Abstract
A rapid and reliable method based on liquid chromatography with UV detection has been developed here to determine the main organic acids in base and sparkling wines of the protected designation of origin Cava. Compounds have been separated by reversed-phase mode with a water/acetonitrile solution (95:5 v/v adjusted to pH 2). Figures of merit established at 210 nm are fully compatible with the wine analysis, with correlation coefficients better than 0.996, repeatabilities around 2%, and detection limits generally below 1 g L−1. A total of 53 base wine and 140 cava samples from different coupages have been analyzed. Compositional profiles of organic acids have been used as the source of analytical information for characterization and classification purposes. Results have shown that varietal and blending issues, malolactic fermentation, and tartaric acid stabilization affect the composition of organic acids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low molecular weight organic acids are important natural constituents of wines. Some of them are originally present in the grape while others appear during subsequent fermentation processes as a consequence of (bio)chemical reactions. For instance, tartaric, gluconic, malic, and citric acids come directly from the grape while succinic, fumaric, lactic, and acetic acids are mainly produced during the winemaking processes (Chidi et al. 2015). Tartaric acid is the main acid of wine, accounting for ca. 30% of the total acids (Sweetman et al. 2009). Tartaric acid is resistant to decomposition by bacteria, so its transformation into lactic and acetic acid is quite residual. Malic acid is microbiologically labile, thus resulting in lactic acid in the course of malolactic fermentation (Maicas 2001; Versari et al. 1999). Citric acid is another subtract of lactic bacteria so its concentration typically decays in the course of winemaking processes. Succinic and acetic acids are other secondary fermentative products, the latter being related to unwanted vinegary spoilage (Chidi et al. 2018). Gluconic acid is a minor component typically associated with an excessive fruit ripening so its occurrence at high concentration is often a sign of poor grape quality.
Organic acids strongly influence on some organoleptic features such as taste and equilibrium. In this way, acids give to wines a slightly tart flavor, but this can be modulated by alcohol, sugars, minerals, and other components. Organic acids are also relevant chemical descriptors of interest for quality control purposes (Ragone et al. 2015; Saurina 2010), providing information on origin, grape variety, microbiological growth, and oenological practices. Levels of acids may affect the color, taste, and aroma of the wine. Also, they influence on the stability and microbiological quality of the wine, stopping or, at least, delaying the growth of harmful microorganisms that could cause wine spoilage. The evolution of the acidity during the several stages of wine and cava production is used by the winemakers to know about the quality of the final product.
Traditionally, the wineries used potentiometric and volumetric methods to assess the total and volatile acidity of wines. The quantification of individual compounds such as tartaric, malic, lactic, acetic, and gluconic acids has been carried out by enzymatic, spectroscopic, and chromatographic methods (Mato et al. 2005; Sochorova et al. 2018). Enzymatic approaches are highly selective but may result in time-consuming and expensive analyses due to the need of specific reagents for each species (Sochorova et al. 2018; Zeravik et al. 2016; Mazzei et al. 2007). New devices based on gold and nanocomposite technologies have contributed to improve the detection (Monosik et al. 2012). Flow injection analysis has been used to facilitate the automation of the enzymatic processes combined with spectroscopic detection (Mataix and Luque de Castro 2001). Spectroscopic methods for multianalyte determination rely on Fourier transform infrared (FTIR) with further chemometric analysis by partial least squares (PLS) regression (Regmi et al. 2012; Pizarro et al. 2011).
Separation techniques result in one of the most convenient approaches for the simultaneous determination of a wide range of the organic acids in wine samples (Mato et al. 2005; Sochorova et al. 2018). Among them, HPLC is the most common technique since the pioneering studies by Palmer and Lis (1973). Regarding the separation in HPLC, reversed-phase (RP) and ion exchange modes have been extensively used, combined with UV spectrophotometric, refractive index, and electrochemical detection (Li et al. 2018; Coelho et al. 2018; Zheng et al. 2009; Kerem et al. 2004). Recently, new RP stationary phases with alkyl (e.g., C18) groups have been especially designed to retain a wide range of hydrophilic species using eluents with high percentages of water (up to 100%). These RP alkyl columns rely on silanol endcapping with trimethylsilyl groups to provide good stability and full compatibility with polar solvents like water. As a result, the use of this type of columns in current analytical laboratories has been consolidated and numerous studies have been published in this regard (Long et al. 2009; Dos Santos Lima et al. 2019). Anyway, despite the excellent performance of these columns, the separation of food components is difficult and various analytical issues remain still unresolved, such as the complex retention behavior of analytes as a function of the pH of the mobile phase, and the diversity of interfering species occurring in the sample. In this regard, sample pretreatments such as dialysis and electrodialysis coupled to HPLC can provide better results (Kritsunankul et al. 2009; Ohira et al. 2014). Apart from HPLC, gas chromatography (GC) and capillary electrophoresis (CE) have also been used for the determination of organic acids in wines. In the case of GC, analytes must be derivatized to decrease their polarity and increase volatility using, for instance, silanization reactions (Zhang et al. 2018). CE, in contrast, is envisaged as a natural separation mode for charged molecules such as organic acids so that several papers have been published on this topic (Rovio et al. 2011; Peres et al. 2009; Mato et al. 2007).
