Abstract
A UPLC-fluorescence method has been developed to evaluate the concentration of ten biogenic amines, as dansylated derivatives, in food. The sample preparation consisted of a solid-liquid extraction with perchloric acid, followed by the dansylation of the extracted amines. The analysis was performed using an Acquity UPLC BEH C18 column on a UPLC Acquity system (Waters) with a fluorescence detector. Two reference materials consisting of tuna muscle incurred with histamine were used to evaluate the performances of the following method: selectivity, specificity, linearity, limits of detection and quantification, precision, and accuracy. The averages of the measured values were evaluated at 98.7% and 96.8% of the expected values, for the two materials. The developed method was applied to quantify biogenic amines in grilled meat from Beninese markets. The biogenic amines index was calculated for each sample. In this study, ten samples can be considered as fresh with values lower than 5 mg/kg, while one sample is considered as acceptable (16.9 mg/kg) and one sample is considered as spoiled (82.8 mg/kg). Tryptamine and 2-phenylethylamine samples were not detected but methylamine and putrescine were detected at concentrations lower than their limit of quantification. Serotonin, spermidine, and spermine were detected in all samples. No link between the biogenic amine concentrations and the cooking conditions was observed. Because the biogenic amines are not heat sensitive, the measured concentrations of biogenic amines in this study could be explained by bad hygienic conditions during meat storage before cooking. It means that the Beninese population may be exposed to sometimes high biogenic amines content, leading to allergies or other more serious health problems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biogenic amines (AB) are basic organic compounds, of low molecular weight, non-volatile and stable to heat (Alvarez and Moreno-Arribas 2014). AB can be aliphatic (methylamine, putrescine, cadaverine), aromatic (tyramine, 2-phenylethylamine), or heterocyclic (histamine) (Mohammed et al. 2016; Sagratini et al. 2012). These amines are called “biogenic” because they are formed by the action of living organisms. They can take part in different physiological processes such as the regulation of temperature, appetite, and blood pressure (Silla Santos 1996).
In food, they are formed by microorganisms either by enzymatic decarboxylation of amino acids or by the transamination of aldehydes and ketones (Alvarez and Moreno-Arribas 2014; Bilgin and Genccelep 2015). Several factors play an important role in the formation of biogenic amines in food. In addition to the availability of precursors (free amino acids), the accumulation of biogenic amines in food requires the presence of microorganisms with decarboxylation activity and favorable conditions to their growth and activity of decarboxylation (temperature, pH, salt concentration) (Yongsawatdigul et al. 2004).
Many symptoms are caused by AB-related intoxication; their severity varies depending on the amount of AB ingested: nausea and migraines, vomiting, diarrheas, hypotension, and cardiac arrhythmia (Tittarelli et al. 2019). The toxicity of histamine appeared to be enhanced by the presence of other amines such as cadaverine, putrescine, and tyramine (Linares et al. 2016; del Rio et al. 2017). Biogenic amines may also be considered as possible carcinogens because of their ability to form nitrosamines, which are known to be potentially carcinogenic (De Mey et al. 2014; Silla Santos 1996).
Some food products may contain high levels of these compounds; these products are dairy products, fish and its derivatives, meat and flesh products, cheese, and fermented beverages such as wine and beer (Mohammed et al. 2016; Bilgin and Genccelep 2015; Aquiles Lazaro et al. 2015). In non-fermented foods, the presence of biogenic amines above a certain threshold is considered as an indication of undesirable microbial activity and loss of freshness or too long shelf-life (Simon Sarkadi 2019).
According to literature, 8 biogenic amines are often found in food: histamine, tyramine, putrescine, cadaverine, tryptamine, 2-phenylethylamine, spermidine, and spermine (Lapa-Guimarães and Pickova 2004; Kim et al. 2009; Naila et al. 2010).
What is more, in fish and fishery products, the presence of serotonin associated with the presence of amine-forming bacteria with strong decarboxylation activity was observed (Kim et al. 2009). Similarly, trimethylamine oxide (TMAO) produced by the action of those bacteria may undergo different transformations depending on the storage conditions of fishery products and lead to the formation of compounds such as methylamine (Baliño-Zuazo and Barranco 2016).
In Europe, like all over the world, there is a serious concern about the consumption of food containing dangerous biocontaminants such as histamine and tyramine. Nowadays, histamine is regularly detected in food present in the European Union, according to the Rapid Alert System for Feed and Food (RASFF) of the European Commission (RASFF, 2019). Indeed, for the last 12 years, 674 notifications about histamine have been reported in the RASFF database, with histamine content up to 10000 mg kg−1. Among those 674 notifications, 665 were concerning fish, fish products, and fish sauce imported from Asian countries, while nine were concerning soy sauce (5), cheese (2), yeast extract (1), and canned pet food (1).
There is no legal limit fixed for histamine in other food or feed products than fishery products. Indeed, according to the Commission Regulation (EC) No 2073/2005, the amount of histamine is acceptable if the average level is less than 100 mg/kg for fishery products from species associated with a large quantity of histidine that is the species from the Scambidae, Clupeidae, Engaulidae, Coryphaenidae, Pomatomidae, or Scomberesocidae families (Commission of the European Communities 2005).
In meat and meat products, the most prevalent biogenic amines are putrescine, tyramine, cadaverine, and histamine. The only amines present at significant levels in fresh meat are spermidine and spermine (Cheng et al. 2016; Papageorgiou et al. 2018). The sum of putrescine, tyramine, cadaverine, and histamine levels, calculated as biogenic amines index (BAI), is often used to evaluate the freshness and quality of pork (Cheng et al. 2016).
