Abstract
Chronic lymphocytic leukemia (CLL) shows a remarkable heterogeneity, with some patients having an almost normal lifespan, others surviving only several months after diagnosis despite intensive therapy. The aim of this study was to investigate the serum thymidine kinase 1 (TK1) concentration in Chinese patients with CLL and its correlation with well-established other prognostic factors. Enhanced chemiluminescent dot blot assay was performed to measure serum TK1 concentration in 80 CLL patients. The concentration of TK1 was significantly increased in patients with Binet C (P = 0.002), higher levels of serum lactate dehydrogenase (LDH) (P = 0.012) and β2-microglobulin (β2-MG) (P = 0.025), unmutated IGHV status (P < 0.001), or higher expression levels of ZAP-70 (P = 0.014) and CD38 (P = 0.018) groups compared to the patients with Binet A, lower levels of serum LDH and β2-MG, mutated IGHV status, or lower expression levels of ZAP-70 and CD38 groups, respectively. Strong correlation of TK1 level with IGHV mutations (r = 0.412, P < 0.001) or ZAP-70 (r = 0.263, P = 0.024) was observed. According to receiver operating characteristic curve analysis for serum TK1 concentration and IGHV mutational status, area under the curve was 0.757 (P = 0.001) and the optimal cut-off value of serum TK1 concentration level was 1.75 pM, with a 87.8% specificity, a 63.6% sensitivity. It was showed that serum TK1 concentration could be a predictive marker of IGHV mutational status, and might be applied for the assessment of prognosis in patients with CLL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Although chronic lymphocytic leukemia (CLL) is often considered as an indolent disease, it can have an extremely variable course, with the life expectancy in patients ranging from as long as that in a healthy age-matched normal population to a median of 1.5 years. Because of this variability, in the past few years, several clinical and hematological parameters have been evaluated as possible indicators of prognosis. One such indicator is the thymidine kinase (TK) level. TK is a pyrimidine metabolic pathway enzyme involved in a salvage pathway of DNA synthesis, and is thus a cell cycle-dependent marker. The TK in human cells occurs as two isozymes: a cytoplasmic (TK1) and a mitochondrial (TK2) form. The level of TK1 rises at the G1/S phase boundary and increases dramatically at late S/early G2 phase during the cell cycle. TK1 is present in proliferating normal and tumor cells, but is virtually absent from quiescent cells. However, TK2 activity is about 5% of TK1 and it is only found in resting cells [1, 2]. Serum TK1 levels in malignant cells are proportional to cellular proliferation rates. TK1 activity in serum has been used as a proliferation marker since 1980 [1–5]. Several studies in patients with lymphoproliferative diseases have shown the prognostic value of this enzyme. For example, in patients with non-Hodgkin’s lymphoma (NHL) [5, 6] and multiple myeloma (MM) [7], the activity of TK1 correlates with the grade of malignancy, the stage of disease, and the length of survival. In CLL, the activity of TK1 correlates not only with the stage of disease but also with the disease status, which in turn has allowed a distinction to be made between aggressive and indolent disease [3, 8], and can be an independent predictor of the duration of the progression-free survival (PFS) in CLL [9, 10]. Furthermore, commercially available anti-TK1 antibodies have recently provided an attractive alternative in clinical cancer applications. In patients with solid tumors, serum TK1 concentration is a more sensitive and reliable marker than TK1 activity [11, 12].
It remains unclear, however, whether the serum TK1 concentration can be used to predict the prognosis in patients with CLL. In the present study, we therefore measured the serum TK1 concentration in 80 Chinese patients with CLL, correlated the serum TK1 concentration with other well-established prognostic indicators such as clinical stage, immunoglobulin heavy chain variable gene (IGHV) mutational status, ZAP-70, CD38, and chromosomal abnormalities. As we know, this is the first report about the clinical significance of serum TK1 concentration in patients with CLL.
2 Materials and methods
2.1 Patients
Our study population consisted of 80 consecutive patients with newly diagnosed CLL between January 2000 and March 2009. All the patients provided informed consent and the University and Institutional Review Boards approved this research. The diagnosis of CLL was based on clinical characteristics, peripheral blood and bone marrow morphology, immunophenotype, and peripheral blood absolute lymphocyte count (ALC) ≥ 5.0 × 109/l according to the National Cancer Institute (NCI) criteria [13]. The immunophenotype features of CLL cells were co-expression of the T-cell antigen CD5 and B-cell surface antigens CD19, CD20 and CD23, and weak expression of surface immunoglobulin, CD20 and CD79b. The staging of CLL was performed according to the Binet staging system [14]. Data collected included: age at diagnosis, gender, date of diagnosis, ALC, Binet stages, β2-microglobulin (β2-MG), lactate dehydrogenase (LDH). A range of other prognostic markers was also analyzed for the majority of patients: IGHV mutational status, CD38 and ZAP-70 expression, and cytogenetics by florescence in situ hybridization (FISH).
