Abstract
The epidemic of obesity and overweight is spreading worldwide. Excessive adiposity is associated with a myriad of adverse health outcomes, leading to increased health care expenditures and shortened life expectancy. In contrast to overeating, calorie restriction (CR), defined as a reduction in food intake without malnutrition, increases both mean and maximum lifespan in a variety of species by reducing the incidence of several chronic degenerative diseases, including cardiovascular disease. The constellation of health benefits brought about by CR results from biological and physiologic changes affecting fundamental processes underlying aging and age-related pathologies. Despite the beneficial properties of CR, it is likely that most people will not engage in such a dietary regimen for the long-term. Supplementation with specific compounds mimicking CR may represent a more feasible means to improve health and prolong life. However, evidence on long-term effectiveness and safety of these compounds is not yet available in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
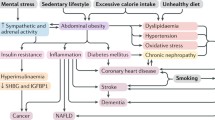
Over the past few decades, excessive consumption of calorie-dense, nutrient-poor foods with high levels of sugar and saturated fats, combined with a sedentary lifestyle, has provoked a global obesity epidemic. The World Health Organization (WHO) estimated that over 1.6 billion adults worldwide were overweight in 2005, among whom 400 million were clinically obese [1]. Furthermore, WHO projections indicate that by 2015 approximately 2.3 billion adults will be overweight and over 700 million will be obese [1]. Excessive body fat is of great concern in the United States, where approximately 65% adults are overweight and over 30% are obese [2]. Extremely obese adults, defined as those having a body mass index (BMI) ≥40 kg/m2, account for almost 5% of the U.S. population [2].
Overweight and obesity are major contributors to the global burden of chronic diseases and disability. Indeed, excessive adiposity is associated with a broad range of pathologies, cardiovascular risk factors, and health complaints, including coronary heart disease, hypertension, congestive heart failure (CHF), stroke, some cancers, diabetes mellitus, dyslipidemia, metabolic syndrome, asthma, osteoarthritis, depression, sleep disorders, and chronic fatigue. Given the multitude of diseases linked with excess body fat, it is not surprising that obesity is associated with reduced life expectancy. Indeed, obesity ranks among the leading causes of premature death in Western countries. Allison et al. [3] estimated that between 280,000 and 325,000 deaths could be attributed to obesity annually in the United States. The reduction in life expectancy due to excessive adiposity is directly proportional to body weight, with 8 and 13 years of life lost by white men and women with a BMI >45 kg/m2, respectively [4].
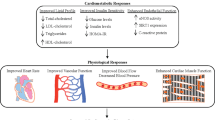
In striking contrast with the adverse health outcomes associated with obesity, calorie restriction (CR), defined as a reduction in calorie intake below usual ad libitum (AL) consumption without malnutrition, improves health and extends lifespan in a multitude of taxonomically diverse organisms [5•]. This remarkable effect resides in the ability of CR to prevent or delay the onset of several chronic degenerative diseases, including cardiovascular disease (CVD), cancer, neurodegenerative disorders, diabetes, and autoimmune diseases [6]. The life-extending properties of CR are also observed in primates and humans. A recent study at the Wisconsin National Primate Research Center concluded that long-term 30% CR delays the onset of age-related diseases (eg, diabetes, CVD, and malignancies) and reduces mortality in rhesus monkeys [7•]. Strikingly, inhabitants of Okinawa Island, whose traditional diet contains about 20% and 40% fewer calories compared with inland Japan and the United States, respectively, have the longest life expectancy and the highest centenarian rate in the world. The extraordinary longevity of Okinawans results from decreased incidence of conditions such as CVD, stroke, and malignancies, which is at least partly attributable to their nutrient-dense, low-calorie diet [8].
Although the benefits of CR have been known for many years, the underlying mechanisms are not fully understood. However, a wide consensus exists supporting the notion that dietary restriction affects global and fundamental biological processes underlying aging and age-related diseases, through complex metabolic and neuroendocrine adaptations. In this review, we discuss the most relevant mechanisms of action of CR, with a special focus on the effects of dietary restriction on the cardiovascular system.
How Does CR Work?
