Abstract
Progressive multifocal leukoencephalopathy (PML) remains a life-threatening central nervous system infection in immunocompromised patients. Although outcomes have improved in cases that immune reconstitution is feasible with anti-retroviral therapy (ART) in HIV + patients or natalizumab removal in those with multiple sclerosis, in individuals with hematological malignancies, the prognosis is usually dismal. Anti-viral treatments have been largely ineffective, but immunotherapy-based approaches with checkpoint inhibitors and adoptive virus-specific T cells’ transfer are currently explored in clinical trials. PML has not been described as a cause of encephalopathy after CAR T therapy. We report the first case of PML 7 months after lymphodepleting chemotherapy with fludarabine/cyclophosphamide and anti-CD19-directed CAR T therapy in a patient with relapsed diffuse large B-cell lymphoma who relapsed fast after a previous autologous hematopoietic stem cell transplant. She remains alive 12 months after diagnosis with stabilization of her symptoms with a combination of therapies targeting viral replication and immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Progressive multifocal leukoencephalopathy (PML) is a demyelinating infection of the central nervous system caused by reactivation of the JC virus (JCV). JCV belongs to the Polyomaviridae family together with BK virus, a common cause of hemorrhagic cystitis in hematopoietic stem cell transplant (HCT) recipients, and Merkel cell polyomavirus (MCPyV) that causes Merkel cell carcinoma of the skin. PML is exclusively described in patients with underlying immunosuppression, most commonly with advanced acquired immunodeficiency syndrome (AIDS), or in recipients of solid organ transplants (SOT) or HCT or after use of immunomodulatory medications, especially in multiple sclerosis like natalizumab, a monoclonal antibody against the α4 integrin subunit [1].
Viral reactivation leads to a lytic infection of mainly oligodendrocytes (but astrocytes as well) that is usually slowly fatal through demyelination unless immunosuppression can be reversed. One of the receptors facilitating entrance of JCV into the target cells is the serotonin receptor 5-HT2 and therapeutically mirtazapine; a 5-HT2 (and 5-HT3) antagonist has been tried in an attempt to delay the PML progression. When there is restoration of the immune system, the immune reconstitution inflammatory syndrome (IRIS) can ensue like in other opportunistic infections. In such cases, IRIS can cause a catastrophic brain edema that can be fatal if not treated promptly with corticosteroids [2].
Despite some promising activity of brincidofovir against polyomaviruses, the parent molecule cidofovir has marginal, if any value in PML whereas other anti-viral medications have shown disappointing results [3, 4]. The pyrimidine analogue cytarabine is not effective [5], whereas the cytotoxic topoisomerase I inhibitor topotecan has shown some possibility of benefit but with significant toxicity in such patients [6]. Despite an established anti-BK activity of the dihydroorotate dehydrogenase inhibitor leflunomide, its utility against JCV has not been established [7]. The antimalarial mefloquine has some in vitro activity, and due to its favorable toxic profile, it is often used despite lack of evidence for clinical benefit [8, 9, 10].
If natalizumab has precipitated PML, the drug is stopped and removed by plasma exchange. Non-specific immune “boosters” like IFNa or intravenous immunoglobulin (IVIG) are frequently used empirically despite lack of proof for benefit [11]. Nevertheless, the anti-Programmed Death-1 receptor IgG4 monoclonal antibodies (anti-PD1 Abs) pembrolizumab and nivolumab have recently shown impressive activity against PML and, probably, will become the standard of care due to their ease of administration and the relatively favorable side-effect profile [12]. Promising results after adoptive immunotherapy with third-party BK virus-specific cytotoxic T lymphocyte lines (BKV-CTLs) have been reported in small series of patients after allogenic stem cell transplantation [13].
We report here the first case of PML after anti-CD19 chimeric antigen receptor T-cell therapy (CAR-T) in a patient with relapsed diffuse large B-cell lymphoma who had relapsed after high-dose chemotherapy and autologous HCT.