2.2 Enhanced chemiluminescent dot blot assay of serum TK1 concentration
Enhanced chemiluminescent (ECL) dot blot assay was performed according to the manufacturer’s protocol (SSTK Inc., Shenzhen, China). Fasting blood samples (2 ml) were collected from individuals. The drawn venous blood was stored for less than 2 h at room temperature (RT) in non-heparin tubes, then centrifuged at 4000 rpm for 10 min and stored at −70°C until analysis. Three μl of serum was directly applied onto a nitrocellulose membrane (HybandTM-C, Amersham). TK1 standards (20, 6.6, 2.2 pM) were used as extrapolation standards. The membrane was blocked in TBS (Tris-buffered saline) with 10% non-fat milk for 4 h and incubated at RT over night after the addition of primary anti-TK1 antibody. After incubation with a biotinylated secondary antibody for 1 h at RT, the membrane was incubated in TBS buffer with avidin–HRP–streptavidin, followed by the addition of ECL substrate. The light intensity of a single spot on the membrane was detected using a CCD imaging system (SSTK Inc., Shenzhen, China). Based on the light intensities of the known concentration of TK1 standards, the light intensities of the serum TK1 spots were re-calculated and expressed as pM. The sensitivity of the assay was 0.2 pM, its reliability 98% and the CV value of the duplicates 8.6%. All the experiments were performed in a blinded manner and in duplicate.
2.3 The analysis of IGHV somatic mutational status
To determine IGHV gene somatic mutational status in CLL cells, the cDNAs were amplified using IGHV primers, a Somatic Hypermutation Assay for Gel Detection kit including MIX I and MIX II, purchased from InVivoScribe, USA. The Hypermutation Mix I targets sequences between the leader and joining regions. Therefore, the amplicon product(s) span the entire variable (V) region, which contains the framework region 1 (FR1), complementarity determining region 1 (CDR1), FR2, CDR2, and FR3 regions. The Hypermutation Mix II targets sequences were between the FR1 and joining (J) regions. The resulting amplicons include a portion of the FR1 region to the downstream J region. MIX I was first applied for IGHV gene amplification. The samples that failed to amplify with MIX I were amplified again with Mix II. PCR amplification was performed as previously described [15]. The majority of samples were sequenced directly using an automated DNA sequencer (ABI 377; Applied Biosystems, Foster City, CA, USA). In case of the failure of direct sequencing, the sequence was determined by cloning. Nucleotide sequences were aligned, and IGHV somatic mutational status was analyzed using one of the following tools: IMGT’s V-QUEST analysis tool (http://imgt.cines.fr) and NCBI’s IgBlast tool (http://www.ncbi.nlm.nih.gov/igblast/). Two percentage deviation from a germline VH sequence was used to determine the mutational status of IGHV gene.
2.4 Detection of ZAP-70 and CD38 by flow cytometry
Flow cytometry was used to detect ZAP-70 and CD38 expression on fresh samples stained with CD5-FITC (BD Biosciences, San Jose, CA, USA), CD19-PerCPCy5.5 (BD Biosciences, San Jose, CA, USA), ZAP-70-PE (clone 1F7.2; Caltag, Burlingame, CA, USA) and CD38-PE (clone HB-7; BD Biosciences, San Jose, CA, USA). This technique was performed as previously described [16]. Isotype controls were run with each sample to distinguish positive from negative cells. To detect ZAP-70, lymphocyte cells were gated further to select CD5+CD19− cells (T cells), which were used as an internal positive control, and CD5+CD19+ cells (CLL cells). After appropriate lymphocytes gating, cytoplasmic ZAP-70 expression was determined in CD5+CD19+ CLL cells. CD38 expression was measured in CD5+CD19+ CLL cells population. Data acquisition and analysis were performed using a FACSCalibur flow cytometer (BD Biosciences) and Cell Quest software (BD Biosciences). The cut-off point for ZAP-70-positive in CLL cells was >20% and CD38-positive was >30%, respectively.