The potential of CR to improve health and prolong life has instigated intense research on the mechanisms underlying these effects. Hormesis, defined as the beneficial adaptation resulting from the exposure to low doses of toxins or other stressors, has been proposed as a basic mechanism mediating CR’s benefits [9]. According to this proposition, CR would put forward a range of evolutionary conserved adaptations, originally developed to help the organism survive in periods of food deprivation. In particular, the reduced flux through the glycolytic pathway elicited by CR and by intermittent feeding is considered a key hormetic mechanism. Indeed, the glycolytic intermediates glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone-phosphate (DHAP) are potentially harmful because of their ability to spontaneously decompose into methylglyoxal (MG) [9]. This latter, in turn, represents a major source of protein advanced glycation end products (AGEs). CR, by suppressing glycolysis, decreases the generation of MG, whose reduced levels act hormetically, enhancing the expression and activity of stress proteins [9]. The high glycolytic flux occurring in AL feeding conditions promotes the accumulation of G3P and DHAP also by lowering nicotinamide adenine dinucleotide (NAD) availability, which is required for their metabolism. A high NAD/NADH ratio decreases the activity of sirtuins, a family of histone deacetylases (HDACs), which are considered central for the control of cell senescence and organism lifespan [10]. In fact, NAD-dependent histone deacetylation catalyzed by sirtuins slows down cellular aging through a wide range of metabolic adaptations, including reduced generation of free radicals, enhanced disposal of damaged proteins and organelles, and reduced levels of apoptosis [10]. In contrast, reductions in sirtuin activity, resulting from NAD shortage as a consequence of a high glycolytic throughput, promote cellular aging, thus shortening the organism’s lifespan [10]. Well-functioning mitochondria are essential for maintaining adequate levels of NAD through NADH oxidation during respiration, which is consistent with the major role postulated for mitochondrial dysfunction in aging [11].
Protection against oxidative stress is considered another key mechanism underlying CR’s benefits. Free radicals and other reactive species are continuously generated by several biological processes, with mitochondrial respiration being the main source. Detoxification of free radicals is performed via enzymatic (eg, superoxide dismutase [SOD], glutathione peroxidase [GPx], catalase, thioredoxins) as well as nonenzymatic (eg, glutathione, vitamin E, vitamin C, β-carotene, uric acid) mechanisms. The accumulation of oxidative damage to cellular macromolecules, due to increased oxidant generation and/or reduced antioxidant capacity, is considered a fundamental mechanism responsible for structural and functional cellular alterations, eventually leading to aging and diseases [11].
Elevations in oxidative damage biomarkers over the course of aging have been detected in a variety of tissues, including the heart [12•]. Furthermore, oxidative stress is involved in the pathogenesis of myocardial ischemia-reperfusion injury, cardiac remodeling after myocardial infarction, left ventricular hypertrophy, and CHF [13]. In addition, oxidative damage plays a role in endothelial dysfunction during aging and in CVD [13].
CR has repeatedly been shown to mitigate or even reverse the age-related accrual of oxidative damage in the cardiovascular system. For instance, elevation in cardiac DNA oxidative damage was greatly attenuated in older mice subjected to lifelong 40% CR [14]. Moreover, rats kept on an alternate-day fasting regimen displayed reduced levels of cardiac oxidative damage and myocardial fibrosis compared with AL-fed controls [15]. The age-related increase in protein oxidative damage in the rat heart was also significantly mitigated by lifelong 40% CR [16]. A similar dietary regimen prevented the accumulation of o-tyrosine and o,o′-dityrosine adducts in aged murine hearts [17]. Reductions in oxidative damage to heart constituents observed in CR animals have been mainly attributed to improvements in cardiac mitochondrial bioenergetic efficiency. Indeed, studies have shown reduced mitochondrial generation of hydrogen peroxide (H2O2) and superoxide anion (O • −2 ) as well as decreases in mitochondrial free radical leak in the heart of CR rodents [13]. Increased activity of cardiomyocyte antioxidant enzymes has also been reported in experimental animals subjected to various CR regimens [13]. Of note is that lifelong mild CR (ie, 8% calorie intake reduction) combined with voluntary wheel running reduced mitochondrial H2O2 generation [18] and increased plasma total antioxidant capacity [19] in older rats.
