp40, b GmHsp60, c GmHsc70, and d GmHsp70. The G. molesta Hsps are labeled in boxes
Transcriptional profiles of GmHsp in tissue distribution
Different tissues of OFM fifth instar larvae including the head, thorax, abdomen, fat body, and midgut were dissected and examined for the transcriptional profiles of five GmHsp genes, including GmHsp90. Patterns were consistent regardless of whether they were analyzed in comparison with housekeeping genes or by the NORMA-gene normalization method, and only the former are therefore presented. All GmHsps were detected in each tissue but were expressed at different levels (Fig. 2). GmHsc70 was expressed at the highest level in the head and midgut (4.21-fold and 3.89-fold) but was low in the fat body (0.82-fold) and midgut (0.18-fold). GmHsp70 exhibited higher expression levels in the abdomen and lower in midgut, with 3.99- and 0.78-fold increased expression respectively. GmHsp40, GmHsp60, and GmHsp90 all showed significant differences in expression among tissues. Within the Hsp70 family, GmHsc70 was higher in the head and midgut than other tissues, and Hsp70 was higher in the abdomen.
Transcriptional profiles of GmHsp across developmental stages
The GmHsps were also examined across OFM developmental stages (Fig. 3). The overall expression patterns of each gene differed across the stages, while most of the Hsps were overexpressed in first instar and pupal stages. The expression level of GmHsp60 varied no more than 4-fold across life stages. In contrast, GmHsp40 and GmHsc70 showed relatively higher variation in expression, with the highest 13-fold change in GmHsp40 in first instars and a 12-fold change of GmHsc70 in pupae. The expression patterns of GmHsp70 and GmHsp90 showed similar trends across life stages. Significantly higher level of expressions were found both in first instar and pupal stages, and expression then decreased until the pupal stage.
Expression in response to diapause
Expression differences of five GmHsps in diapausing OFM showed three genes with upregulated expression (GmHsp40, GmHsc70, and GmHsp70) and one significantly downregulated gene (GmHsp90) at the diapause stage (Fig. 4). GmHsc70 exhibited the highest fold change (10×) in diapausing individuals, and this difference was significant (t = −22.55, df = 8, p < 0.001), in contrast with the lowest fold change for GmHsp90 (t = 3.04, df = 8, p = 0.016).
Mean normalized expression (with standard errors) of GmHsps in diapausing G. molesta, based on log2 transformation of qRT-PCR of the assayed Hsps. Asterisks indicate mean values that are different significantly (p < 0.01) in fifth instar larvae between diapausing and control groups. A value of 0 indicates no difference in expression, whereas positive and negative values indicate upregulation and downregulation, respectively
Expression in response to cold and heat treatments
GmHsp40 and GmHsp90 were significantly upregulated under 1 or 2-h cold stress (Fig. 5). Expression of GmHsp60, GmHsc70, and GmHsp70 were not significantly changed under cold stresses compared with controls. Expression trends were consistent between heat and cold treatments, except for GmHsp70 which only responded to heat (Fig. 6). GmHsp60 and GmHsc70 expression did not change in response to any of the thermal stresses. Furthermore, although 1 and 2-h stress treatments both caused upregulation of expression, the 1-h treatment led to higher expression than the 2-h treatment.
Hsp expression patterns under constant and ramping heat stress
The hierarchical cluster analysis revealed changing patterns of GmHsp gene expression for most genes in response to acute and ramping heat stresses (Fig. 7). Almost all GmHsp genes showed lowest expression levels in control samples (green color), and all genes were strikingly upregulated under heat stress (red color). In terms of the response similarity under heat stress, acute and ramping stresses clustered together, although the ramping heat treatment also included a 2-h recovery period at 25 °C. Based on gene clustering, sHsp genes showed patterns that were intermixed with the large Hsp families, suggesting that these did not represent a unique group.
We characterized expression patterns of 11 GmHsp genes under three heat exposure regimes (Fig. 8). All genes were shown to be significantly increased under the three treatments compared with the controls. GmHsp11.1 presented a unique pattern compared to the other genes, in showing the highest level of expression under ramping heat stress (Fig. 8a). On the other hand, the highest expression of large GmHsps occurred under acute stress, although 2-h recovery in both GmHsp70s did not affect expression. The sHsp genes changed rapidly under acute heat stress and fell into two clusters depending on their responses to the recovery period (Fig. 8b). GmHsp21.4, 19.9, and 31.8 displayed highest expression immediately after heat stress, while the other three sHsp were expressed at a higher level in the recovery stage.
