Abstract
Temperature-induced conformational changes of reduced and oxidized HspB1 crosslinked by disulfide bond between single Cys137 of neighboring monomers were analyzed by means of different techniques. Heating of reduced HspB1 was accompanied by irreversible changes of Trp fluorescence, whereas oxidized HspB1 underwent completely reversible changes of fluorescence. Increase of the temperature in the range of 20–70 °C was accompanied by self-association of both reduced and oxidized protein. Further increase of the temperature led to formation of heterogeneous mixture of large self-associated complexes of reduced HspB1 and to formation of smaller and less heterogeneous complexes of oxidized HspB1. Heat-induced changes of oligomeric state of reduced HspB1 were only partially reversible, whereas the corresponding changes of oligomeric state of oxidized HspB1 were almost completely reversible. Oxidation resulted in decrease of chaperone-like activity of HspB1. It is concluded that oxidative stress, inducing formation of disulfide bond, can affect stability and conformational mobility of human HspB1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large family of small heat shock proteins (sHsp) combines a number of proteins expressed in practically all kingdoms including viruses, bacteria, plants, and animals (Maaroufi and Tanguay 2013; Basha et al. 2012; Kriehuber et al. 2010). Monomers of small heat shock protein have small molecular weight and conservative structure consisting of the so-called α-crystallin domain (ACD) containing ~90 residues and forming immunoglobulin-like β-sandwich structure flanked by less ordered and more variable N- and C-terminal extensions (Basha et al. 2012; McHaourab et al. 2009; Mymrikov et al. 2011). As a rule, sHsp tend to form large homo- or heterooligomers and possess the so-called holdase activity, i.e., ability to bind improperly folded proteins preventing their aggregation (Basha et al. 2012; McHaourab et al. 2009; Mymrikov et al. 2011). The human genome contains ten genes encoding small heat shock proteins which slightly differ in molecular weight, stress inducibility, oligomeric structure, and tissue distribution (Taylor and Benjamin 2005; Arrigo 2013; Mymrikov et al. 2011). HspB1 (Hsp27) is ubiquitously expressed in practically all human tissues (Mymrikov et al. 2011; Taylor and Benjamin 2005). In addition to its well-recognized holdase activity, HspB1 is involved in regulation of many vital functions. For instance, HspB1 seems to participate in regulation and stabilization of cytoskeleton (Mounier and Arrigo 2002; Wettstein et al. 2012), possesses anti-apoptotic activity (Acunzo et al. 2012; Kampinga and Garrido 2012), and protects the cell against oxidative stress (Arrigo 2007; Christians et al. 2012). Expression of HspB1 is increased upon different stress conditions (Arrigo 2013) including oxidative stress which is accompanied by partial oxidation of the single Cys residue and formation of crosslinked dimer (Zavialov et al. 1998a). Replacement of the single Cys residue of human or murine HspB1 by Ala decreases its ability to interact with cytochrome c and its anti-apoptotic activity (Bruey et al. 2000; Diaz-Latoud et al. 2005; Pasupuleti et al. 2010). Cardiac ischemia and reperfusion are accompanied by S-thiolation of the single SH group and partial dissociation of large HspB1 oligomers (Eaton et al. 2002a, b). These data indicate that modification of the single Cys might affect the structure and properties of HspB1. Thermal transitions were widely used for analysis of the structure of HspB1 (Dudich et al. 1995; Ehrnsperger et al. 1999; Lelj-Garolla and Mauk 2012; Skouri-Panet et al. 2012). However, to our knowledge, effect of disulfide crosslinking on the thermal transitions and the overall structure of HspB1 were not analyzed in detail. Therefore, this paper deals with investigation of disulfide crosslinking on thermal-induced changes of HspB1 structure.
Materials and methods
Proteins
Recombinant wild-type human HpsB1 was expressed and purified as described earlier (Nefedova et al. 2013). Protein samples in buffer B (20 mM Tris/acetate pH 7.6, 10 mM NaCl, 0.1 mM EDTA, 0.1 mM phenylmethanesulfonyl fluoride (PMSF), 1 mM dithiothreitol (DTT)) were aliquoted and stored at −20 °C. According to the SDS gel electrophoresis (Laemmli 1970), the purity of all protein samples exceeded 95 %.
Disulfide crosslinking
Protein (1 mg/ml) was dialyzed against 20 mM 4(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES)/NaOH (pH 7.5) containing 100 mM NaCl, 0.1 mM PMSF, and 1 mM MgCl2 overnight at room temperature. As found earlier (Mymrikov et al. 2010), this procedure leads to crosslinking of more than 80 % of the wild-type HspB1 and formation of crosslinked dimers.