2.5 Detection of molecular cytogenetic aberrations by FISH
Florescence in situ hybridization analysis was performed on the sample for conventional cytogenetic studies. In order to detect prognostically relevant anomalies of chromosomal regions 6q, 11q, 13q, 14q, 17p and chromosome 12, the following fluorescent-labeled probes were used in interphase cytogenetic analyses: LSI MYB (6q23), LSI ATM (11q22), LSI D13S319 (13q14), LSI IGHC/IGHV (14q32), LSI p53 (17p13) and CEP12 (centromere 12) (all probes were purchased from Vysis, Downers Grove, IL, USA). FISH was performed as previously described [17]. The cut-off levels for positive values (mean of normal control ±3 SD), determined from samples of 8 cytogenetically normal persons, was 7.5, 7.7, 10.3, 8.9, 5.2 and 3.0% for del(6q23), del(11q22), del(13q14), 14q32 translocation, del(17p13) and trisomy 12, respectively.
2.6 Statistical analysis
All statistical analyses were performed using the SPSS program for Windows (version 15.0). An effect was considered statistically significant at P < 0.05. Variables examined were age at diagnosis, gender, ALC, Binet stages, β2-MG, LDH, IGHV mutational status, ZAP-70 protein, CD38 expression level, and molecular cytogenetic aberrations. The differences of serum TK1 concentrations between various groups of patients were analyzed using the Student’s t test. The correlations of serum TK1 concentration with other known prognostic factors were described using the Spearman correlation coefficient. The receiver operating characteristic (ROC) curve was used to determine the cut-off value of serum TK1 concentration level, the positive and negative predictive value of IGHV mutational status.
3 Results
3.1 Patients
The characteristics of 80 patients with CLL are summarized in Table 1. Fifty-two patients were males and 28 were females (male:female ratio, 1.9), and the median age at diagnosis was 61 years (range 35–82 years). According to the Binet clinical staging system, 40 (50.0%) patients were in Binet A, 19 (23.8%) in Binet B and 21 (26.3%) in Binet C. With a median follow-up of 35 months (range 1–112 months) from CLL diagnosis in this series, only two patients died (CLL-related deaths) at overall survival of 28 and 48 months, respectively.
3.2 Concentration of TK1 in serum
Enhanced chemiluminescent dot blot assay was used to detect the concentration of TK1 in serum. The mean concentration of TK1 in 10 normal controls was 0.63 ± 0.44 pM (range 0–2.2 pM; 95% CI, 0.21–1.04 pM). Of the 80 patients, the mean serum TK1 concentration was 1.56 ± 1.17 pM (range 0.13–5.23 pM; 95% CI, 1.28–1.80 pM).
3.3 The differences of serum TK1 concentration between various groups of patients
As shown in Table 2, there were no significant differences of TK1 level between groups of sex (male vs. female) (P = 0.805), age (≥60 vs. <60 years) (P = 0.075), ALC (>50 × 109/l vs. ≤50 × 109/l) (P = 0.499), and cytogenetic abnormalities (deletion in 17p13 or 11q22 vs. deletion in 13q14 as the sole abnormality) (P = 0.140). The concentration of TK1 was significantly increased in the patients with Binet stage C (P = 0.002), higher levels of serum LDH (P = 0.012) and β2-MG (P = 0.025), unmutated IGHV status (P < 0.001), or higher expression levels of ZAP-70 (P = 0.014) and CD38 (P = 0.018) groups compared to the patients with Binet stage A, lower levels of serum LDH and β2-MG, mutated IGHV status, or lower expression levels of ZAP-70 and CD38 groups, respectively.
3.4 The correlations between serum TK1 concentration and other prognostic factors
The possibility of interaction between concentration of TK1 and other known prognostic factors, such as IGHV mutational status, ZAP-70 and CD38 expression, and cytogenetic abnormalities, was analyzed in our cohort. Strong correlations of TK1 level with the presence or absence of IGHV mutations (r = 0.412, P < 0.001), and positive or negative expression of ZAP-70 (r = 0.263, P = 0.024) were observed. But there was not noticeable correlations between TK1 and CD38 (r = 0.209, P = 0.074), as well as between TK1 and cytogenetic abnormalities (r = 0.090, P = 0.448).
3.5 Serum TK1 concentration can predict IGHV mutational status
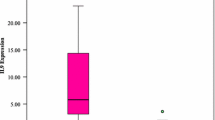
Maximum Youden index values were used to determine optimal threshold cut-off values for prognostic factors as continuous variables based on the ROC curve. This allowed for optimizing the cut points for outcome of CLL subtypes based on IGHV mutational status. According to ROC curve analysis for serum TK1 concentration and IGHV mutational status (Fig. 1), AUC was 0.757 (95% CI, 0.632–0.883; P = 0.001) and the optimal cut-off value of serum TK1 concentration level was 1.75 pM, with a 87.8% specificity, a 63.6% sensitivity. Serum TK1 concentration could be a predictive marker of IGHV mutational status. The AUC of ZAP-70 and CD38 to IGHV mutational status were 0.741 (P = 0.002) (Fig. 2) and 0.783 (P < 0.001) (Fig. 3), respectively.