Discussion
We have identified large Hsps from OFM and shown how their expression varies across tissues, developmental stages, and in response to stressful conditions. The results build on previous findings based on the characterization of sHsps in OFM (Zhang et al. 2015) which highlighted that Hsps can show quite different patterns of upregulation or downregulation under heat, cold, and diapause and that these patterns are not necessarily similar across insects. Unlike the sHsps which show a relatively low level of phylogenetic conservation across Lepidoptera and insects generally (Zhang et al. 2015), the large Hsps show strong sequence conservation within families; nevertheless, expression patterns appear much less conserved despite this pattern.
All GmHsps revealed typical signatures or conserved domains of each Hsp family and grouped together in phylogenetic analyses with corresponding Hsps of other insects, suggesting high congruence in sequences. One of GmHsp70 members previously uploaded in GenBank JX678981 (Chen et al. 2014) showed 84 % similarity of full-length amino acid sequence with the gene we studied. Both our GmHsp70 and the one studied by Chen et al. (2014) have a conserved C-terminal motif “EEVD” with a potential binding function, suggesting a role in protein folding/refolding (Michels et al. 1999; Vabulas et al. 2010). Information on Hsp genes from model species might help to identify functional roles of GmHsps, such as the stress-inducible pattern in other species.
The reasons why most GmHsp genes are highly expressed in the first instar and pupal stages are unclear and may reflect a protective function at these stages, given that these stages of OFM are exposed to ambient conditions, while larvae live in fruit and are likely to evade abiotic stress. This may reflect the role of molecular chaperons in the first line of defense of an insect to prevent irreversible denaturation of proteins and vital enzymes (Arrigo 2013; King and MacRae 2015). GmHsp70/90 were also highly expressed in the fat body, suggesting a role of chaperone function in stress signal detection in tissue during development (Krebs and Feder 1998), but these functional roles remain speculative in the absence of additional data including changes in protein levels.
Three GmHsps (GmHsp40, 70, and 90) were heat inducible, which was also the case for Hsps isolated from other insects in these families. Hsp70 is considered as an important stress chaperone under adverse conditions, being expressed at a high level under thermal stress (Colinet et al. 2010; Fu et al. 2014; King and MacRae 2015; Moribe et al. 2010). However, unlike in several other insects (Colinet et al. 2010; Wang and Kang 2005; Zhang and Denlinger 2010), GmHsp70 was not upregulated under cold stress. The similar expression patterns of GmHsp70 and GmHsp40 and rapid upregulation of the expression of these genes suggest a potential role of these co-chaperones in stress responses (Huang and Kang 2007; Kampinga and Craig 2010; Shu et al. 2011; Xu et al. 2011), but this needs further investigation such as through the use of RNAi to alter expression patterns. On the other hand, the absence of large changes in GmHsc70 expression under both heat and cold conditions, which has also been observed in other insects (Bettencourt et al. 2008; Zhang and Denlinger 2010), suggests that this Hsp cognate is not thermally inducible and may not play a chaperone role under adverse conditions.
Some Hsp genes are also upregulated during insect diapause (Rinehart et al. 2007; Zhang and Denlinger 2010), and in our study, we found that GmHsp40 and GmHsp70/GmHsc70 were induced under larval diapause of OFM. Hsp70 in diapausing OFM could be involved in resisting severe cold temperatures in winter and in the repair of freezing damage during recovery (Rinehart et al. 2007). We also observed a decrease of GmHsp90 expression which may relate to a decline of steroid receptor or ecdysteroid/Hsp90 heterocomplex in diapausing individuals (Pratt and Toft 1997, 2003; Zhang and Denlinger 2010). Hsp90 inhibition reduces cell growth signals and growth factors (Peterson and Blagg 2009), perhaps contributing to the maintenance of a diapause state in OFM. The changes in expression pattern of Hsp70 and Hsp90 in our study are not consistent with patterns in other Lepidopteran insects (Gkouvitsas et al. 2008; Rinehart et al. 2007; Sonoda et al. 2006; Tachibana et al. 2005; Tungjitwitayakul et al. 2008), and it is not clear if this reflects functional redundancy among the Hsp genes.