Fluorescence measurements
HspB1 (0.1 or 0.3 mg/ml) in buffer F (20 mM HEPES/NaOH pH 7.5, 100 mM NaCl) in the presence or in the absence of 2 mM DTT was heated with a constant rate of 1 °C/min from 20 up to 90 °C and cooled back with the same rate by automatic Peltier cell holder of Cary Eclipse spectrofluorometer. In some experiments, this procedure, i.e., the cycle of heating and cooling, was repeated two times. Fluorescence was excited at 297 and recorded at 320 and/or 365 nm (slits width 5 nm). In the range of thermal transition, the intensity of fluorescence (I) is equal to I = (1 − α) × I n + α × I d, where I n and I d correspond to intensity of fluorescence of native and denatured protein, respectively, and α is fraction of conversion from native to denatured state. As described earlier (Bushueva et al. 1980), I n and I d can be estimated from the linear portion of the plot 1/I T = a + b × T/η, where I T is fluorescence, T is absolute temperature, η is viscosity at given T (in cP), and a and b are constants. The so-called parametric plots were also used for quantitative analysis of thermal unfolding recorded by Trp fluorescence (Permyakov and Burstein 1984). In this case, we plotted intensity of fluorescence at 365 nm on the intensity of fluorescence at 320 nm. The experimental curve consisted of two linear parts corresponding to common thermal transitions of native and unfolded protein and a curve connecting these two linear parts and corresponding to the region of thermal transition. This approach also provided quantitative determination of the temperature of half-transition from native to unfolded state of HspB1. The temperature of half-transition determined by dependence of 1/I on temperature or by parametric plots was practically indistinguishable. Spectra of fluorescence (excited at 297 nm and recorded in the range of 300–400 nm, slits width 5 nm) were recorded before heating (at 20 °C), immediately after heating up to 90 °C and after cooling back to 20 °C.
Light scattering
Light scattering was measured on Eclipse spectrofluorometer with excitation and emission wavelength set to 340 nm (slits width 2.5 nm). Reduced or oxidized HspB1 (0.3 mg/ml) in buffer F (in the presence or in the absence of 2 mM DTT) was heated with constant rate of 1 °C/min from 20 up to 90 °C and cooled back with the same rate.
Dynamic light scattering (DLS)
Dynamic light scattering was measured on Zetasizer Nano (Malvern) at protein concentration of 0.3 mg/ml in buffer F either in the presence of 2 mM DTT (reduced HspB1) or in the absence of DTT (oxidized HspB1). Each measurement lasted for 15 s and was repeated five times. Five cycles of these measurements were repeated and thus collected 25 measurements were analyzed. DLS measurements were performed at 30, 40, 50, 60, 70, 75, 80, 85, and 90 °C, and the protein sample was cooled back in the same order. The whole cycle of DLS measurements lasted for ~120 min, and the total duration of DLS measurements was comparable with that of fluorescence or light-scattering measurements. Particle size was estimated from intensity distribution by the build-in instrument program.
Size-exclusion chromatography (SEC)
One hundred thirty microliter of the HspB1 (0.3 mg/ml) in buffer F either in the presence of 2 mM DTT (reduced) or in the absence of DTT (oxidized) was subjected to size-exclusion chromatography on Superdex 200 HR 10/30 column equilibrated with buffer S (20 mM Tris/acetate pH 7.6, 150 mM NaCl with 15 mM β-mercaptoethanol (ME) (reduced HspB1) or without ME (oxidized HspB1) and run at the rate of 0.5 ml/min). The protein samples were loaded on the column before heating (sample 1), immediately after heating with the rate of 1 °C/min from 20 up to 90 °C (sample 2), after cooling with the same rate from 90 to 20 °C (sample 3), 2 h after cooling and keeping at 20 °C (sample 4), and 24 h after cooling and keeping at 20 °C (sample 5). In all cases, equal quantities of protein (~40 μg) were loaded on the column which was calibrated by protein markers: thyroglobulin (669 kDa), ferritin (440 kDa), rabbit skeletal muscle glyceraldehyde-3-phosphate dehydrogenase (144 kDa), and bovine serum albumin (68 kDa).
Chaperone-like activity
Two substrates, porcine heart mitochondria malate dehydrogenase (Serva) and subfragement-1 (S1) of rabbit skeletal muscle myosin, were used as model protein substrates. In the first case, HspB1 in 10 mM phosphate pH 7.4 in the absence or in the presence of 2 mM DTT was incubated at 45 °C for 20 min. Afterwards, the incubation was continued for 5 min at 55 °C, and malate dehydrogenase (dissolved in 10 mM phosphate pH 7.4 and clarified by centrifugation for 5 min at 14,000 g) was added up to the final concentration equal to 0.2 mg/ml. The weight ratio malate dehydrogenase/HspB1 was varied in the range of 4:1 up to 10:1.
In the second case, S1 obtained by the method of Weeds and Taylor (1975) (0.4 mg/ml) in 20 mM HEPES/NaOH (pH 7.0) containing 115 mM NaCl was incubated at 42 °C either in the absence or in the presence of HspB1. All experiments with oxidized HspB1 were performed in the absence of DTT; the same experiments with reduced HspB1 were performed in the presence of 20 mM DTT. In both cases, the heat-induced aggregation of the model protein substrates was followed by measuring optical density at 340 nm.