4 Discussion
Chronic lymphocytic leukemia is the most common type of adult leukemia in the Western countries, however, infrequent in the Eastern. Our findings demonstrated that the frequency of IGHV gene usage was significantly different in Chinese CLL patients compared with Western patients, suggesting involvement of ethnic and/or environmental factors in CLL disease initiation [15]. The clinical course of CLL patients is highly variable; while some patients show an indolent disease and never require treatment, others suffer from a much more aggressive course requiring intensive treatment immediately after diagnosis. For these reasons, the markers giving prognostic information for the individual patient are needed. The limitation of clinical staging systems (Rai and Binet staging systems) in CLL [14, 18], which fail to identify early stage patients most likely to progress, has lead to the search for new prognostic markers with highly predictive capabilities.
Mutational status of the IGHV gene was proved to be a strong prognostic parameter identified in CLL, even among cases with a good prognosis (Binet stage A patients). The determination of the presence of somatic mutations is a difficult, expensive and time-consuming technique which will not be readily available for most of the patients. Therefore, to look for surrogates for IGHV mutation is tempting.
Human cells contain at least two TK isoenzymes, which differ in their biochemical properties and their cellular distribution. The cytosolic isozyme (TK1), is found in the G1/S phase of dividing cells, but is absent in resting cells. Kallander et al. [3] examined the prognostic significance of the serum TK1 activity in a series of 55 CLL patients and found a significant correlation between the TK1 activity and the Rai stage. In addition, longitudinal studies showed the level of TK1 activity to increase parallel with the transition from indolent to active disease. Hallek et al. [9, 10] further found that the serum TK1 activity can predict the duration of PFS of CLL. Magnac et al. [19] reported that elevated TK1 activity was found in patients with unmutated IGHV genes. In addition, elevated TK1 activity was also found in patients with intermediate and poor prognosis chromosomal abnormalities, namely trisomy 12, del(11q23) and del(17p13). The prognostic value of CD38 and ZAP-70 on the CLL cells by flow cytometry has been confirmed as an independent prognostic marker in several studies. As expected, patients with CD38-positive were associated with significantly elevated TK1 values (P = 0.004). Significantly elevated TK1 levels were present in ZAP-70-positive patients, in comparison with those who were ZAP-70 negative (P < 0.001).
Recently, commercially available anti-TK1 antibodies have provided to measure the concentration of TK1 in serum. In patients with solid tumors, serum TK1 concentration is a more sensitive and reliable marker than TK1 activity [11, 12]. However, it remains unclear whether the serum TK1 concentration can be used to predict the prognosis in patients with CLL. The aim of this study was to investigate the associations between elevated serum TK1 concentration and other well-established poor prognosis markers, namely unmutated IGHV genes, specific chromosomal abnormalities and increased ZAP-70 and CD38 expression.
In the current study, we have analyzed TK1 concentration in serum with a recently developed method. The results showed that the serum TK1 concentration reflected the stages of disease, especially the Binet stage; thus, serum TK1 concentration remained low as long as the disease was at early stage and increased at advanced stage. We further found that the TK1 level correlated with measures of tumor burden, such as serum β2-MG and LDH levels. We also confirmed that elevated serum TK1 concentration was associated with other prognostic indicators such as unmutated IGHV genes, increased ZAP-70 and CD38 expression. According to ROC curve analysis for serum TK1 concentration and IGHV mutational status, serum TK1 concentration could be a predictive marker of IGHV mutational status. Unfortunately, since no commercially available radioenzyme assay provided to measure the TK1 activity in China, we could not compare the prognostic significance of TK1 concentration with TK1 activity.
In conclusion, this study demonstrated the value of determination of serum TK1 concentration, to provide useful prognostic information in CLL patients. Due to the simplicity of the ECL dot blot assay to measure serum TK1 concentration, it can be easily applied in routine clinical work.
References
He Q, Skog S, Wang N, Eriksson S, Tribukait B. Characterization of a peptide antibody against a C-terminal part of human and mouse cytosolic thymidine kinase, which is a marker for cell proliferation. Eur J Cell Biol. 1996;70:117–24.