Cava is a type of sparkling wine of high quality with protected designation of origin (PDO) produced by the Champenoise method. Cava is gaining popularity in our society because of its excellent organoleptic features thus, currently, resulting in the most exported Spanish wine (Buxaderas and Lopez-Tamames 2012; http://www.institutdelcava.com/en/, 2020). Cava starts from base wines conveniently blended which are subjected to a second fermentation taking place in the bottle, followed by an aging period for a minimum of 9 months in the cellar before commercialization. Although the classical coupage is composed of Macabeu (Ma), Xarel.lo (Xa), and Parellada (Pa) varieties, in the last years, new varieties of white and red grapes have been introduced, such as Chardonnay (Cha), Pinot Noir (PN), Trepat (Tr), and Garnacha (Ga), the latter producing rosé products (Izquierdo-Llopart and Saurina 2019).
In this paper, a new HPLC method with UV detection has been developed to determine organic acids of low molecular weight in base wine and cava samples. The analytical method has been optimized carefully to improve detection and separation features. Analytical parameters such as linearity, detection limits, and repeatability have been established under optimal working conditions. Here, fingerprints by HPLC-UV and compositional profiles related to organic acids have been exploited as the source of information for characterization purposes. The corresponding data sets have been analyzed using radial diagrams and principal component analysis (PCA). Patters among chemical composition and oenological features have been encountered, thus demonstrating the applicability of the method to the characterization and quality control of these products.
Materials and Methods
Chemicals and Solutions
Phosphoric acid (85% w/w, Merck), acetonitrile (UHPLC PAI-ACS SuperGradient, Panreac, Castellar de Valles, Barcelona, Spain) and Milli-Q water (Millipore Bedford) were the components of the mobile phase. Reagents for the preparation of organic acid standards were tartaric, malic, citric, succinic, fumaric, gluconic, acetic, and lactic acids (analytical reagent grade, Merck, Darmstadt, Germany). Stock solutions at a concentration of 10 g L−1 were prepared in Milli-Q water (from Milli-Q system, Millipore Bedford, USA). Standard working solutions were prepared in the range 1 to 8000 mg L−1. The highest one was prepared with Milli-Q water and the others by the appropriate dilution with the mobile phase.
Samples
Fifty-three base wines and 140 cava samples of different blends (coupages) were kindly provided by the winery Raventós Codorníu (Sant Sadurni d’Anoia, Barcelona, Spain). Base wines resulting from a first alcoholic fermentation in tanks were made with 10 different blends (see Table 1) of the following grape varieties: Macabeu (Ma), Xarel·lo (Xa), Parellada (Pa), Chardonnay (Cha), Monastrell (Mo), Pinot Noir (PN), Garnatxa negra (Ga), and Trepat (Tr). All blends were subjected to malolactic fermentation (MLF), with the exception of coupage I. Cava samples resulting from a second fermentation of base wines consisted of 11 coupages as indicated in Table 2. They were the same as those previously defined in Table 1 except for the additional coupage K, analogous to coupage A but with 15–30 months of aging period. A quality control (QC) for the set of base wines was prepared by mixing 100 μL of each wine sample. In the same way, another QC for the series of cava samples was prepared. QCs were analyzed repeatedly every 10 sample injections to detect and minimize possible chromatographic variations and evaluate the soundness of PCA models. All the samples were degasified and filtered through 0.45-μm nylon filters (Whatman, Clifton, NJ, USA) prior to the analysis.
Chromatographic Method
An Agilent HPLC 1100 LC system (Agilent Technologies, Santa Clara, CA, USA) equipped with quaternary pump (G1311A model), vacuum degasser (G1379A model), autosampler (G1392A model), and diode array detector (DAD, G1315B model) was used. Data was processed with an Agilent ChemStation for LC 3D (Rev. A. 10.02) offline software.