Results
Temperature-induced changes of intrinsic fluorescence of reduced and oxidized HspB1
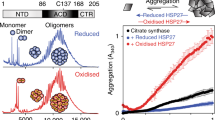
Heating of reduced HspB1 was accompanied by monotonous decrease of Trp fluorescence in the range of 20–65 °C, cooperative transition in the range of 65–75 °C, followed by farther monotonous decrease of fluorescence in the range of 75–90 °C (Fig. 1a). Half-maximal change of intrinsic fluorescence of reduced HspB1 was detected at 69.7 ± 0.5 °C. Cooling back from 90 to 20 °C was accompanied by monotonous increase of fluorescence in the range of 90–75 °C, cooperative transition in the range of 75–65 °C followed by monotonous increase of fluorescence in the range of 65–20 °C. Half-maximal changes of cooperative part of fluorescence curve in the course of cooling were observed at 69.9 ± 0.5 °C, i.e., at the temperature identical to that measured in the course of heating of HspB1 sample. Reversion of fluorescence of reduced HspB1 upon cooling was incomplete (Fig. 1a). If the cycle of heating and cooling of reduced HspB1 was repeated, we observed similar dependence of fluorescence intensity on temperature with small cooperative transitions observed at the temperatures similar to that determined in the course of the first heating (Fig. 1a). Again, the changes of fluorescence were incompletely reversible, and the intensity of fluorescence after the second heating was lower than that before or after the first heating. It is worthwhile to mention that in the presence of 2 mM DTT, heating up to 90 °C and cooling back to 20 °C were not accompanied by oxidation of the single SH group of HspB1 and formation of any quantities of HspB1 dimers crosslinked by disulfide bond (see Fig. 4c).
Thermally induced changes of fluorescence intensity at 320 nm for reduced (a) and oxidized (b) HspB1. The solid lines indicate dependence of fluorescence upon heating, and the dotted lines indicate dependence of fluorescence upon cooling. The data of two cycles of heating and cooling (numbered 1 and 2) are presented. Data are representative of six independent experiments
Heating of oxidized HspB1 resulted in monotonous decrease of fluorescence without any cooperative transitions and cooling back from 90 to 20 °C resulted in practically complete reversion of fluorescence intensity (Fig. 1b). The second cycle of heating and cooling was accompanied by the same practically completely reversible changes of fluorescence of oxidized HspB1 (Fig. 1b).
Fluorescence spectra of reduced and oxidized HspB1 at 20 °C were practically indistinguishable and were characterized by a broad maximum at about 345 nm (Fig. 2, curves 1 and 2). Heating up to 90 °C resulted in decrease of intensity of fluorescence and shifting of the maximum of fluorescence from 345 up to about 355 nm for both reduced and oxidized HspB1 (Fig. 2, curves 3 and 4). However, at high temperature, the intensity of fluorescence of reduced HspB1 was smaller than that of the oxidized sample. Cooling back resulted in complete reversion of the amplitude and position of maximum of fluorescence of oxidized HspB1 (Fig. 2, curve 6). At the same time, the amplitude of fluorescence of reduced HspB1was reversed only partially, although the shape of fluorescence spectra returned to its initial state with maximum of fluorescence at about 345 nm (Fig. 2, curve 5).
Fluorescence spectra of reduced (1, 3, 5, black lines) and oxidized (2, 4, 6, gray lines) HspB1. Fluorescence spectra were recorded before heating at 20 °C (curves 1 and 2), immediately after heating up to 90 °C (curves 3 and 4), and after cooling back to 20 °C (curves 5 and 6). Representative results of three independent experiments are shown
Thermal transitions of reduced and oxidized HspB1 followed by light scattering
Heating of oxidized HspB1 was accompanied by comparative small increase, and cooling back resulted in practically complete reversion of light scattering at 340 nm (Fig. 3, curves 2 and 4). In the case of reduced HspB1, the dependence of light scattering on temperature was more complicate (Fig. 3, curves 1 and 3). In the range of 20–75 °C, the light scattering was slowly increased and this increase was comparable with that observed in the case of oxidized HspB1. In the range of 75–80 °C, the light scattering was sharply increased, whereas in the range of 80–90 °C, the light scattering was decreased to the level detected at the beginning of the thermal transition at 75 °C (Fig. 3, curve 1). Cooling back (in the range of 90–70 °C) was accompanied at first by steep increase of light scattering (probably reflecting aggregation or self-association) followed by slow decrease of light scattering in the range of 70–20 °C (Fig. 3, curve 3). In this case, the changes of light scattering were irreversible and the light scattering did not return to the level detected before heating (Fig. 3, curve 3).
Temperature-dependent changes of light scattering of reduced (1 and 3) and oxidized (2 and 4) HspB1. Solid lines (1 and 2) represent dependence of light scattering in the course of heating, and dashed curves (3 and 4) represent dependence of light scattering in the course of cooling. Data are representative of five different experiments
Temperature-dependent changes of oligomeric structure of HspB1
Size-exclusion chromatography was used for investigation of probable changes of oligomeric structure of HspB1 induced by heating. Unfortunately, we were unable to perform size-exclusion chromatography at a very high temperature, and therefore, we analyzed protein samples collected at different temperatures and performed gel-filtration at constant room temperature. At low temperature, both reduced and oxidized HspB1 form large oligomers with an apparent molecular weight of 650–700 kDa (elution volume 10.3 ml) (Fig. 4a, b, curve 1). Two peaks were detected in the sample of reduced HspB1 heated under usual conditions (1 °C/min) from 20 up to 90 °C. The first small peak was eluted at exclusion volume of the column (7.9 ml) and probably corresponds to very large oligomers (or aggregates) of HspB1 formed upon heating (Fig. 4a, curve 2). The second peak eluted at ~10 ml probably corresponds to self-associated oligomers formed upon heating of reduced HspB1 (Fig. 4a, curve 2). The chromatographic run takes about 40 min; therefore, the results obtained under these conditions demonstrate distribution of oligomers resulting from heating and uncontrolled cooling back to room temperature in the course of chromatography. These processes can be accompanied by irreversible aggregation and formation of oligomers (aggregates) eluted in the void volume of the column. The sample collected immediately after cooling to 20 °C was also represented by two peaks on the size-exclusion chromatography (Fig. 4a, curve 3). Cooling was accompanied by an increase of the size of the peak eluted at exclusion volume (7.9 ml) and decrease of the size of the second peak with elution volume identical to that of unheated sample. Further incubation of heated reduced HspB1 sample at 20 °C for 2 or 24 h was not accompanied by any significant changes of oligomeric state (Fig. 4a, curve 4).