Ke PY, Chang ZF. Mitotic degradation of human thymidine kinase 1 is dependent on the anaphase-promoting complex/cyclosome-CDH1-mediated pathway. Mol Cell Biol. 2004;24:514–26.
Kallander CF, Simonsson B, Hagberg H, Gronowitz JS. Serum deoxythymidine kinase gives prognostic information in chronic lymphocytic leukemia. Cancer. 1984;54:2450–5.
Kauffman MG, Kelly TJ. Cell cycle regulation of thymidine kinase: residues near the carboxyl terminus are essential for the specific degradation of the enzyme at mitosis. Mol Cell Biol. 1991;11:2538–46.
Hallek M, Wanders L, Strohmeyer S, Emmerich B. Thymidine kinase: a tumor marker with prognostic value for non-Hodgkin’s lymphoma and a broad range of potential clinical applications. Ann Hematol. 1992;65:1–5.
Suki S, Swan F Jr, Tucker S, Fritsche HA, Redman JR, Rodriguez MA, et al. Risk classification for large cell lymphoma using lactate dehydrogenase, beta-2 microglobulin, and thymidine kinase. Leuk Lymphoma. 1995;18:87–92.
Luoni R, Ucci G, Riccardi A, Gobbi P, Avato FM, Vignale C, et al. Serum thymidine kinase in monoclonal gammopathies. A prospective study. The Cooperative Group for Study and Treatment of Multiple Myeloma. Cancer. 1992;69:1368–72.
Kallander CF, Simonsson B, Gronowitz JS, Nilsson K. Serum deoxythymidine kinase correlates with peripheral lymphocyte thymidine uptake in chronic lymphocytic leukemia. Eur J Haematol. 1987;38:331–7.
Hallek M, Wanders L, Ostwald M, Busch R, Senekowitsch R, Stern S, et al. Serum beta(2)-microglobulin and serum thymidine kinase are independent predictors of progression-free survival in chronic lymphocytic leukemia and immunocytoma. Leuk Lymphoma. 1996;22:439–47.
Hallek M, Langenmayer I, Nerl C, Knauf W, Dietzfelbinger H, Adorf D, et al. Elevated serum thymidine kinase levels identify a subgroup at high risk of disease progression in early, nonsmoldering chronic lymphocytic leukemia. Blood. 1999;93:1732–7.
He Q, Fornander T, Johansson H, Johansson U, Hu GZ, Rutqvist LE, et al. Thymidine kinase 1 in serum predicts increased risk of distant or loco-regional recurrence following surgery in patients with early breast cancer. Anticancer Res. 2006;26:4753–9.
He Q, Zhang P, Zou L, Li H, Wang X, Zhou S, et al. Concentration of thymidine kinase 1 in serum (S-TK1) is a more sensitive proliferation marker in human solid tumors than its activity. Oncol Rep. 2005;14:1013–9.
Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O’Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–7.
Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–206.
Chen L, Zhang Y, Zheng W, Wu Y, Qiao C, Fan L, et al. Distinctive IgVH gene segments usage and mutation status in Chinese patients with chronic lymphocytic leukemia. Leuk Res. 2008;32:1491–8.
Xu W, Li JY, Wu YJ, Yu H, Shen QD, Tian T, et al. CD38 as a prognostic factor in Chinese patients with chronic lymphocytic leukaemia. Leuk Res. 2009;33:237–43.
Qiu HX, Xu W, Cao XS, Zhou M, Shen YF, Xu YL, et al. Cytogenetic characterisation in Chinese patients with chronic lymphocytic leukemia: a prospective, multicenter study on 143 cases analysed with interphase fluorescence in situ hybridisation. Leuk Lymphoma. 2008;49:1887–92.
Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–34.
Magnac C, Porcher R, Davi F, Nataf J, Payelle-Brogard B, Tang RP, et al. Predictive value of serum thymidine kinase level for Ig-V mutational status in B-CLL. Leukemia. 2003;17:133–7.
Acknowledgments
This study was supported by National Natural Science Foundation of China (30871104), Jiangsu Province’s Outstanding Medical Academic Leader Program (LJ200623), Jiangsu Province’s Medical Elite Program (RC2007042), Natural Science Foundation of Jiangsu Province (BK2007249), “Qing Lan” project of Jiangsu Province, and “Liu Da Ren Cai Gao Feng” of Jiangsu Province.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Xu, W., Cao, X., Miao, KR. et al. Serum thymidine kinase 1 concentration in Chinese patients with chronic lymphocytic leukemia and its correlation with other prognostic factors. Int J Hematol 90, 205–211 (2009). https://doi.org/10.1007/s12185-009-0380-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-009-0380-8