We contrasted our patterns of expression of large Hsp genes with small Hsp genes. As ATP-independent Hsps, the sHsps bind denaturing proteins and further facilitate subsequent refolding by other ATP-dependent Hsp chaperons (Basha et al. 2012). The high expression we found for sHsp under heat stress highlights the potential for these genes to show a rapid response and may attest to their functional importance. However, our data also highlight that sHsps do not fall into one group; two clear patterns were identified when ramping, and acute heat responses were compared, suggesting that the expression of some sHsps is rapidly altered in the absence of a stress and may have a short-lived protective function in contrast to those that show persistently high expression during the recovery phase. These contrasting patterns need to be further investigated at the protein level.
The number and sequence of sHsps are different among orders and even between closely related species; sHsp represent a heterogeneous group that lacks phylogenetic conservation (Basha et al. 2012; Morrow and Tanguay 2012; Zhang et al. 2015). The highly variable N- and C-terminal sequences of sHsp might result in diversified protein substrates, such as metabolic enzymes and proteins involved in translation (Fu et al. 2014; Morrow and Tanguay 2015), contributing to the lack of functional conservation in this family. Some conserved large Hsps do not have these highly variable terminal sequences and may show slower rates of evolution because they have pleiotropic effects on development and because there is less redundancy in this group due to the smaller number of genes comprising the various families; in contrast, sHsps can be deleted without marked deleterious effects, at least in Drosophila (Morrow and Tanguay 2015). Homologous sequences of Hsp70 and Hsp90 can show similar functions across different insects (King and MacRae 2015).
Finally, we suggest that the various Hsps may provide useful biomarkers for detecting the diapause state and stressful conditions. Both copies of GmHsp70 were good indicators of stressful conditions given that they were expressed at a much lower base level than GmHsp90, which may associate with stress signal detection in tissue during development (Krebs and Feder 1998). On the other hand, GmHsp90 may be a biomarker of diapause in OFM, being downregulated specifically when entering diapause. This gene may be useful for differentiating diapause forms from normally developing fifth instar larvae, as these classes cannot be distinguished by morphological criteria. This may help when timing chemical sprays to coincide with the last field generation. The sHsps may also act as indicators of stressful conditions, but lack of phylogenetic signals and consistent expression changes among species unlike Hsp70 (Shatilina et al. 2011; Zhang et al. 2015). Recovery-inducible sHsp (e.g., GmHsp18.9, 22.5, and 19.8b) are likely to be the most useful in this context. In making connections between gene expression and functional traits, it will be important to consider changes in protein levels as well as mRNA. The latter has been proved to be representative of changes at the protein level in some exposures to acute stress although a direct connection will not necessarily always be evident (Sørensen 2010; Gao et al. 2014). To understand how expression of large and small Hsps might influence diapause and stress responses, further investigations at the protein and metabolomic levels are required.
In conclusion, we have found that several members of Hsp40, 70, and 90 families are expressed under thermal extremes in OFM, while members of two of these families as well as Hsc70 show increased expression under diapause. The strong sequence conservation of large Hsps was not matched by functional conservation of expression patterns. These results as well as the different impact of acute and ramping stress on Hsps provides the basis for future functional studies in this important invasive pest which has developed a worldwide distribution from the early twentieth century and continues to cause significant economic damage on a global scale (Kirk et al. 2013). Hsps may have played a role in the rapid establishment and/or spread of OFM in temperate and tropical areas to help counter both heat waves in summer and cold environments in winter. We have described Hsps and their expression from a native OFM population, providing a basis for further comparisons of populations from across the invasive and native range of OFM. The acute and ramping heat treatments we have used match the increasingly common hot conditions encountered in the field in fruit-producing areas of Northern China under climate warming, and Hsp expression may become important in countering increasingly stressful conditions, raising the possibility of future evolutionary changes in Hsp families affecting expression patterns.