Analytes were separated in a C18 polar analytical column Zorbax SB-Aq (4.6 mm ID × 150 mm, 5-μm particle size, Agilent Technologies) under isocratic elution with acidified water/acetonitrile solution (95/5, v/v) adjusted to pH 2 with phosphoric acid. The column was set at room temperature, the injection volume was 10 μL, the flow rate was 1 mL min−1, and the run time was 5 min. The UV detection was performed at 210 nm. Apart from the selected column, the performance of the following columns was investigated during the optimization process: Kinetex C18 polar (Phenomenex, Torrance, CA, 100 mm × 4.6 mm I.D. with 2.6-μm particle size), Spherisorb S10 NH2 (Waters Corporation, Milford, MA, 250 mm × 4.6 mm I.D. with 5-μm particle size), XTerra® C18 (Waters, 150 mm × 4.6 mm I.D. with 3.5-μm particle size), Rezex Roa (Phenomenex, 150 mm × 7.8 mm I.D. with 8-μm particle size), and Syncronis TM HILIC (Thermo Fisher Scientific Inc., 100 mm × 4.6 mm I.D. with 5-μm particle size).
Data Analysis
Base wines and cavas were characterized according to their levels of organic acids as the source of analytical information. Samples were preliminary evaluated by radial plots obtained with Excel (Microsoft, Redmon WA, USA). PCA using the PLS-Toolbox (working under MATLAB, Applied Chemometrics, Inc., PO Box 100 Sharon, USA) was further applied to relate the organic acid contents with the wine classes.
Two different types of data matrices were analyzed by PCA under profiling and fingerprinting approaches, which consisted of organic acid concentrations and chromatograms at 210 nm, respectively. As the pretreatment, concentrations were autoscaled to equalize the descriptive ability of each variable; in fingerprinting, data was smoothed with a Savitzky–Golay filter (second degree fitting, 11-point window) and normalized (vector normalization of each chromatogram within the working time window). In any case, the plot of scores showed the distribution of the samples on the principal components (PCs), thus revealing trends on the varieties and blends of base wines and cavas. The variability of the experimental data was assessed from the dispersion of the QCs which should appear in a compact group in the middle of the score plot. The plot of loadings showed the distribution of variables and their impact on the sample features.
Results and Discussion
Optimization of the Chromatographic Conditions
First studies were focused on the optimization of the detection and separation conditions of the HPLC-UV method. The detection of organic acids by UV spectroscopy is, in general, difficult because of the quite poor absorption features of these analytes in UV range. Apart from fumaric and lactic acids which displayed a reasonable absorptivity above 240 nm, the other analytes were detected at 210 nm.
The separation of organic acids by HPLC was envisaged as a complex issue owing to the high polarity of analytes. Here, separation conditions were first optimized using pure analyte standards, including, acetic, lactic, fumaric, tartaric, malic, succinic, gluconic, and citric acids. Several analytical columns were investigated, covering a wide range of interaction mechanisms such as hydrophobicity, hydrophilicity, and anion exchange. A preliminary study was carried out to select the most promising columns and disregard the less satisfactory ones.
In the first screening, RP columns (e.g., Kinetex C18, Kinetex C18 polar, and XTerra C18) were tested using hydro-organic solutions (acetonitrile percentage from 0 to 1% v/v) acidified with phosphoric and sulfuric acids in the pH range from 1.0 to 7.0 (pH adjusted with a sodium hydroxide solution). Some of these columns were successfully proposed for the study of polar compounds so they were considered here for a preliminary evaluation (Snow et al. 2015). In our study, the elution mode was isocratic, the flow rate was 0.5 mL min−1, and the column temperature ranged from 23 to 60 °C. Results obtained indicated that the separation was not entirely satisfactory and several compounds co-eluted.
The weak anion exchange column assayed (Spherisorb S10 NH2) consisted of aminopropyl groups chemically linked on silica particles. The separation was investigated at different pH values in the range from 1.4 to 8.0 using a mobile phase of 0.5 mmol L−1 phosphoric acid (pH adjusted with sodium hydroxide solution). The elution mode was isocratic and the flow rate was 0.5 mL min−1. The retention behavior depended on both the protonation of the exchanger occurring below pH 8 and deprotonation of analytes. As a result, it was found that analytes co-eluted at pH 1.5 because of their poor interaction with the exchanger; the interaction increased with pH up to 6.5 due to the formation of carboxylate anions of analytes, and finally decayed at pH 8.0 due to the loss of exchange ability of the column. The retention behavior was complex, especially for polyprotic compounds as multiple charged species were involved. Besides, proper separation conditions without peak overlapping could not be found. Other conditions such as column temperature (in the range 20 to 80 °C) and addition of acetonitrile as an organic modifier (from 0 to 10% v/v) were also investigated. Anyway, although the retention varied with these factors, the selectivity was seldom modified so that the separation of co-eluting compounds was not improved.