Studies have shown that CR can also attenuate the oxidative damage associated with CVD. For instance, 15% CR reduced cardiac lipid peroxidation in Dahl salt-sensitive rats fed a high-salt diet [20]. This adaptation was accompanied by ameliorations in left ventricular remodeling, diastolic function, and cardiac index, and it delayed the onset of cardiac cachexia. Lifelong 40% CR also attenuated cardiac oxidative damage in middle-aged rats following myocardial ischemia-reperfusion [21]. Furthermore, 3-months of 30% CR abolished the increase in mitochondrial reactive oxygen species (ROS) generation and NADPH-dependent O • −2 production in the coronary endothelium and aortic wall of spontaneously diabetic rats, resulting in reduced levels of lipid peroxidation and increased nitric oxide availability [22]. Finally, CR combined with low-intensity physical activity reduced oxidative stress and improved acetylcholine-dependent vasodilation in middle-aged obese, but otherwise healthy, persons [23].
Chronic low-grade inflammation is thought to play an important role in tissue damage, fibrosis, and organ dysfunction associated with aging and age-related diseases, including CVD [24•]. Importantly, chronic inflammation has been proposed as the converging process linking normal aging with age-related diseases [24•]. According to this hypothesis, the age-dependent elevation in oxidative stress activates redox-sensitive transcription factors (eg, nuclear factor–κB), which in turn enhance the expression of inflammatory cytokines, cellular adhesion molecules (CAMs), and proinflammatory enzymes [24•].
Several studies have shown that CR attenuates the age-associated elevation in systemic inflammation. Old rodents kept on lifelong 40% CR display reduced circulatory levels of various inflammatory biomarkers, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), C-reactive protein (CRP), and several CAMs [24•]. Furthermore, lifelong 8% CR either alone or combined with voluntary wheel running prevented the increase in plasma CRP levels in old rats [19]. Mitigation of systemic inflammation by CR has also been reported in nonhuman primates [25]. Moreover, similar anti-inflammatory effects can be obtained with CR in humans [26–29], even when dietary restriction is initiated late in life [30].
Regarding the effects of CR on inflammation in the presence of CVD, lifelong 40% dietary restriction attenuated the myocardial inflammatory response to ischemia-reperfusion in rats [21]. Furthermore, 15% CR reduced plasma IL-6 and TNF-α levels in salt-sensitive rats fed a high-salt diet [20]. Three-month 30% CR also prevented the increase in transforming growth factor-β1 (TGF-β1) levels in the aorta of spontaneously diabetic rats [22].
An additional mechanism by which CR is thought to protect the cardiovascular system is through the attenuation of cardiomyocyte apoptosis [13]. Cardiac cells are virtually post-mitotic and their slow turnover is supported by resident cardiomyogenic cells. Cardiomyocyte removal through apoptosis increases with advancing age, which, in combination with insufficient stem cell replenishment, may contribute to the age-related heart remodeling [13]. Apart from aging, myocyte loss due to apoptosis is enhanced in diabetic patients and in those with end-stage CHF [13].
A recent study demonstrated that lifelong 40% CR attenuated the age-related increase in mitochondrial permeability transition pore (mPTP) opening susceptibility in the rat heart [31]. Notably, opening of the mPTP is considered a central event for the initiation of mitochondria-mediated apoptosis. Furthermore, microarray analyses revealed that 40% CR started at middle age reduced the expression of proapoptotic genes and upregulated antiapoptotic transcripts in the heart of old mice [32]. Interestingly, 6-month 35% CR reduced the activation of cardiomyocyte apoptosis in rats subjected to ischemia-reperfusion injury [33••]. This adaptation was accompanied by improved recovery of left ventricular function and limitation of infarct size.
In summary, a vast literature exists supporting an antiaging, cardioprotective action by CR. Although some of the mechanisms through which this effect is achieved have been discovered, further research is necessary to disentangle the complex actions of CR on the cardiovascular system and the organism as a whole.
Is Cardioprotection by CR Achievable in Humans?
As previously mentioned, the inhabitants of Okinawa, who are spontaneously calorie restricted, experience a very low incidence of chronic degenerative diseases, including CVD, and have the longest life expectancy in the world. Another classic example of naturally occurring, long-term CR in humans is the “Biosphere 2” experiment. Biosphere 2 is an artificial, closed, self-sustaining ecosystem located in Oracle, Arizona. In 1991, eight persons (four females and four males) entered the biosphere and the complex was physically sealed for 2 years. The anticipated daily calorie intake over the course of the experiment was >2,500 Kcal. However, due to unexpected problems in the growth of crops, biosphere members had limited access to food for 18 months, with an actual caloric intake ~25% lower than anticipated, while sustaining very high levels of physical activity. As a result, all members lost significant amount of body weight (~18% in men and ~10% in women), which was restored within 6 months after exiting the biosphere. The forced CR experienced by these individuals led to a decline in metabolic rate, body temperature, systolic and diastolic blood pressure, and white blood cell (WBC) count [34•]. Blood glucose, cholesterol, insulin, cortisol, and thyroid hormone levels were also reduced [34•]. It is possible that the high levels of physical activity sustained by the crew members over the 2-year experimentation might have contributed to those adaptations. Nevertheless, changes experienced by these individuals closely resembled those observed in laboratory animals subjected to CR.