References
Angilletta MJ (2009) Looking for answers to questions about heat stress: researchers are getting warmer. Funct Ecol 23:231–232. doi:10.1111/j.1365-2435.2009.01548.x
Arbeitman MN, Hogness DS (2000) Molecular chaperones activate the Drosophila ecdysone receptor, an RXR heterodimer. Cell 101:67–77. doi:10.1016/S0092-8674(00)80624-8
Arrigo AP (2013) Human small heat shock proteins: protein interactomes of homo- and hetero-oligomeric complexes: an update. Febs Lett 587:1959–1969. doi:10.1016/j.febslet.2013.05.011
Ashok Kumar K, Somasundram P, RadhaKrishnan R et al (2014) A review on heat shock protein gene expressions and its association with thermo tolerance in the silkworm of Bombyx mori (L). J Entomol Zool Stud 2:170–176
Basha E, O’Neill H, Vierling E (2012) Small heat shock proteins and alpha-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci 37:106–117. doi:10.1016/j.tibs.2011.11.005
Bedulina DS, Evgen’ev MB, Timofeyev MA et al (2013) Expression patterns and organization of the hsp70 genes correlate with thermotolerance in two congener endemic amphipod species (Eulimnogammarus cyaneus and E. verrucosus) from Lake Baikal. Mol Ecol 22:1416–1430. doi:10.1111/Mec.12136
Bettencourt BR, Hogan CC, Nimali M, Drohan BW (2008) Inducible and constitutive heat shock gene expression responds to modification of Hsp70 copy number in Drosophila melanogaster but does not compensate for loss of thermotolerance in Hsp70 null flies. BMC Biol 6:5. doi:10.1186/1741-7007-6-5
Chen H, Xu XL, Li YP, Wu JX (2014) Characterization of heat shock protein 90, 70 and their transcriptional expression patterns on high temperature in adult of Grapholita molesta (Busck). Insect Sci 21:439–448. doi:10.1111/1744-7917.12057
Chen SF, Kang ML, Chen YC et al (2012) Autophagy-related gene 7 is downstream of heat shock protein 27 in the regulation of eye morphology, polyglutamine toxicity, and lifespan in Drosophila. J Biomed Sci 19:52. doi:10.1186/1423-0127-19-52
Choi BG, Hepat R, Kim Y (2014) RNA interference of a heat shock protein, Hsp70, loses, its protection role in indirect chilling injury to the beet armyworm, Spodoptera exigua. Comp Biochem Phys A 168:90–95. doi:10.1016/j.cbpa.2013.11.011
Colinet H, Hoffmann AA (2012) Comparing phenotypic effects and molecular correlates of developmental, gradual and rapid cold acclimation responses in Drosophila melanogaster. Funct Ecol 26:84–93. doi:10.1111/j.1365-2435.2011.01898.x
Colinet H, Lee SF, Hoffmann AA (2010) Temporal expression of heat shock genes during cold stress and recovery from chill coma in adult Drosophila melanogaster. FEBS J 277:174–185. doi:10.1111/j.1742-4658.2009.07470.x
Colinet H, Siaussat D, Bozzolan F, Bowler K (2013) Rapid decline of cold tolerance at young age is associated with expression of stress genes in Drosophila melanogaster. J Exp Biol 216:253–259. doi:10.1242/jeb.076216
Dickson R (1949) Factors govering the induction of diapause in the oriental fruit moth. Ann Entomol Soc Am 42:511–537
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 95:14863–14868
Fischer K, Karl I (2010) Exploring plastic and genetic responses to temperature variation using copper butterflies. Climate Res 43:17–30. doi:10.3354/Cr00892
Fu XM, Chang ZY, Shi XD et al (2014) Multilevel structural characteristics for the natural substrate proteins of bacterial small heat shock proteins. Protein Sci 23:229–237. doi:10.1002/pro.2404
Gao J, Zhang W, Dang W et al (2014) Heat shock protein expression enhances heat tolerance of reptile embryos. Proc Biol Sci 281:20141135. doi:10.1098/rspb.2014.1135
Gillespie DR, Nasreen A, Moffat CE et al (2012) Effects of simulated heat waves on an experimental community of pepper plants, green peach aphids and two parasitoid species. Oikos 121:149–159. doi:10.1111/j.1600-0706.2011.19512.x
Gkouvitsas T, Kontogiannatos D, Kourti A (2008) Differential expression of two small Hsps during diapause in the corn stalk borer Sesamia nonagrioides (Lef.). J Insect Physiol 54:1503–1510. doi:10.1016/j.jinsphys.2008.08.009
Heckmann LH, Sorensen PB, Krogh PH, Sorensen JG (2011) NORMA-Gene: A simple and robust method for qPCR normalization based on target gene data. BMC Bioinformatics 12:250. doi:10.1186/1471-2105-12-250