The possibilities of size exclusion as the separation mechanism were investigated using a sulfonated polymeric Rezex ROA column. The mobile phase consisted of 2.5 mmol L−1 sulfuric acid solutions (pH was adjusted to 1.4, 2.5, and 6.0 using sodium hydroxide solution). The column temperature was set to 20 and 80 °C and the flow rate was 1.5 mL min−1. The separation of most organic acids, evaluated from pure standards, was successful, with chromatograms displaying good resolutions and peak symmetries. The best separation was obtained at pH 2.5, although double peaks were obtained for various compounds. Anyway, chromatographic results were not fully satisfactory when dealing with wine samples. This finding was attributed to the higher complexity of the wine matrix and the occurrence of interferences, possibly from phenolic acids. This column was finally discarded because of the coelution of the analytes with other matrix components.
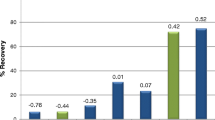
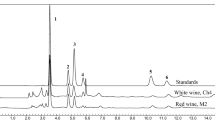
The separation performance of a polar alkyl-based column (Zorbax SB-Aq) was also investigated in detail as follows. In recent years, the use of this type of columns has become more popular and numerous studies have been published in this regard. In particular, this RP stationary phase was conceived to retain a wide range of compounds, especially hydrophilic species, using high percentages of water in the mobile phase (Long et al. 2009). In this case, analytes should be neutral to enhance their interactions with the stationary phase. The mobile phase consisted of 20 mmol L−1 phosphoric acid solution and pH was varied from 2.0 to 3.0 to protonate the carboxylic groups. The flow rate was 1 mL min−1. It was found that at pH 2.0, all the components were reasonably separated while at pH 3.0 the retention decreased and some overlapping peaks occurred. The effect of the addition of acetonitrile to the organic phase was studied in the range 0 to 10% v/v. Results shown in Fig. 1 indicate that retention decreased with increasing the organic solvent content. An optimal compromise among separation and analysis time was obtained with 5% acetonitrile, so this composition was chosen for further experiments. As an example, chromatograms of standard solutions of the organic acids and representative white and rosé cavas are depicted in Fig. 2. It can be seen that compounds were successfully resolved and compositional profiles of samples showed differences that could be exploited for descriptive purposes.
Chromatograms of a standard solution of 200 mg L−1 each organic acid as a function of the acetonitrile percentage in the mobile phase. Conditions: Agilent Zorbax SB-Aq column; 20 mmol L−1 phosphoric acid at pH 2; injection volume 10 μL; flow rate 1 mL min−1; run time 5 min; detection at 210 nm. Peak assignation: (1) gluconic acid, (2) tartaric acid, (3) malic acid, (4) lactic acid, (5) acetic acid, (6) citric acid, (7) succinic acid
Chromatograms of a standard solution (a) and a white base wine (b) and a rosé base wine (c). Conditions: Agilent Zorbax SB-Aq column; 20 mmol L−1 aqueous phosphoric acid (pH 2)/acetonitrile 95:5 v:v; injection volume 10 μL; flow rate 1 mL min−1; run time 5 min; detection at 210 nm. Standard composition: 200 mg L−1 tartaric and succinic acids; 50 mg L−1 acetic acid; 25 mg L−1 citric, lactic, gluconic, and malic acids; and 2.5 mg L−1 fumaric acid. Peak assignation: (8) fumaric acid, see Fig. 1 for the others
Method Validation
Quality parameters of the proposed HPLC-UV method were established with pure organic acid standards and selected wine and cava samples. Results have been summarized in Table 3. The linear range of the calibration was established from the injection of 10 standard solutions with different analyte concentrations, namely 20 to 2000 mg L−1 for acetic, succinic, and gluconic acids; 10 to 2000 mg L−1 for citric acid; 10 to 5000 mg L−1 for lactic acid; 5 to 5000 mg L−1 for malic acid; 10 to 8000 mg L−1 for tartaric acid; and 1 to 500 mg L−1 for fumaric acid. Calibration models obtained by least square regression displayed excellent linearity, with determination coefficients better than 0.993. The repeatability of the method was evaluated from 10 replicated injections of a standard mixture of 200 mg L−1 for tartaric and succinic acids; 50 mg L−1 for acetic acid; 25 mg L−1 for citric, lactic, gluconic, and malic acids; and 2.5 mg L−1 for fumaric acid. The relative standard deviation (RSD%) in terms of retention time was below 0.3% and around 6% in terms of peak area. Limits of detection (LODs) and quantification (LOQs) were estimated from 10 replicated injections of a standard solution of the different analytes at 50 mg L−1 each, except for fumaric acid which was assayed at 5 mg L−1. LODs and LOQs were calculated at signal-to-noise ratios of 3 and 10, respectively. It should be pointed out that these values were in the order of magnitude of milligrams per liter, fully compatible with the typical levels of organic acids in the wine and cava samples. The accuracy of the method was studied from a spiking/recovery procedure in which a representative sample was spiked with the organic acids at the levels specified for the study of the method repeatability. The mean recovered concentration from a series of 6 independent replicates calculated as a percentage (crecovered/cspiked × 100) was used to express the accuracy values, crecovered and cspiked being the calculated and added concentrations, respectively. Results were in the range 89 to 111%, thus indicating that the method proposed was suitable for the analysis of wine and cava samples.