Case
A 68-year-old female who presented with left-neck lymphadenopathy was diagnosed with stage IIA diffuse large B-cell lymphoma (DLBCL) of non-germinal center origin and she achieved complete remission (CR) after 6 cycles of R-CHOP (Rituximab−Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone). She relapsed 3 months later with extensive lymphadenopathy above and below the diaphragm and splenomegaly. She received 3 cycles of R-ICE (Rituximab−Ifosfamide, Carboplatin, and Etoposide) which resulted in CR2. Then, she had myeloablative chemotherapy with R-BEAM (Rituximab−Carmustine, Etoposide, Cytarabine, and Melphalan) and autologous stem cell transplant (auto-HCT). Repeat imaging 2 months later showed intra-abdominal recurrence and she received Rituximab−Gemcitabine and Oxaliplatin to avoid disease progression until the insurance approved axicabtagene ciloleucel (Yescarta) that was infused after lymphodepleting chemotherapy with fludarabine and cyclophosphamide.
The CAR-T infusion was complicated by grade 2 cytokine release syndrome that responded to tocilizumab and grade 2 neurotoxicity that responded to steroids. One month later, PET/CT showed complete metabolic remission and her lymphoma has not relapsed up to this date. The CAR T therapy was further complicated by prolonged moderate neutropenia requiring filgrastim and by severe hypogammaglobulinemia being treated with monthly intravenous immunoglobulin.
She presented 7 month post-CAR T-cell therapy with confusion, aphasia, ataxia, and involuntary athetotic movements, especially of the right arm together with amnesia and cognitive decline. MRI of the brain revealed three enhancing lesions in the left parietal lobe, posterior left frontal lobe, and right occipital lobe. PET/CT showed no FDG avid lymphadenopathy confirming continuous remission of the lymphoma. Lumbar puncture showed no atypical lymphocytes and undetectable JCV DNA by PCR. She had brain biopsy that showed reactive astrocytes including some with fragmented nuclear inclusions (Creutzfeldt−Peter cells). There were cells with viral inclusions positive for SV40, consistent for JCV-induced PML. There was impressive inflammatory infiltrate by small reactive cells in the brain lesions. Absolute lymphocyte count was 593 cells per microliter, peripheral CD4 lymphocyte count was 199 cells per microliter, and JCV DNA level by PCR in serum was 5800 copies per ml.
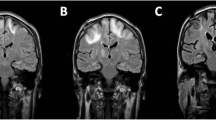
She was started on mefloquine (250 mg weekly), mirtazapine (escalated dose up to 45 mg/d), and IVIG (initially weekly and then monthly infusions of 0.5 g/kg) with partial improvement in her neurological status in the following 6 weeks. An episode of interim worsening happened and 3 day course of dexamethasone was given due to the fear of IRIS without a significant change in her status. Electroencephalogram ruled out seizure activity. A repeat MRI remained unchanged (Fig. 1), and JCV levels in plasma remained positive at 6400 copies per ml and in CSF 130 copies per ml. Infectious disease service recommended against use of cidofovir due to risk of toxicity and lack of clinical data supporting its use for this indication. Although brincidofovir has reported in vitro activity against dsDNA viruses, no clinical data for this indication are currently available. We continued mefloquine, mirtazapine, and IVIG, and the patient was transferred to another hospital for therapy with cytotoxic lymphocytes (CTL).
a MRI brain-T2 with fat suppression series during neurotoxicity after CAR-T cell (left) and about 10 months later (right) when the patient had already been diagnosed with JCV encephalitis. b T1 with IV gadolinium during neurotoxicity after CAR-T cell (left) and about 10 months later (right) when the patient had already been diagnosed with JCV encephalitis
Discussion
We present a patient who had motor and cognitive decline several months after CAR T-cell therapy. Neurotoxicity is a common complication of CAR T-cell therapies and it has been reported up to 60 days after cell infusion. Symptoms typically start within 4–5 days after cell infusion and are usually reversible within 3–8 weeks (with the exception of cerebral edema which is rare but usually rapidly fatal). Although there is a delayed form of CAR T-related encephalopathy described in the third week or fourth week after infusion, cases of neurotoxicity after several months are not reported [14]. Thus, it is unlikely that the presentation of our patient was directly related to CAR-T toxicity.