Thermally induced changes of oligomeric structure of reduced (a) and oxidized (b) HspB1 as determined by size-exclusion chromatography. Elution profiles of unheated (1, black line), heated up to 90 °C (2, dashed line), cooled back to 20 °C (3, light gray), and kept at 20 °C for 24 h after heating (4, dark gray) protein samples. Effect of heating on disulfide crosslinking of reduced (c) and oxidized (d) HspB1. Protein samples were collected before heating (1) after heating up to 90 °C (2), immediately after cooling back to 20 °C (3), and after keeping for 2 (4) or 24 h (5) after cooling to 20 °C. Heating of oxidized HspB1 was performed in the absence of DTT, and heating of reduced HspB1 was performed in the presence of 2 mM DTT. All samples were subjected to SDS gel electrophoresis without addition of DTT to the protein samples. Completely reduced sample of HspB1 was loaded on track marked 0. Positions of molecular weight markers (in kDa) and monomer (M) and disulfide crosslinked dimer (D) of HspB1 are marked by arrows
Heating of oxidized HspB1 was also accompanied by appearance of two peaks on elution profile. The first small peak was eluted at exclusion volume (7.9 ml), and the second broad peak was eluted at ~9.4 ml (Fig. 4b, curve 2). The first peak probably corresponds to very large oligomeric complexes or aggregates of HspB1, and the second peak corresponds to self-associated oligomers formed upon heating or upon heating and uncontrolled cooling in the course of chromatography of oxidized protein. Cooling back from 90 to 20 °C does not affect position or amplitude of the first peak, whereas the elution volume of the second peak was shifted back to 10.3 ml, i.e., to the elution volume characteristic for unheated sample of oxidized HspB1 (Fig. 4b, curves 3 and 4).
We also analyzed effect of heating on the extent of disulfide crosslinking. As already mentioned, heating of reduced HspB1 was performed in the presence of 2 mM DTT. The data of Fig. 4c indicate that heating up to 90 °C, followed by cooling back to 20 °C, and keeping the protein sample at 20 °C for 2 h were not accompanied by disulfide crosslinking of preliminary reduced HspB1 (Fig. 4c). Further incubation for 24 h at 20 °C results in accumulation of crosslinked HspB1 dimers. The extent of crosslinking of preliminary oxidized HspB1 was not changed upon heating and cooling and further incubation at 20 °C (Fig. 4d).
Dynamic light scattering of thermal transitions of HspB1
The method of dynamic light scattering was also applied for investigation of thermal transitions of reduced and oxidized HspB1. In the case of reduced HspB1, intensity distribution at 30 °C revealed the main peak with a particle diameter of ~20 nm (Fig. 5a). Heating of reduced HspB1 was accompanied by shifting of a diameter of the main peak to ~80–90 nm (Fig. 5a). At the beginning, cooling of the sample of reduced HspB1 was accompanied by broadening of the main peak followed by appearance of two badly separated peaks, the smaller one with a particle diameter of ~25–30 nm and the larger one with a diameter of ~100–110 nm (Fig. 5b).
Temperature dependence of size distribution (intensity) of reduced HspB1. a Size distribution measured in the sample of reduced HspB1 (0.3 mg/ml) in the course of heating from 30 to 90 °C. b Size distribution of the same sample cooled back from 90 to 30 °C. Representative results of three independent experiments are shown
The data of DLS indicate that at low temperature, the sample of oxidized HspB1 contained predominantly particles with a diameter of ~20 nm (Fig. 6a). Heating of oxidized HspB1 was accompanied by increase of diameter of particles of the main peak. However, this increase was less pronounced than in the case of reduced protein, and even at the highest temperature, the diameter of particles in the main peak was in the range of 50–60 nm (Fig. 6a). In the course of cooling, the main peak was shifted back to its original position (~20 nm) (Fig. 6b)
Temperature dependence of distribution (intensity) of oxidized HspB1. a Size distribution measured in the sample of oxidized HspB1 (0.3 mg/ml) in the course of heating from 30 to 90 °C. b Size distribution of the same sample cooled back from 90 to 30 °C. Representative results of three independent experiments are shown
Chaperone-like activity of reduced and oxidized HspB1
Heating of isolated malate dehydrogenase (MDH) was accompanied by protein denaturation and aggregation (Fig. 7a, curves 1 and 3). Under conditions used, incubation of isolated HspB1 both in the oxidized or reduced form was not accompanied by any changes of the optical density at 340 nm (Fig. 7a, curve 5). HspB1 retarded and partially decreased MDH aggregation thus demonstrating the chaperone-like activity (Fig. 7a, curves 2 and 4). At all weight ratio analyzed (MDH/HspB1 in the range of 4/1 up to 10/1), the reduced HspB1 was slightly more effective than its oxidized counterpart in prevention of MDH aggregation (Fig. 7a, curves 2 and 4, respectively).