The performance of the proposed method was compared with other recent publications dealing with the determination of organic acids in wines by HPLC and related techniques (see Table 4). As can be seen, tartaric, malic, lactic, acetic, citric, and succinic acids were commonly quantified as they were the most relevant compounds. Anyway, in some cases, other specific acids were investigated such as shikimic, glucuronic, and glucaric. In general, RP mode with stationary especially adapted for polar species was the choice of several authors. Alternatively, ion exchange mode was another explored possibility. Regarding detection, UV at 210 nm was widely used, providing LODs in the range 0.1 to 10 mg L−1; in general, these values were higher than those reported here. Electrochemical and refractive index detectors improved LOD values in one order of magnitude, approx. LC-MS platforms provided additional advantages such as improved sensitivity and selectivity, and allowed new compounds to be identified, of course, at the expense of more complex and expensive assays. Other analytical parameters of our method, such as linearity, repeatability, and accuracy, were similar values previously published (see Table 4). Regarding runtime, our proposed method allowed quite fast analyses thus being especially suitable for dealing with the study of large series of samples for quality control and authentication issues. In summary, our proposal seems to be a good option for the determination of organic acids in wine matrices given its great analytical performance, standing out for its simplicity, low cost, robustness, and speed.
Characterization of Base Wine and Cava Samples from the Protected Designation of Origin Cava
Compositional Profiles of Organic Acids
Average concentrations of organic acids in each coupage of the base wine and cava samples are given in Tables 5 and 6, respectively. This data has been used to plot several radial diagrams of organic acid concentrations depending on the classes (see Fig. 3). By far, tartaric acid is the most abundant acid in base wines and cavas due to the high levels occurring in the grapes. Tartaric acid concentrations in base wines were quite disperse, ranging from 6.48 to 10.18 mg L−1, with highest concentrations for PN and BN (without MLF). More homogeneous values were found in cavas (from 4.6 to 5.7 mg L−1), thus indicating that the descriptive ability of this variable for cava discrimination was poor. Besides, a noticeable decrease in tartaric acid concentration was found (ca. 35% lower) when comparing cavas and base wines. This decay was attributed to the tartaric stabilization process to which the base wine was subjected before performing the second fermentation. This oenological process was focused on limiting the quantity of potassium bi-tartrate and neutral calcium tartrate in the final products to avoid further precipitation.
Radial diagrams of organic acid concentrations in the different coupages. Coupage: Ma/Xa/Pa, Macabeu, Xarel·lo and Parellada; Cha, Chardonnay; PN, Pinot Noir; Mo/Ga/Tr, Monastrell, Garnatxa and Trepat; BN, Blanc de Noirs; ML, Malolactic fermentation. Compound assignation: a tartaric acid, b malic acid, c lactic acid, d citric acid, e succinic acid, f gluconic acid. Black color corresponds to base wines and orange to cava samples
From the quantitative point of view, malic acid was the second most important acid in these samples. Before MLF, this compound occurred at concentrations from 2 to 4 g L−1, approximately, in base wines and below 1 g L−1 in cavas. Exceptionally, coupage I, which was not subjected to MLF, displayed similar concentrations in both base wine and in cava samples. Regarding the influence of grape varieties on the malic acid content, it was evidenced that base wines from Cha variety were especially rich while the classical blend (Ma, Xa, and Pa) and the rosé combination (Mo, Ga, and Tr) showed the lowest levels. Inversely correlated with the evolution of malic acid, lactic acid was mainly generated by the action of lactic bacteria during the MLF process. Thus, the amount of lactic acid in coupage I was lower than that in other blends because of the absence of MLF.
Citric acid was present in base wines at concentrations ranging from 0.5 to 1.7 g L−1 depending on the blend. This acid decays significantly when the wine is fermented by lactic bacteria because it is a sensitive substrate to this type of microorganisms. Levels from 0.10 to 0.32 g L−1 were found in cava samples. For varietal comparison, base wines of coupages elaborated with high percentages of the Cha variety (e.g., G, S, and A) displayed high values of citric acid since the corresponding grapes are richer in this component.
Succinic acid is very stable in front of microbiological processes, so its evolution throughout vinification and aging is quite irrelevant. As it can be seen, concentrations in base wines and cavas are similar, in the range from 0.5 to 0.7 g L−1, approximately. In cavas, for instance, the comparison of 9-month and 18-month aged samples with the same varietal composition (e.g., coupages A, G and K) revealed almost identical concentrations.