Hoffmann AA, Blacket MJ, McKechnie SW et al (2012) A proline repeat polymorphism of the Frost gene of Drosophila melanogaster showing clinal variation but not associated with cold resistance. Insect Mol Biol 21:437–445. doi:10.1111/j.1365-2583.2012.01149.x
Huang LH, Kang L (2007) Cloning and interspecific altered expression of heat shock protein genes in two leafminer species in response to thermal stress. Insect Mol Biol 16:491–500. doi:10.1111/j.1365-2583.2007.00744.x
Jones MM (2010) Susceptibility of oriental fruit moth, (Grapholita molesta (Busck)) to selected insecticides and mixtures. PhD thesis. University of Illinois at Urbana-Champaign
Jones MM, Robertson JL, Weinzierl RA (2010) Susceptibility of oriental fruit moth (Lepidoptera: Tortricidae) larvae to selected reduced-risk insecticides. J Econ Entomol 103:1815–1820. doi:10.1603/Ec10029
Kampinga HH, Craig EA (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11:579–592. doi:10.1038/nrm2941
Kim BM, Rhee JS, Jeong CB et al (2014) Heavy metals induce oxidative stress and trigger oxidative stress-mediated heat shock protein (hsp) modulation in the intertidal copepod Tigriopus japonicus. Comp Biochem Phys C 166:65–74. doi:10.1016/j.cbpc.2014.07.005
King AM, MacRae TH (2015) Insect heat shock proteins during stress and diapause. Ann Rev Entomol 60:59–75. doi:10.1146/annurev-ento-011613-162107
Kirk H, Dorn S, Mazzi D (2013) Worldwide population genetic structure of the oriental fruit moth (Grapholita molesta), a globally invasive pest. BMC Ecol 13:12. doi:10.1186/1472-6785-13-12
Krebs RA, Feder ME (1998) Hsp70 and larval thermotolerance in Drosophila melanogaster: how much is enough and when is more too much? J Insect Physiol 44:1091–1101
Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92:2177–2186. doi:10.1152/japplphysiol.01267.2001
Kvist J, Wheat CW, Kallioniemi E et al (2013) Temperature treatments during larval development reveal extensive heritable and plastic variation in gene expression and life history traits. Mol Ecol 22:602–619. doi:10.1111/j.1365-294X.2012.05521.x
Ma G, Rudolf VH, Ma CS (2015) Extreme temperature events alter demographic rates, relative fitness, and community structure. Glob Chang Biol 21:1794–1808. doi:10.1111/gcb.12654
Michels AA, Kanon B, Bensaude O, Kampinga HH (1999) Heat shock protein (Hsp) 40 mutants inhibit Hsp70 in mammalian cells. J Biol Chem 274:36757–36763
Mitchell KA, Hoffmann AA (2010) Thermal ramping rate influences evolutionary potential and species differences for upper thermal limits in Drosophila. Funct Ecol 24:694–700. doi:10.1111/j.1365-2435.2009.01666.x
Moribe Y, Oka K, Niimi T et al (2010) Expression of heat shock protein 70a mRNA in Bombyx mori diapause eggs. J Insect Physiol 56:1246–1252. doi:10.1016/j.jinsphys.2010.03.023
Morrow G, Tanguay RM (2012) Small heat shock protein expression and functions during development. Int J Biochem Cell Biol 44:1613–1621. doi:10.1016/j.biocel.2012.03.009
Morrow G, Tanguay RM (2015) Drosophila small heat shock proteins: an update on their features and functions. In: Hightower LE (ed) Tanguay RM. Springer International Publishing Switzerland, The big book on small heat shock proteins, pp 579–606
Peterson LB, Blagg BS (2009) To fold or not to fold: modulation and consequences of Hsp90 inhibition. Future Med Chem 1:267–283. doi:10.4155/fmc.09.17
Pratt WB, Toft DO (1997) Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev 18:306–360. doi:10.1210/edrv.18.3.0303
Pratt WB, Toft DO (2003) Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med 228:111–133
Rezende EL, Tejedo M, Santos M (2011) Estimating the adaptive potential of critical thermal limits: methodological problems and evolutionary implications. Funct Ecol 25:111–121. doi:10.1111/j.1365-2435.2010.01778.x
Rinehart JP, Li A, Yocum GD et al (2007) Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc Natl Acad Sci U S A 104:11130–11137. doi:10.1073/pnas.0703538104
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg180
Russell DA (1986) Ecology of the oriental fruit moth, Grapholita molesta (Busck) in New Zealand. PhD thesis. University of Auckland