Apart from these sporadic cases of naturally occurring CR, a growing number of clinical studies indicate that dietary restriction results in significant improvements in traditional cardiovascular risk factors (eg, blood pressure, blood glucose, lipids, body composition) among overweight and obese persons as well as in lean individuals. Furthermore, recent studies suggest that CR may favorably affect biomarkers of oxidative stress and inflammation. Indeed, fat loss induced by negative energy balance via either CR or physical exercise ameliorated glucose tolerance, improved the lipoprotein profile, and reduced plasma CRP levels in middle-aged non-obese individuals [35]. In addition, lower levels of CRP, TNF-α, and TGF-β1 have been detected in middle-aged healthy persons on long-term CR (ie, 3-15 years) compared with age- and gender-matched healthy controls consuming typical Western diets [28]. In the same study population, CR ameliorated glucose tolerance and blood lipids, reduced systolic and diastolic blood pressure, and decreased plasma CRP and platelet-derived growth factor AB (PDGF-AB) levels [26]. Importantly, carotid artery intima-media thickness was approximately 40% less in the CR group than in the control group [26]. Moreover, in a 6-month randomized controlled trial, CR (25% of baseline energy requirements) reduced DNA damage in WBCs [36], improved whole body insulin sensitivity [36], enhanced skeletal muscle mitochondrial biogenesis [37], and produced favorable changes in systemic inflammation, coagulation, lipid, and blood pressure [38••, 39] in healthy, non-obese adults. In the same study population, CR, particularly when combined with physical exercise, reduced the sympathetic nervous system drive and increased the activity of the parasympathetic system, resulting in an overall improvement in autonomic control [40•]. Interestingly, decline in left ventricular diastolic function, an early marker of cardiac aging, was significantly attenuated in middle-aged healthy persons kept on CR for 1 year [27]. In another recent study, a 16-week very low-calorie diet (ie, 450 kcal/d) decreased myocardial triglyceride content and improved diastolic function in obese, middle-aged individuals with type II diabetes mellitus [41•]. In contrast, progressive implementation of severe CR in healthy lean men increased the myocardial triglyceride content and decreased the diastolic performance in a dose-dependent fashion [42•].
Dietary restriction retains the ability of ameliorating inflammatory biomarkers, even when started in advanced age. Indeed, in obese older adults (≥60 years of age), 18-month diet-induced weight loss reduced several markers of systemic inflammation (eg, CRP, IL-6, and soluble TNF-α receptor 1) [30].
In conclusion, the available evidence indicates that CR is effective in reducing CVD risk in both younger and older persons as well as in normal weight and overweight individuals. However, important research questions remain unanswered: which is the optimal degree of CR to obtain beneficial physiologic changes without incurring adverse events? At what age and for how long should an individual engage in CR to maximize the benefits? Can CR be safely implemented in older people? Is large-scale CR implementation feasible?
Mimicking CR: Is the “Magic Pill” Possible?
Despite undisputed health benefits brought about by CR, it is likely that most people will not be able to sustain substantial food restrictions for the long-term. Furthermore, persons practicing long-term severe CR may experience several adverse events, including undesired changes in physical appearance, loss of strength and stamina, menstrual irregularities, infertility, loss of libido, osteoporosis, cold sensitivity, slower wound healing, and psychological conditions such as food obsession, depression, and irritability [13]. Moreover, weight loss may not be advisable in non-obese older persons, as it can accelerate age-related muscle loss and increase the risk of disability and mortality [43]. Difficulties adhering to long-term food intake reductions and health concerns intrinsic to the adoption of CR regimens have sparked a great interest in the field of CR mimetics. In fact, these agents could reproduce the effects of CR without requiring modifications in food intake. The first CR mimetic identified was 2-deoxy-d-glucose (2DG), an analog of glucose. Once ingested, 2DG is absorbed by the intestines and taken up by cells via glucose importers. Within cells, 2DG is converted into 2-deoxyglucose-6-phosphate, which blocks glycolysis. In keeping with its CR-mimicking properties, 2DG was shown to extend both mean and maximum lifespan in Caenorhabditis elegans [44]. However, a recent study demonstrated that chronic 2DG administration to rats, although reproducing a CR-like phenotype, caused cardiotoxicity and increased mortality [45••]. Even though it is currently unknown if long-term dietary supplementation with 2DG is cardiotoxic in humans, these results have raised serious concerns regarding the safety of 2DG as a CR mimetic.