Fumaric and acetic acids were below the detection limits so they were irrelevant for descriptive purposes. In the acetic acid case, this finding indicates that MLF was done under optimal conditions. Gluconic acid appears in ripe fruits as an indicator of grape putrefaction. Values higher than 0.6 g L−1 have been associated with spoiled grape which is not recommendable for wine production. In our sets of samples, gluconic acid concentrations were lower than 0.5 g L−1. For descriptive purposes, for instance, combinations with red grapes and Cha varieties presented higher values than those from classical blends.
Principal Component Analysis
In order to carry out a more comprehensive characterization of wines as a function of the contents of organic acids, PCA was applied using both chromatographic fingerprints and concentration profiles as the source of information.
First models were established from chromatograms recorded at 210 nm. Data was preprocessed by smoothing and normalization to minimize the influence of the overall intensity on the description. In the case of base wines, the distribution of samples as a function of blends was better visualized from the scatter plot of PC1 versus PC4 (see Fig. 4a). Results showed that QC samples appeared in a compact group in the middle of the graph, thus indicating the excellent reproducibility of chromatographic data as well as the descriptive ability of the model. A good separation between rosé and white wines was observed across PC1, with wines rich in red grape varieties predominating on the left part and those from white varieties (blends of Ma, Xa, Pa, and Cha) located on the right side. PC4 mainly discriminated among white coupages, with the three classical varieties to the bottom and Cha to the top part.
Regarding cava wines, Fig. 4 b shows the corresponding plot of scores. In contrast to the base wine description, here the discrimination of samples as a function of grape varieties and blends was not so well defined. This finding was attributed to the correction in the organic acid contents before the second fermentation of the traditional Champenoise method from which levels of acids tended to be more similar. As a result, although samples belonging to the same coupage were clustered, some overlapping among classes was found so that the relevance of organic acids as the descriptors of cava classes was limited.
Profiling data consisting of concentrations of organic acids in base wine and cava samples was also evaluated by PCA. For base wines, the data matrix consisted of the contents of the 8 acids in the 53 samples plus the QCs regularly injected (every 10 samples). Data was autoscaled before PCA to equalize the influence of major and minor components on the description. Three PCs were able to explain ca. 90% of the experimental variance (with PC1 retaining a 36.98%, PC2 a 25.76%, and PC3 a 19.71%). The plot of scores (Fig. 5a) suggested that PC1 mainly separated wines according to the content of Cha in the blends. Hence, the monovarietal Cha was on the right while the Ma, Xa, and Pa mixing appeared in the opposite site. Correspondingly, coupages with a low percentage of Cha appeared close to the classical varieties while those richer in Cha tended to the right. The application of MLF was clearly distinguished form PC3, with base wines, with BN without MLF found in a compact group to the top and the treated ones to the bottom part. Information gained for the loading plot indicated that malic acid was abundant in BN without MLF, citric acid in classes with the Cha, and succinic acid in classical coupages (Ma, Xa, and Pa). Finally, wines from red grapes presented increased levels of gluconic acid. These results were in agreement with preliminary conclusions extracted from radial diagrams (see in the “Compositional Profiles of Organic Acids” section).
Results of principal component analysis from the study of organic acid of base wines. Plot of scores (a) and plot of loading (b). Wine assignation: see Fig. 4
In the case of cavas, the corresponding matrix was composed of organic acid concentrations of 140 samples belonging to 11 classes of different blends and the QCs. Results by PCA (not given here) showed that PC1 and PC2 explained 38.80% and 17.94% of the experimental variance, respectively. Regarding the sample distribution, QCs were clustered in the center of the model and cavas from the same coupage were grouped together. Unfortunately, although some general patterns could be deduced, no clear separation among blends was encountered with the exception of non-MLF (right side) versus MLF (left side) classes. As above-indicated, this finding was attributed to the fact that organic acid concentrations in the cava samples were quite similar regardless of blends since they were oenological corrected to obtain more homogeneous lots from an organoleptic point of view.
Conclusions
The HPLC-UV method developed here was applied successfully to the determination of organic acids in base wine and cava samples. Among the diverse separation possibilities that could be suitable to address the separation, including reversed-phase, HILIC, and anion exchange, the reversed-phase mode especially adapted to polar compounds provided the best results. Hence, analytes were chromatographically resolved without interferences from other endogenous wine species. The exploratory study of compositional profiles of organic acids revealed important differences among base wines and cavas. In particular, analyte amounts in base wines depended on the blends and grape varieties while their composition in the set of cavas was quite homogeneous (and lower). This behavior was attributed to the corrective actions applied to the cava production (including malolactic fermentation, tartaric stabilization, second fermentation, etc.) which equalized the acidity features.