Effect of reduced and oxidized HspB1 on the heat-induced aggregation of malate dehydrogenase (a) or subfragment-1 (S1) of rabbit skeletal myosin (b). The experiments were performed either in the presence of 2 mM DTT (1, 2) or in the absence of DTT (3, 4). The incubation mixture contained either isolated model protein substrates (curves 1 (black square) and 3 (black circle)), protein substrates in the presence of reduced (curve 2 (inverted black triangle)) or oxidized (curve 4 (black triangle)) HspB1, or isolated oxidized or reduced HspB1 (curve 5 (black hexagon)). Concentrations of malate dehydrogenase and HspB1 were equal to 0.2 and 0.05 mg/ml, respectively, and that of S1 and HspB1 were equal to 0.4 and 0.1 mg/ml, respectively. Results of at least three independent experiments are shown with errors bars indicating standard deviation
Heating of isolated S1 both in the presence and in the absence of 2 mM DTT was accompanied by thermal denaturation and aggregation recorded by increase of the optical density at 340 nm (Fig. 7b, curves 1 and 3). The kinetics and amplitude of aggregation of isolated S1 were similar both in the presence and in the absence of DTT. Long incubation (more than 80 min) of isolated S1 either in the absence or in the presence of DTT was accompanied by formation of large protein aggregates and their precipitation leading to decrease of the optical density at 340 nm which becomes very noisy (Fig. 7b, curves 1 and 3). Addition of reduced HspB1 was accompanied by significant retardation of S1 aggregation (Fig. 7b, curve 2). The oxidized HspB1 was less effective, and although it retarded the onset of aggregation, the optical density after long incubation in the presence of oxidized HspB1 was even larger than in the case of isolated S1 (Fig. 7, curve 4). This seems to be due to co-aggregation of oxidized HspB1 and S1. Thus, using two different model protein substrates, we found that reduced HspB1 possessed higher chaperone-like activity than its oxidized counterpart.
Discussion
Increase of the temperature in the range of 20–60 °C is accompanied by HspB1 self-association. This self-association was recorded earlier by means of light scattering (Ehrnsperger et al. 1999), sedimentation velocity (Lelj-Garolla and Mauk 2006, 2012), and small-angle X-ray scattering (SAXS) (Skouri-Panet et al. 2012). Deletion of the first 14 or 24 residues completely prevented temperature-dependent self-association of HspB1 (Lelj-Garolla and Mauk 2012), and therefore, it was supposed that the N-terminal extension plays an important role in HspB1 self-association. Similar temperature-dependent self-association was also reported for αB-crystallin (Spinozzi et al. 2006; Skouri-Panet et al. 2006; Aquilina et al. 2013).
In our case, heating in the range of 20–60 °C was accompanied by monotonous decrease of Trp fluorescence and small increase of the light scattering (Figs. 1 and 3) and the behavior of reduced and oxidized HspB1 was very similar. According to the data of DLS, increase of the temperature from 30 up to 60 °C was accompanied by increase of diameter of reduced HspB1 particles from ~18 up to ~31 nm. In the case of oxidized HspB1, increase of the temperature from 30 up to 60 °C induced similar increase of particle diameter from ~21 up to ~29 nm (Figs. 5a and 6a).
Further increase of the temperature in the range of 60–75 °C was accompanied by sharp decrease of Trp fluorescence of reduced HspB1, whereas fluorescence of oxidized HspB1 was only monotonously decreased (Fig. 1). Thus, the data of fluorescence indicate that the reduced HspB1 undergoes thermal transition with the temperature of half-maximal transition ~69.5 °C. Five out of six Trp residues are located in the N-terminal extension, and therefore, we hypothesize that the thermal transition observed at 69.5 °C is due either to the change of location or orientation of the N-terminal extension of reduced HspB1. At the same time, oxidized HspB1 did not undergo this thermal transition. Thus, the N-terminal domain of reduced HspB1 is less resistant to thermal transitions than the corresponding domain of the oxidized protein. Earlier, Zavialov et al. (1998a) also concluded that the disulfide crosslinking stabilizes the N-terminal domain of HspB1 against urea-induced unfolding.