In recent years, intensive research has been devoted to resveratrol. Resveratrol is a plant-derived polyphenol found in grapes, red wine, peanuts, and some berries. CR-mimicking properties of resveratrol are thought to be linked with its ability of activating sirtuins. Preclinical studies have revealed that resveratrol extends the lifespan and delays the onset of aging phenotypes in short-lived organisms [46]. Furthermore, studies in rodents have shown that resveratrol inhibits cardiomyocyte apoptosis, protects the myocardium against ischemia-reperfusion injury, prevents left ventricle hypertrophy, improves endothelial function, inhibits platelet aggregation, and reduces inflammation [13]. In addition, resveratrol improved survival and insulin sensitivity and reduced the prevalence of cardiac pathology in mice fed a high-calorie diet [47]. Strikingly, short-term supplementation with a nutraceutical mixture containing resveratrol induced a transcriptional shift in the mouse heart resembling that elicited by long-term CR [48••].
Preliminary studies in humans appear to support a beneficial effect of resveratrol on the cardiovascular system. For instance, Lekakis et al. [49] reported that consumption of a red grape polyphenol extract containing resveratrol improved endothelial function in patients with coronary heart disease. Furthermore, 4-week supplementation with a lyophilized grape powder reduced blood lipids and systemic inflammatory and oxidative stress biomarkers in both pre- and postmenopausal women [50].
In summary, although preliminary evidence indicates that resveratrol might be cardioprotective, additional research is needed to determine whether this compound may effectively and safely mimic CR in humans.
Conclusions
A wealth of evidence is available supporting the effectiveness of CR in delaying aging and improving cardiovascular health both in experimental animals and humans. However, most persons are reluctant or unable to engage in long-term sustained CR. It is likely that milder CR regimens combined with physical exercise may convey most of the benefits observed in experimental settings adopting stricter CR protocols. CR mimetics, among which resveratrol has emerged as a leading candidate, might overcome the difficulty adhering to long-term dietary restriction. Although preliminary studies show promises, no evidence supporting long-term efficacy and safety of CR mimetics is yet available in humans. Furthermore, cardiotoxicity and increased mortality associated with long-term administration of 2DG in rats indicate that CR mimetics might not represent a panacea for aging and age-related diseases [45••].
In conclusion, the adoption of healthier eating habits and a more active lifestyle appears at present as the only means for promoting cardiovascular health, increasing life expectancy, and improving quality of life, at least until the “magic pill” becomes available.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
World Health Organization, Office of Health Communications and Public Relations: Obesity and overweight. 2006. Available at http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed May 3, 2010.
Ogden CL, Carroll MD, Curtin LR, et al.: Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006, 295:1549–1555.
Allison DB, Fontaine KR, Manson JE et al.: Annual deaths attributable to obesity in the United States. JAMA 1999, 282:1530–1538.
Fontaine KR, Redden DT, Wang C, et al.: Years of life lost due to obesity. JAMA 2003, 289:187–193.
• Fontana L, Partridge L, Longo VD: Extending healthy life span--from yeast to humans. Science 2010, 328:321–326. This concise yet exhaustive review describes the mechanisms of lifespan extension by CR and genetic and pharmacologic manipulations of nutrient-sensing pathways in various species. The article also describes findings in humans with growth hormone and/or insulin-like growth factor 1 deficiency as well as in persons subjected to calorie restriction.
Weindruch R: The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol Pathol 1996, 24:742–745.
• Colman RJ, Anderson RM, Johnson SC, et al.: Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325:201–204. This 20-year longitudinal study shows that long-term, moderate CR reduces the incidence of age-related diseases (CVD, cancer, and diabetes) and mortality in rhesus monkeys. These findings support the effectiveness of CR in nonhuman primates.
Kagawa Y: Impact of Westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Prev Med 1978, 7:205–217.