Cases of JC encephalopathy have not been reported to our knowledge after CAR-T therapy. The lymphodepletion regimens given prior to CAR T-cell infusions can lead to profound immunosuppression especially in heavily pretreated lymphoma patients. Furthermore, hypogammaglobulinemia is almost unanimous due to the direct effect of anti-CD19 CAR-T cells on normal B cells. Finally, 30–40% of patients treated with CAR-T develop prolonged cytopenias including absolute lymphopenia [15]. It is unknown if cytopenias result from previous chemotherapies including fludarabine and cyclophosphamide or from inflammatory damage to the bone marrow stroma caused during killing of the lymphoma or during CRS. The functional status of virus-specific T cells after recovery from CAR T therapy is largely unknown. We know that CAR-T cells themselves upregulate PD-1 and exhaustion markers few weeks after being in the recipient body [16]. We also know that frequently after allo-HCT, we see viral-including CMV reactivations despite the presence of anti-viral T cells and this phenomenon is due to a dysfunction or exhaustion of such cells apart from the presence of immunosuppressive medications like calcineurin inhibitors and corticosteroids [17]. What it is known is that anti-PD1 therapies help patients with JC encephalitis by activating previously exhausted polyoma-specific T cells. Our patient had hypogammaglobulinemia and lymphopenia after CAR T therapy and we assume that these factors together with a dysfunction of polyoma-specific T cells and old age (immunosenescence) all contributed to JC viral reactivation. The use of empiric therapy including mirtazapine, mefloquine, and IVIG did not reverse the patient’s neurologic syndrome; however, it is possible that they may have halted the progression of her disease, since no new lesions appeared on neuroimaging. It is unknown what will be the outcome of adoptive polyoma-specific CTL therapy that is given on this patient by a different center. We know from the literature that such cells are crossing the blood–brain barrier and can be retrieved in the cerebrospinal fluid. A clinical trial of adoptive cellular immunotherapy for PML with ex vivo generated polyomavirus-specific T cells using HLA-matched first-degree relatives is currently conducted by the National Institute of Neurological Disorders and Stroke (NCT02694783). Checkpoint inhibitors are also being tested for treatment of PML [18, 19]. In a series of 8 patients treated with pembrolizumab, 5 showed clinical improvement and 4 had persistent reduction in JC viral load for more than a year after last pembrolizumab dose [20]. PD-1 expression is found to be elevated on the CD4 + and CD8 + T lymphocytes of patients with PML and is particularly elevated on JC virus-specific CD8 + T cells
(21). Combining checkpoint inhibitors with adoptive cellular therapies may augment the immune response and prove an effective therapy for PML. Our patient has shown stabilization up to this date, but if the treatment fails, then pembrolizumab or nivolumab will be tried.
Our case teaches us to consider opportunistic infections even rare ones like JC encephalitis after CAR-T therapy. It also emphasizes the importance of early referral for CAR T therapy to not damage the marrow stem cells and the marrow stroma and the T-cell functionality and repertoire with futile chemotherapeutic attempts in refractory aggressive B-cell lymphomas. It also underlines the importance of supportive care after CAR-T-cell therapy with prophylactic antimicrobials (at least against pneumocystis, HSV, and VZV) and intravenous immunoglobulin. Any additional immunosuppressive medication should be stopped after CAR-T cells and corticosteroids should be used carefully for CAR-T toxicity management. Finally, after identification of such rare infections should call for institution of multidisciplinary care and infectious disease physicians should be contacted fast to give specific recommendations. Referral to centers with clinical trials, especially targeting immune reconstitution or adoptive immunotherapy, should be considered early. It is noteworthy that our patient is relatively stable without significant residual neurological deficits 12 months after the identification of this infection that is usually lethal.
References
Major EO, Yousry TA, Clifford DB. Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: a decade of lessons learned. Lancet Neurol. 2018;17(5):467–80.
Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9(4):425–37.