Further increase of the temperature in the range of 75–90 °C was accompanied by similar monotonous decrease of fluorescence of both reduced and oxidized HspB1 (Fig. 1). The shape of fluorescence spectra of reduced and oxidized samples was practically identical and was characterized by shifting of fluorescence maxima from ~345 up to ~355 nm. However, the intensity of fluorescence of reduced sample was roughly two times smaller than that of oxidized sample (Fig. 2). Moreover, the amplitude of fluorescence of reduced HspB1 did not return back to its initial value upon cooling, whereas the changes of fluorescence of oxidized HspB1 were practically completely reversible (Figs. 1 and 2). These facts agree with a suggestion that the N-terminal domain of the oxidized protein is more resistant to thermal transitions than that of the reduced HspB1. The question arises how formation of disulfide bond in the middle of α-crystallin domain can affect the structure and properties of remote N-terminal domain. At present, there is no a clear-cut answer on this question; however, the recently published data obtained on αB-crystallin clearly show that there are tight allosteric communications between different parts of αB-crystallin molecules involved in formation of large oligomers of this protein (Hochberg and Benesch 2014; Aquilina et al. 2004; Delbecq and Klevit 2013). In any case, our suggestion that the disulfide bond affects the thermal transition of the N-terminal domain requires further experimental verification.
In the range of 20–70 °C, the light scattering of reduced and oxidized HspB1 was similar and the size of particles formed by reduced HspB1 was only slightly larger than that of oxidized protein (Figs. 3, 5, and 6). However, in the range of 75–90 °C, the light scattering of reduced HspB1 was dramatically different from that of oxidized sample. Heating from 75 up to 80 °C was accompanied by sharp increase of the light scattering and particle size of reduced HspB1 and much smaller increase of light scattering and particle size of oxidized HspB1 (Figs. 3, 5, and 6). Further increase of the temperature from 80 up to 90 °C practically did not affect the light scattering and particle size of oxidized HspB1 (Figs. 3 and 6). In this temperature range, the particle size of reduced HspB1 was not practically changed (Fig. 5), whereas the light scattering of reduced HspB1 was significantly decreased (Fig. 3). We hypothesize that this paradoxical behavior can be due either to the changes in the density, shape, and/or refractive index of particles formed by reduced HspB1.
In the case of reduced HspB1, cooling from 90 to 20 °C was accompanied by at first a large increase followed by a small decrease of light scattering (Fig. 3). In this case, the changes of light scattering were irreversible and the light scattering at the end of cooling was about three times larger than that at the beginning of the experiment (Fig. 3). The data of DLS indicate that cooling of reduced HspB1 led at first to broadening of the distribution curve followed by splitting of distribution curve into two poorly separated peaks with a diameter of ~28 and ~105 nm (Fig. 5). These data indicate that at the beginning of cooling (in the range of 90–70 °C), we observed either change in the density, shape, and/or refractive index of reduced HspB1 particles or partial dissociation of large ensembles formed by these particles. This is reflected by increase of light scattering and broadening of distribution of particle size. On the later stages of cooling, large particles formed by self-associated reduced HspB1 started to dissociate; however, even at 20 °C, this dissociation was not complete. The data of SEC indicate that upon cooling, the amplitude of the peak eluted in exclusion volume (~7.9 ml) was significantly increased, thus indicating that the large complexes or self-associated oligomers of reduced HspB1 cannot completely dissociate and are accumulated in the sample (Fig. 4).
Cooling of oxidized HspB1 was accompanied by monotonous decrease of light scattering which practically completely returned to its initial level (Fig. 3). Similar results were obtained by the method of DLS. In this case, upon cooling, the size of oxidized HspB1 particles decreased from ~43 to ~24 nm, i.e., to level characteristic for the unheated sample (Fig. 6). Upon cooling of oxidized HspB1, the amplitude of the peak eluted in exclusion volume was not practically changed thus indicating that in the case of oxidized HspB1, heat-induced changes of aggregation state are practically completely reversible (Fig. 4).
Two different model protein substrates were used for investigation of chaperone-like activity of reduced and oxidized HspB1. Under conditions used, reduced HspB1 was more effective than its oxidized counterpart in prevention of MDH aggregation, and although this difference was not very large, it was well reproduced at different weight ratios of MDH/HspB1 (Fig. 7a). Even a larger difference was observed if S1 of skeletal muscle myosin was used as a model protein substrate. In this case, reduced HspB1 effectively retarded aggregation of S1 even at long times of incubation. At the same time, oxidized HspB1 slightly retarded aggregation of S1 at short times of incubation but was ineffective at long times of incubation, when the amplitude of the optical density at 340 nm in the presence of oxidized HspB1 became larger than that in the absence of HspB1 (Fig. 7b, curve 4). Our data disagree with the earlier published results of Zavialov et al. (1998a) and Pasupuleti et al. (2010). By using citrate synthase and insulin as model protein substrates, these groups failed to detect pronounced difference in the chaperone-like activity of reduced and oxidized HspB1. Thus, depending on the nature of protein substrate disulfide crosslinking either does not affect or decreases the chaperone-like activity of HspB1. Decrease of the chaperone-like activity of HspB1 is probably due to a more rigid and less flexible structure of oxidized HspB1 making it less able to adopt the structure required for effective interaction with certain (but not with all) target proteins.