Data from both chromatographic fingerprints and compositional profiles were treated by PCA to gain overall information on organic acid descriptors. Results from the two approaches were similar, in agreement with previous conclusions extracted from radial diagrams. Hence, we believe that organic acids may result in useful descriptors of varieties and blends at the stage of base wine but they offer limited possibilities to discriminate among cava classes because of the corrective oenological processes applied to the winemaking procedure.
References
Buxaderas S, Lopez-Tamames E (2012) Sparkling wines: features and trends from tradition. In: Henry J (ed) Book Series: Advances in Food and Nutrition Research, vol 66, pp 1–45
Chidi BS, Rossouw D, Buica AS, Bauer FF (2015) Determining the impact of industrial wine yeast strains on organic acid production under white and red wine-like fermentation conditions. S Afr J Enol Vitic 36:316
Chidi BS, Bauer FF, Rossouw D (2018) Organic acid metabolism and the impact of fermentation practices on wine acidity - a review. S Afr J Enol Vitic 39:315
Coelho EM, Padilha CVD, Miskinis GA, de So AGB, Pereira GE, de Azevedo LC, Lima MD (2018) Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: method validation and characterization of products from northeast Brazil. J Food Compos Anal 66:160–167
de Souza JC, da Silva JL, Fabrao RM, Stradiotto NR, Zanoni MVB (2019) Electroactive sugars, organic acids and sugar alcohol analysis in wine using anion-exchange chromatography with electrochemical detection. Microchem J 147:972–978. https://doi.org/10.1016/j.microc.2019.04.010
http://www.institutdelcava.com/en/ (accessed 24th January, 2020).
Ivanova-Petropulos V, Petruseva D, Mitrev S (2020) Rapid and simple method for determination of target organic acids in wine using HPLC-DAD analysis. Food Anal Methods 13:1078–1087. https://doi.org/10.1007/s12161-020-01724-4
Izquierdo-Llopart A, Saurina J (2019) Characterization of sparkling wines according to polyphenolic profiles obtained by HPLC-UV/Vis and principal component analysis. Foods 8:22
Kerem Z, Bravdo BA, Shoseyov O, Tugendhaft Y (2004) Rapid liquid chromatography-ultraviolet determination of organic acids and phenolic compounds in red wine and must. J Chromatogr A 1052:211–215
Kritsunankul O, Pramote B, Jakmunee J (2009) Flow injection on-line dialysis coupled to high performance liquid chromatography for the determination of some organic acids in wine. Talanta 79:1042–1049
Li FF, Bao Y, Yu YX, Cui H, Fa Y, Liu H (2018) Simultaneous determination of organic acids, inorganic anions, and alditols in wine with valve-switching ion chromatography. Chromatographia 81:1103–1108
Lima MD, Nunes PC, Silva BDD, Padilha CVD, do Bonfim THF, Stamford TLM, Vasconcelos MAD, Aquino JD (2019) Determining 1-kestose, nystose and raffinose oligosaccharides in grape juices and wines using HPLC: method validation and characterization of products from Northeast Brazil. J Food Sci Technol 56:4575–4584. https://doi.org/10.1007/s13197-019-03936-x
Long WJ, Brooks AE, Biazzo W (2009) Analysis of polar compounds using 100% aqueous mobile phases with Agilent ZORBAX Eclipse Plus Phenyl-Hexyl and other ZORBAX Phenyl Columns. Application note. Agilent Technologies Inc., Santa Clara, CA, USA, pp. 1-7. Available at https://www.agilent.com/cs/library/applications/5990-3616EN.pdf.