Chery GS, Palmer SM, Snyder LD, Todd JL, Wolfe C. Brincidofovir in progressive multifocal leukoencephalopathy after lung transplant. Am J Respir Crit Care Med. 2015;191:A1432.
Eckert S, Weinstock-Guttman B, Kolb C, Hojnacki D (2018) Treatment of natalizumab induced PML with cidofovir or CMX001 (brincidofovir) and mirtazapine. Neurology 90:P5–380
Hall ODH, Dafni RD, Patricia P, Wetherill EW, McArthur CA, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with HIV infection. N Engl J Med. 1998;338:1345–51.
Royal W, Dupont B, McGuire D, Chang L, Goodkin K, Ernst T, et al. Topotecan in the treatment of acquired immunodeficiency syndrome-related progressive multifocal leukoencephalopathy. J Neurovirol. 2003;9(3):411–9.
Williams JW, Javaid B, Kadambi PV, Gillen D, Harland R, Thistlewaite JR, et al. Leflunomide for polyomavirus type BK nephropathy. N Engl J Med. 2005;352(11):1157–8.
Brickelmaier M, Lugovskoy A, Kartikeyan R, Reviriego-Mendoza MM, Allaire N, Simon K, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother. 2009;53(5):1840–9.
Kobayashi Z, Akaza M, Numasawa Y, Ishihara S, Tomimitsu H, Nakamichi K, et al. Failure of mefloquine therapy in progressive multifocal leukoencephalopathy: Report of two Japanese patients without human immunodeficiency virus infection. J Neurol Sci. 2013;324(1–2):190–4.
Epperla N, Medina-Flores R, Mazza JJ, Yale SH. Mirtazapine and mefloquine therapy for non-AIDS-related progressive multifocal leukoencephalopathy. Wis Med J. 2014;113(6):242–5.
Wenning W, Haghikia A, Laubenberger J, Clifford DB, Behrens PF, Chan A, et al. Treatment of progressive multifocal leukoencephalopathy associated with natalizumab. N Engl J Med. 2009;361(11):1075–80.
Koralnik IJ. Can immune checkpoint inhibitors keep JC virus in check? N Engl J Med. 2019;380(17):1667–8.
Muftuoglu M, Olson A, Marin D, et al. Allogeneic BK virus-specific T cells for progressive multifocal leukoencephalopathy. N Engl J Med. 2018;379:1443–511.
Hunter BD, Jacobson CA. CAR T-cell associated neurotoxicity: mechanisms, clinicopathologic correlates, and future directions. JNCI J Natl Cancer Inst. 2019;11(7):646–54.
Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–30.
Kasakovski D, Xu L, Li Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J Hematol Oncol. 2018;11(1):91.
Kato T, Nishida T, Ito Y, Murase M, Murata M, Naoe T. Correlations of programmed death 1 expression and serum IL-6 level with exhaustion of cytomegalovirus-specific T cells after allogeneic hematopoietic stem cell transplantation. Cell Immunol. 2014;288(1–2):53–9.
Hoang E, Bartlett NL, Goyal MS, Schmidt RE, Clifford DB. Progressive multifocal leukoencephalopathy treated with nivolumab. J Neurovirol. 2019;25(2):284–7.
Walter O, Treiner E, Bonneville F, Mengelle C, Vergez F, Lerebours F, et al. Treatment of progressive multifocal leukoencephalopathy with nivolumab. N Engl J Med. 2019;380(17):1674–6.
Cortese I, Muranski P, Enose-Akahata Y, Ha S-K, Smith B, Monaco M, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med. 2019;380(17):1597–605.
Tan CS, Bord E, Broge TA, Glotzbecker B, Mills H, Gheuens S, et al. Increased program cell death-1 expression on T lymphocytes of patients with progressive multifocal leukoencephalopathy. J Acqui Immune Defic Syndr. 2012;60(3):244–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sdrimas, K., Diaz-Paez, M., Camargo, J.F. et al. Progressive multifocal leukoencephalopathy after CAR T therapy. Int J Hematol 112, 118–121 (2020). https://doi.org/10.1007/s12185-020-02840-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-02840-x