Saldanha AJ (2004) Java Treeview—extensible visualization of microarray data. Bioinformatics 20:3246–3248. doi:10.1093/bioinformatics/bth349
Shatilina ZM, Riss HW, Protopopova MV et al (2011) The role of the heat shock proteins (HSP70 and sHSP) in the thermotolerance of freshwater amphipods from contrasting habitats. J Therm Biol 36:142–149. doi:10.1016/j.jtherbio.2010.12.008
Shu YH, Du Y, Wang JW (2011) Molecular characterization and expression patterns of Spodoptera litura heat shock protein 70/90, and their response to zinc stress. Comp Biochem Phys A 158:102–110. doi:10.1016/j.cbpa.2010.09.006
Sonoda S, Fukumoto K, Izumi Y et al (2006) Cloning of heat shock protein genes (hsp90 and hsc70) and their expression during larval diapause and cold tolerance acquisition in the rice stem borer, Chilo suppressalis Walker. Arch Insect Biochem Physiol 63:36–47. doi:10.1002/arch.20138
Sørensen JG (2010) Application of heat shock protein expression for detecting natural adaptation and exposure to stress in natural populations. Curr Zool 56:703–713
Tachibana S, Numata H, Goto SG (2005) Gene expression of heat-shock proteins (Hsp23, Hsp70 and Hsp90) during and after larval diapause in the blow fly Lucilia sericata. J Insect Physiol 51:641–647. doi:10.1016/j.jinsphys.2004.11.012
Takahashi KH, Rako L, Takano-Shimizu T et al (2010) Effects of small Hsp genes on developmental stability and microenvironmental canalization. BMC Evol Biol 10:284. doi:10.1186/1471-2148-10-284
Tamura K, Peterson D, Peterson N et al (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Tower J (2011) Heat shock proteins and Drosophila aging. Exp Gerontol 46:355–362. doi:10.1016/j.exger.2010.09.002
Tungjitwitayakul J, Singtripop T, Nettagul A et al (2008) Identification, characterization, and developmental regulation of two storage proteins in the bamboo borer Omphisa fuscidentalis. J Insect Physiol 54:62–76. doi:10.1016/j.jinsphys.2007.08.003
Vabulas RM, Raychaudhuri S, Hayer-Hartl M, Hartl FU (2010) Protein folding in the cytoplasm and the heat shock response. Cold Spring HarbPerspect Biol 2:a004390. doi:10.1101/cshperspect.a004390
Wang XH, Kang L (2005) Differences in egg thermotolerance between tropical and temperate populations of the migratory locust Locusta migratoria (Orthoptera : Acridiidae). J Insect Physiol 51:1277–1285. doi:10.1016/j.jinsphys.2005.07.010
Xu Q, Zou Q, Zheng H et al (2011) Three heat shock proteins from Spodoptera exigua: Gene cloning, characterization and comparative stress response during heat and cold shocks. Comp Biochem Physiol B 159:92–102. doi:10.1016/j.cbpb.2011.02.005
Yocum GD, Joplin KH, Denlinger DL (1998) Upregulation of a 23 kDa small heat shock protein transcript during pupal diapause in the flesh fly, Sarcophaga crassipalpis. Insect Biochem Mol Biol 28:677–682
Zhang B, Zheng JC, Peng Y et al (2015) Stress responses of small heat shock protein genes in Lepidoptera point to limited conservation of function across phylogeny. Plos One 10:e0132700. doi:10.1371/journal.pone.0132700
Zhang Q, Denlinger DL (2010) Molecular characterization of heat shock protein 90, 70 and 70 cognate cDNAs and their expression patterns during thermal stress and pupal diapause in the corn earworm. J Insect Physiol 56:138–150. doi:10.1016/j.jinsphys.2009.09.013
Zhao L, Jones WA (2012) Expression of heat shock protein genes in insect stress responses. Invertebr Surviv J 9:93–101
Acknowledgments
We thank for Dr. Wei Zhang for assistance with experiments and topic discussion. This research was supported by the National Natural Science Foundation of China (31400323) and the National Special Fund for Agro-scientific Research in the Public Interest (201103024) as well as an ARC Fellowship to AAH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 133 kb).
Rights and permissions
About this article
Cite this article
Zhang, B., Peng, Y., Zheng, J. et al. Response of heat shock protein genes of the oriental fruit moth under diapause and thermal stress reveals multiple patterns dependent on the nature of stress exposure. Cell Stress and Chaperones 21, 653–663 (2016). https://doi.org/10.1007/s12192-016-0690-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-016-0690-8