Maicas S (2001) The use of alternative technologies to develop malolactic fermentation in wine. Appl Microbiol Biotechnol 56:35
Mataix E, Luque de Castro MD (2001) Determination of L-(-)-malic acid and L-(+)-lactic acid in wine by a flow injection-dialysis-enzymic derivatisation approach. Anal Chim Acta 428:7–14
Mato I, Suarez-Luque S, Huidobro JF (2005) A review of the analytical methods to determine organic acids in grape juices and wines. Food Res Int 38:1175–1188
Mato I, Suarez-Luque S, Huidobro JF (2007) Simple determination of main organic acids in grape juice and wine by using capillary zone electrophoresis with direct UV detection. Food Chem 102:104–112
Mazzei F, Botre F, Favero G (2007) Peroxidase based biosensors for the selective determination of D,L-lactic acid and L-malic acid in wines. Microchem J 87:81–86
Monosik R, Stred’ansky M, Greif G, Sturdik E (2012) Comparison of biosensors based on gold and nanocomposite electrodes for monitoring of malic acid in wine. Cent Eur J Chem 10:157
Ohira SI, Kuhara K, Shigetomi A, Yamasaki T, Kodama Y, Dasgupta PK, Toda K (2014) On-line electrodialytic matrix isolation for chromatographic determination of organic acids in wine. J Chromatogr A 1372:18–24
Palmer JK, Lis DM (1973) Determination of organic acids in foods by liquid chromatography. J Agric Food Chem 21:903–906
Pereira V, Camara JS, Cacho J, Marques JC (2010) HPLC-DAD methodology for the quantification of organic acids, furans and polyphenols by direct injection of wine samples. J Sep Sci 33:1204–1215. https://doi.org/10.1002/jssc.200900784
Peres RG, Moraes EP, Micke GA, Tonin FG, Tavares MFM, Rodriguez-Amaya DB (2009) Rapid method for the determination of organic acids in wine by capillary electrophoresis with indirect UV detection. Food Control 20:548–552
Pizarro C, Gonzalez-Saiz JM, Esteban-Diez I, Orio P (2011) Prediction of total and volatile acidity in red wines by Fourier-transform mid-infrared spectroscopy and iterative predictor weighting. Anal Bioanal Chem 399:2061–2072
Ragone R, Crupi P, Piccinonna S, Bergamini C, Mazzone F, Fanizzi FP, Schena FP, Antonacci D (2015) Classification and chemometric study of Southern Italy monovarietal wines based on NMR and HPLC-DAD-MS. Food Sci Biotechnol 24:817–826
Regmi U, Palma M, Barroso CG (2012) Direct determination of organic acids in wine and wine-derived products by Fourier transform infrared (FT-IR) spectroscopy and chemometric techniques. Anal Chim Acta 732:137–144
Ricciutelli M, Moretti S, Galarini R, Sagratini G, Mari M, Lucarini S, Vittori S, Caprioli G (2019) Identification and quantification of new isomers of isopropyl-malic acid in wine by LC-IT and LC-Q-Orbitrap. Food Chem 294:390–396. https://doi.org/10.1016/j.foodchem.2019.05.068F
Rovio S, Siren K, Siren H (2011) Application of capillary electrophoresis to determine metal cations, anions, organic acids, and carbohydrates in some Pinot Noir red wines. Food Chem 124:1194–1200
Saurina J (2010) Characterization of wines using compositional profiles and chemometrics. Trends Anal Chem 29:234–245
Snow L, Trass M, Klein M, Orlowicz S, Rivera B (2015) Fast and robust analysis of organic acids from wine using HPLC-UV. Application note TN-1189, pp.1-8. Phenomenex, Torrance, CA, USA. Available at https://az621941.vo.msecnd.net/documents/0d4a0bb0-f83c-4a26-b928-43649c3ed58a.pdf.
Sochorova L, Torokova L, Baron M, Sochor J (2018) Electrochemical and others techniques for the determination of malic acid and tartaric acid in must and wine. Int J Electrochem Sci 13:9145
Sweetman C, Deluc LG, Cramer GR, Ford CM, Soole KL (2009) Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 70:1329–1344
Tasev K, Stefova M, Ivanova-Petropulos V (2016) HPLC method validation and application for organic acid analysis in wine after solid-phase extraction. Maced J Chem Chem Eng 35:225–233. https://doi.org/10.20450/mjcce.2016.1073
Versari A, Parpinello GP, Cattaneo M (1999) Leuconostoc oenos and malolactic fermentation in wine: a review. J Ind Microbiol Biotechnol 23:447–455
Zeravik J, Fohlerova Z, Milovanovic M, Kubesa O, Zeisbergerova M, Lacina K, Petrovic A, Glatz Z, Skladal P (2016) Various instrumental approaches for determination of organic acids in wines. Food Chem 194:432–440
Zhang XK, Lan YB, Zhu BQ, Xiang XF, Duan CQ, Shi Y (2018) Changes in monosaccharides, organic acids and amino acids during Cabernet Sauvignon wine ageing based on a simultaneous analysis using gas chromatography-mass spectrometry. J Sci Food Agric 98:104–112
Zheng YJ, Duan YT, Zhang YF, Pan QH, Li JM, Huang WD (2009) Determination of organic acids in red wine and must on only one RP-LC-column directly after sample dilution and filtration. Chromatographia 69:1391–1395
Zotou A, Loukou Z, Karava O (2004) Method development for the determination of seven organic acids in wines by reversed-phase high performance liquid chromatography. Chromatographia 60:39–44. https://doi.org/10.1365/s10337-004-0330-9
Acknowledgments
The authors thank Raventós Codorníu SA for kindly providing the cava samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Anaïs Izquierdo Llopart declares that she has no conflict of interest. Aida Carretero declares that she has no conflict of interest. Javier Saurina declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with humans and animals performed by any of the authors.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Izquierdo-Llopart, A., Carretero, A. & Saurina, J. Organic Acid Profiling by Liquid Chromatography for the Characterization of Base Vines and Sparkling Wines. Food Anal. Methods 13, 1852–1866 (2020). https://doi.org/10.1007/s12161-020-01808-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01808-1