On the cellular level, oxidative stress is accompanied by oxidation and disulfide crosslinking of HspB1 (Zavialov et al. 1998a). Therefore, the question arises what is the effect of oxidation on the structure, properties, and physiological activity of HspB1. Under normal conditions, formation of a disulfide bond does not affect secondary, tertiary, or quaternary structure of HspB1 (Zavialov et al. 1998a; Pasupuleti et al. 2010; and this investigation) and only minor changes in the properties of the N-terminal domain of HspB1 were observed at increased temperature (Figs. 1 and 2) or in the presence of urea (Zavialov et al. 1998a). Anyhow, under normal conditions, it is difficult to reveal any changes in the structure of reduced and oxidized HspB1. However, analysis of thermal stability at very high unphysiological temperatures clearly discloses the differences in the structure and properties of reduced and oxidized protein. Indeed, reduced, un-crosslinked HspB1 possesses higher flexibility and higher susceptibility to irreversible thermal transition, whereas oxidized HspB1, containing a single disulfide bond, is more rigid and stable. These minor changes in HspB1 structure might affect its interaction with certain target proteins and this is reflected by decreased chaperone-like activity with certain model protein substrates (Fig. 7). At the same time, replacement of Cys137 by Ala (C137A mutation) is accompanied by a decrease of anti-apoptotic activity (Bruey et al. 2000; Pasupuleti et al. 2010) and the oxidized wild-type HspB1 is more effective than its C137A mutant in prevention of apoptosis induced by oxidative stress in CHO and HeLa cells (Pasupuleti et al. 2010). This effect can be due to the ability of oxidized HspB1 specifically interact with certain target proteins (for instance, with cytochrome c) or to the buffering of sulfhydryl groups in the course of oxidative stress. The intracellular concentration of HspB1 is rather high, and therefore, it can participate in sulfhydryl buffering. As already mentioned, oxidation of SH groups does not dramatically affect HspB1 structure and can be easily reversed by intermediate thiolations (Eaton et al. 2002a, b; Zavialov et al. 1998b) followed by complete reduction after ending of oxidative stress. Thus, reversible oxidation and reduction can be important for functioning of HspB1 in the cell.
Abbreviations
- ACD:
-
α-Crystallin domain
- DLS:
-
Dynamic light scattering
- DTT:
-
Dithiothreitol
- HEPES:
-
4(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid
- MDH:
-
Malate dehydrogenase
- ME:
-
β-Mercaptoethanol
- PMSF:
-
Phenylmethanesulfonyl fluoride
- SEC:
-
Size-exclusion chromatography
- sHsp:
-
Small heat shock proteins
- WT:
-
Wild type
References
Acunzo J, Katsogiannou M, Rocchi P (2012) Small heat shock proteins HSP27 (HspB1), alphaB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol 44(10):1622–1631
Aquilina JA, Benesch JL, Ding LL, Yaron O, Horwitz J, Robinson CV (2004) Phosphorylation of alphaB-crystallin alters chaperone function through loss of dimeric substructure. J Biol Chem 279(27):28675–28680. doi:10.1074/jbc.M403348200
Aquilina JA, Shrestha S, Morris AM, Ecroyd H (2013) Structural and functional aspects of hetero-oligomers formed by the small heat shock proteins alphaB-crystallin and HSP27. J Biol Chem 288(19):13602–13609. doi:10.1074/jbc.M112.443812
Arrigo AP (2007) The cellular “networking” of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv Exp Med Biol 594:14–26. doi:10.1007/978-0-387-39975-1_2
Arrigo AP (2013) Human small heat shock proteins: protein interactomes of homo- and hetero-oligomeric complexes: an update. FEBS Lett 587(13):1959–1969
Basha E, O'Neill H, Vierling E (2012) Small heat shock proteins and alpha-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci 37(3):106–117
Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C (2000) Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2(9):645–652. doi:10.1038/35023595
Bushueva TL, Busel EP, Burstein EA (1980) Some regularities of dynamic accessibility of buried fluorescent residues to external quenchers in proteins. Arch Biochem Biophys 204(1):161–166
Christians ES, Ishiwata T, Benjamin IJ (2012) Small heat shock proteins in redox metabolism: implications for cardiovascular diseases. Int J Biochem Cell Biol 44(10):1632–1645
Delbecq SP, Klevit RE (2013) One size does not fit all: the oligomeric states of alphaB crystallin. FEBS Lett 587(8):1073–1080
Diaz-Latoud C, Buache E, Javouhey E, Arrigo AP (2005) Substitution of the unique cysteine residue of murine Hsp25 interferes with the protective activity of this stress protein through inhibition of dimer formation. Antioxid Redox Signal 7(3–4):436–445. doi:10.1089/ars.2005.7.436
Dudich IV, Zav'yalov VP, Pfeil W, Gaestel M, Zav'yalova GA, Denesyuk AI, Korpela T (1995) Dimer structure as a minimum cooperative subunit of small heat-shock proteins. Biochim Biophys Acta 1253(2):163–168
Eaton P, Byers HL, Leeds N, Ward MA, Shattock MJ (2002a) Detection, quantitation, purification, and identification of cardiac proteins S-thiolated during ischemia and reperfusion. J Biol Chem 277(12):9806–9811. doi:10.1074/jbc.M111454200