Hipkiss AR: Dietary restriction, glycolysis, hormesis and ageing. Biogerontology 2007, 8:221–224.
Dali-Youcef N, Lagouge M, Froelich S, et al.: Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann Med 2007, 39:335–345.
Harman D: Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956, 11:298–300.
• Wong YT, Gruber J, Jenner AM et al.: Elevation of oxidative-damage biomarkers during aging in F2 hybrid mice: protection by chronic oral intake of resveratrol. Free Radic Biol Med 2009, 46:799–809. This study shows that chronic, low-dose administration of resveratrol attenuates the age-dependent increase in oxidative damage in various mouse tissues, including the heart. These findings add to a growing literature supporting the effectiveness of resveratrol as a CR mimetic in rodents.
Marzetti E, Wohlgemuth SE, Anton SD et al.: Cellular mechanisms of cardioprotection by calorie restriction: state of the science and future perspectives. Clin Geriatr Med 2009, 25:715–732.
Sohal RS, Agarwal S, Candas M et al.: Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev 1994, 76:215–224.
Castello L, Froio T, Maina M, et al.: Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kB activation. Free Radic Biol Med 2010, 48:47–54.
Sohal RS, Ku HH, Agarwal S, et al.: Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev 1994, 74:121–133.
Leeuwenburgh C, Wagner P, Holloszy JO, et al.: Caloric restriction attenuates dityrosine cross-linking of cardiac and skeletal muscle proteins in aging mice. Arch Biochem Biophys 1997, 346:74–80.
Judge S, Judge A, Grune T et al.: Short-term CR decreases cardiac mitochondrial oxidant production but increases carbonyl content. Am J Physiol Regul Integr Comp Physiol 2004, 286:R254–R259.
Kalani R, Judge S, Carter C, et al.: Effects of caloric restriction and exercise on age-related, chronic inflammation assessed by C-reactive protein and interleukin-6. J Gerontol A Biol Sci Med Sci 2006, 61:211–217.
Seymour EM, Parikh RV, Singer AA, et al.: Moderate calorie restriction improves cardiac remodeling and diastolic dysfunction in the Dahl-SS rat. J Mol Cell Cardiol 2006, 41:661–668.
Chandrasekar B, Nelson JF, Colston JT, et al.: Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2001, 280:H2094–H2102.
Minamiyama Y, Bito Y, Takemura S, et al.: Calorie restriction improves cardiovascular risk factors via reduction of mitochondrial reactive oxygen species in type II diabetic rats. J Pharmacol Exp Ther 2007, 320:535–543.
Sciacqua A, Candigliota M, Ceravolo R, et al.: Weight loss in combination with physical activity improves endothelial dysfunction in human obesity. Diabetes Care 2003, 26:1673–1678.
• Chung HY, Cesari M, Anton S, et al.: Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 2009, 8:18–30. This comprehensive review summarizes the most recent findings on the role played by chronic inflammation in aging and major age-related diseases. Modulation of molecular inflammation by CR and physical exercise is also described.
Lane MA, Reznick AZ, Tilmont EM, et al.: Aging and food restriction alter some indices of bone metabolism in male rhesus monkeys (Macaca mulatta). J Nutr 1995, 125:1600–1610.
Fontana L, Meyer TE, Klein S, et al.: Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A 2004, 101:6659–6663.
Meyer TE, Kovacs SJ, Ehsani AA, et al.: Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol 2006, 47:398–402.
Fontana L, Klein S, Holloszy JO, et al.: Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab 2006, 91:3232–3235.
Bosutti A, Malaponte G, Zanetti M, et al.: Calorie restriction modulates inactivity-induced changes in the inflammatory markers C-reactive protein and pentraxin-3. J Clin Endocrinol Metab 2008, 93:3226–3229.
Nicklas BJ, Ambrosius W, Messier SP, et al.: Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr 2004, 79:544–551.
Hofer T, Servais S, Seo AY, et al.: Bioenergetics and permeability transition pore opening in heart subsarcolemmal and interfibrillar mitochondria: effects of aging and lifelong calorie restriction. Mech Ageing Dev 2009, 130:297–307.
Lee CK, Allison DB, Brand J, et al.: Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci U S A 2002, 99:14988–14993.