Eaton P, Fuller W, Shattock MJ (2002b) S-thiolation of HSP27 regulates its multimeric aggregate size independently of phosphorylation. J Biol Chem 277(24):21189–21196. doi:10.1074/jbc.M200591200
Ehrnsperger M, Lilie H, Gaestel M, Buchner J (1999) The dynamics of Hsp25 quaternary structure. Structure and function of different oligomeric species. J Biol Chem 274(21):14867–14874
Hochberg GK, Benesch JL (2014) Dynamical structure of alphaB-crystallin. Prog Biophys Mol Biol. doi:10.1016/j.pbiomolbio.2014.03.003
Kampinga HH, Garrido C (2012) HSPBs: small proteins with big implications in human disease. Int J Biochem Cell Biol 44(10):1706–1710
Kriehuber T, Rattei T, Weinmaier T, Bepperling A, Haslbeck M, Buchner J (2010) Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J 24(10):3633–3642
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Lelj-Garolla B, Mauk AG (2006) Self-association and chaperone activity of Hsp27 are thermally activated. J Biol Chem 281(12):8169–8174
Lelj-Garolla B, Mauk AG (2012) Roles of the N- and C-terminal sequences in Hsp27 self-association and chaperone activity. Protein Sci 21(1):122–133. doi:10.1002/pro.761
Maaroufi H, Tanguay RM (2013) Analysis and phylogeny of small heat shock proteins from marine viruses and their cyanobacteria host. PLoS ONE 8(11):e81207. doi:10.1371/journal.pone.0081207
McHaourab HS, Godar JA, Stewart PL (2009) Structure and mechanism of protein stability sensors: chaperone activity of small heat shock proteins. Biochemistry 48(18):3828–3837. doi:10.1021/bi900212j
Mounier N, Arrigo AP (2002) Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones 7(2):167–176
Mymrikov EV, Bukach OV, Seit-Nebi AS, Gusev NB (2010) The pivotal role of the beta 7 strand in the intersubunit contacts of different human small heat shock proteins. Cell Stress Chaperones 15(4):365–377. doi:10.1007/s12192-009-0151-8
Mymrikov EV, Seit-Nebi AS, Gusev NB (2011) Large potentials of small heat shock proteins. Physiol Rev 91(4):1123–1159
Nefedova VV, Datskevich PN, Sudnitsyna MV, Strelkov SV, Gusev NB (2013) Physico-chemical properties of R140G and K141Q mutants of human small heat shock protein HspB1 associated with hereditary peripheral neuropathies. Biochimie 95(8):1582–1592. doi:10.1016/j.biochi.2013.04.014
Pasupuleti N, Gangadhariah M, Padmanabha S, Santhoshkumar P, Nagaraj RH (2010) The role of the cysteine residue in the chaperone and anti-apoptotic functions of human Hsp27. J Cell Biochem 110(2):408–419. doi:10.1002/jcb.22552
Permyakov EA, Burstein EA (1984) Some aspects of studies of thermal transitions in proteins by means of their intrinsic fluorescence. Biophys Chem 19(3):265–271
Skouri-Panet F, Quevillon-Cheruel S, Michiel M, Tardieu A, Finet S (2006) sHSPs under temperature and pressure: the opposite behaviour of lens alpha-crystallins and yeast HSP26. Biochim Biophys Acta 1764(3):372–383. doi:10.1016/j.bbapap.2005.12.011
Skouri-Panet F, Michiel M, Ferard C, Duprat E, Finet S (2012) Structural and functional specificity of small heat shock protein HspB1 and HspB4, two cellular partners of HspB5: role of the in vitro hetero-complex formation in chaperone activity. Biochimie 94(4):975–984. doi:10.1016/j.biochi.2011.12.018
Spinozzi F, Mariani P, Rustichelli F, Amenitsch H, Bennardini F, Mura GM, Coi A, Ganadu ML (2006) Temperature dependence of chaperone-like activity and oligomeric state of alphaB-crystallin. Biochim Biophys Acta 1764(4):677–687. doi:10.1016/j.bbapap.2006.02.003
Taylor RP, Benjamin IJ (2005) Small heat shock proteins: a new classification scheme in mammals. J Mol Cell Cardiol 38(3):433–444
Weeds AG, Taylor RS (1975) Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature 257(5521):54–56
Wettstein G, Bellaye PS, Micheau O, Bonniaud P (2012) Small heat shock proteins and the cytoskeleton: an essential interplay for cell integrity? Int J Biochem Cell Biol 44(10):1680–1686
Zavialov A, Benndorf R, Ehrnsperger M, Zav'yalov V, Dudich I, Buchner J, Gaestel M (1998a) The effect of the intersubunit disulfide bond on the structural and functional properties of the small heat shock protein Hsp25. Int J Biol Macromol 22(3–4):163–173
Zavialov AV, Gaestel M, Korpela T, Zav’yalov VP (1998b) Thiol/disulfide exchange between small heat shock protein 25 and glutathione. Biochim Biophys Acta 1388(1):123–132
Acknowledgments
The authors are grateful to Professor D.I. Levitsky and Dr. O.P. Nikolaeva (A.N. Bach Institute of Biochemistry, Russian Academy of Sciences) who kindly provided subfragment-1 of myosin. This investigation was supported by the Russian Foundation for Basic Research (13-04-00015 to NBG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chalova, A.S., Sudnitsyna, M.V., Semenyuk, P.I. et al. Effect of disulfide crosslinking on thermal transitions and chaperone-like activity of human small heat shock protein HspB1. Cell Stress and Chaperones 19, 963–972 (2014). https://doi.org/10.1007/s12192-014-0520-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-014-0520-9