• Shinmura K, Tamaki K, Bolli R: Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol 2008, 295:H2348–H2355. This study demonstrates that 6-month CR attenuates the activation of cardiomyocyte apoptosis, improves the recovery of left ventricular function, and reduces infarct size after ischemia-reperfusion in middle-aged rats. Amelioration of myocardial ischemic tolerance is sustained by increased nuclear levels of Sirt1, a histone-deacetylase belonging to the sirtuin family.
Walford RL, Mock D, Verdery R, et al.: Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci 2002, 57:B211–B224.
Fontana L, Villareal DT, Weiss EP, et al.: Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab 2007, 293:E197–E202.
Heilbronn LK, de JL, Frisard MI, et al.: Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 2006, 295:1539–1548.
Civitarese AE, Carling S, Heilbronn LK, et al.: Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med 2007, 4:e76.
•• Lefevre M, Redman LM, Heilbronn LK, et al.: Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis 2009, 203:206–213. This study shows that 6-month negative energy balance obtained via CR either alone or combined with aerobic exercise reduces blood pressure and improves blood lipid profile in healthy non-obese individuals. These favorable changes translate in a significant reduction of the estimated 10-year CVD risk in both intervention groups.
Larson-Meyer DE, Newcomer BR, Heilbronn LK, et al.: Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 2008, 16:1355–1362.
• de Jonge L., Moreira EA, Martin CK, et al.: Impact of 6-month caloric restriction on autonomic nervous system activity in healthy, overweight, individuals. Obesity (Silver Spring) 2010, 18:414–416. This study shows that 6-month moderate CR, particularly if combined with aerobic exercise, ameliorates autonomic nervous system activity, as determined via spectral analysis of heart rate variability, in healthy overweight individuals.
• Hammer S, Snel M, Lamb HJ, et al.: Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol 2008, 52:1006–1012. This study shows that 4-month very low-calorie diet reduces myocardial triglyceride stores and improves left ventricular diastolic function in obese diabetic persons. These changes are accompanied by ameliorations in glucoregulation and body composition.
• Hammer S, van der Meer RW, Lamb HJ, et al.: Progressive caloric restriction induces dose-dependent changes in myocardial triglyceride content and diastolic function in healthy men. J Clin Endocrinol Metab 2008, 93:497–503. This study shows that severe CR induces unfavorable changes in myocardial triglyceride content and diastolic function in lean healthy men. These findings indicate that CR should be implemented cautiously in normal-weight individuals.
Landi F, Zuccala G, Gambassi G et al.: Body mass index and mortality among older people living in the community. J Am Geriatr Soc 1999, 47:1072–1076.
Schulz TJ, Zarse K, Voigt A, et al.: Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 2007, 6:280–293.
•• Minor RK, Smith DL Jr, Sossong AM, et al.: Chronic ingestion of 2-deoxy-D-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol 2010, 243:332–339. This study shows that chronic 2DG ingestion, although reproducing a CR-like phenotype, induces cardiomyocyte vacuolization and increases mortality in rats. These findings raise serious concerns about the use of 2DG as a CR mimetic in humans.
Valenzano DR, Terzibasi E, Genade T et al.: Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol 2006, 16:296–300.
Baur JA, Pearson KJ, Price NL, et al.: Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444:337–342.
•• Barger JL, Kayo T, Pugh TD et al.: Short-term consumption of a resveratrol-containing nutraceutical mixture mimics gene expression of long-term caloric restriction in mouse heart. Exp Gerontol 2008, 43:859–866. This study shows that 3-month supplementation with a nutraceutical mixture containing resveratrol induces a shift in the gene expression profile in the mouse heart closely resembling that obtained with long-term CR. These findings confirm the CR mimicking effects of resveratrol, which may be achieved, at least at the gene expression level, even with a short-term treatment.
Lekakis J, Rallidis LS, Andreadou I et al.: Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil 2005, 12:596–600.
Zern TL, Wood RJ, Greene C et al.: Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr 2005, 135:1911–1917.
Acknowledgments
The authors recognize that not all of the excellent scientific work in this area could be included or cited due to the vast literature on the subject and space limitations. This research was supported by grants to CL (NIA R01-AG17994 and AG21042) and the University of Florida Institute on Aging and Claude D. Pepper Older Americans Independence Center (1 P30AG028740).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Marzetti, E., Wohlgemuth, S.E., Aulisa, A.G. et al. Calorie Restriction for Optimal Cardiovascular Aging: The Weight of Evidence. Curr Cardio Risk Rep 4, 340–346 (2010). https://doi.org/10.1007/s12170-010-0114-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12170-010-0114-8