In Benin, pork is highly consumed. Indeed, according to Ayssiwede et al. (2009), 48.40% of the population eats from 1.5 to 3 kg of pork per week, with an average level of consumption estimated at 1.2 kg meat/week or around 58 kg/person/year.
Considering all this, in this work, we shall present a quantitative method of analysis of the ten biogenic amines (Fig. 1) in meat, using Ultra-Performance Liquid Chromatography combined with fluorescence detection (UPLC-FLD). The method was applied to grilled pork samples purchased in Beninese markets, with 2 different traditional grilling techniques, to evaluate the amount of biogenic amines that could be found in grilled pork available in Benin.
Materials and Methods
Chemicals, Solvents, and Materials
Analytical standards of biogenic amines (tryptamine hydrochloride, tyramine hydrochloride, cadaverine dihydrochloride, spermine tetrahydrochloride, spermidine trihydrochloride, 2-phenylethylamine hydrochloride, putrescine dihydrochloride, histamine dihydrochloride, serotonin hydrochloride, and methylamine hydrochloride), glycine, dansyl chloride, and 1,7-diaminoheptane were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium hydroxide, Normapur quality trichloroacetic acid (TCA), ethanol, and perchloric acid 60% were provided by VWR International (West Chester, PA, USA). Pro analysis quality sodium hydrogen carbonate was from Merck (Darmstadt, Germany). HPLC quality acetone, LC-MS quality acetonitrile, methanol, and UPLC quality water were from Biosolve (Valkenswaard, The Netherlands).
A 15-mL Falcon polypropylene graduated conical tube with cap were commercially available from Greiner Bio-One (Germany). Acrodisc® 25-mm syringe filters (with 5-μm Versapor® membrane) were purchased from Pall Life Sciences, MI, USA.
Putrescine, methylamine, serotonin, cadaverine, histamine, spermine, spermidine, tyramine, 2-phenylethylamine, and 1,7-diaminoheptane individual stock solutions (10 mg/mL) were prepared by dissolving each biogenic amine standard in a 5% trichloroacetic acid (TCA) solution. Tryptamine solution (7.5 mg/ml) was prepared by dissolving tryptamine in ethanol containing 5% trichloroacetic acid (TCA). A working solution of 1,7-diaminoheptane at a concentration of 100 ng/μL was prepared by diluting a 10 mg/ml stock solution with TCA 5%. This solution was used as internal standard. A pool of the ten other amines was obtained by mixing volumes of 150–2000 μL of each stock solution with TCA 5% to 10 ml. All of the standard solutions were kept for a maximum of 6 months at + 4 °C.
Sample Extraction and Derivatization
The analysis of biogenic amines was realized by adapting the method from Kim et al. (2009). Two grams of fresh sample was cut in small pieces with a scalpel or blended until homogeneous in a mechanical blender (Moulinex, Germany) and placed into a 10-mL glass tube. If lyophilized sample were used, 0.4 g sample was weighed into a 10-mL glass tube and 1.6 g water was added to rehydrate the sample. The sample was then vortexed and let stand for 10 min for rehydration. Two hundred microliters of internal standard working solution was added in the tube and the tube was vortexed again. Then 2.3 mL perchloric acid 0.4 M was added and the tube was capped. The mixture was shaken 15 min on a rotating shaker and centrifuged (10 min, 3700g, room temperature). The supernatant was transferred into a 150-mL Falcon tube and 2.5 mL of perchloric acid 0.4 M were added to the sample, which was extracted and centrifuged once again. Both supernatants were combined and volume was adjusted to 6 mL with perchloric acid 0.4 M. The extract was vortexed and was filtered through an Acrodisc® filter. One milliliter was then transferred in a clean 15-mL Falcon tube where 200 μL NaOH 2 N and 300 μL of saturated NaHCO3 were added, with vortex of the tube after each addition.
The dansylation was realized by adding 2 mL dansyl chloride (10 mg/mL in acetone) and incubating the tubes at 70 °C for 15 min in a water bath. After dansylation, 100 μL glycine (150 mg/mL in water) was added to bind to the dansyl chloride in excess, the tube was vortexed and a second incubation during 15 min at 70 °C was realized. The tube was then centrifuged (5 min, 3700g at room temperature). Finally, 500 μL of the solution was poured into an injection vial which was capped and the samples were kept at 5 °C in the autosampler until analysis.
UPLC-Fluorescence Analysis
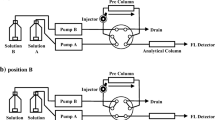
Amines were analyzed on a UPLC Acquity system integrated autosampler (Acquity Sample Manager FTN), solvent delivery system (Acquity QSM H Class), and column heater coupled to an Acquity Fluorescence detector, all from Waters Corporation (Milford, MA, USA). The column used was an Acquity UPLC BEH C18 (2.1 × 100 mm, 1.7 μm), with a UPLC BEH C18 VanGuard pre-column (2.1 × 5 mm, 1.7 μm), both from Waters Corporation. The mobile phase was water (solvent A) and acetonitrile (solvent B). The gradient elution conditions 70% solvent A were maintained for 1 min, from 70 to 15% of solvent A within 22 min, then conditions were held for 0.50 min and the contribution of solvent A was increased to 70% over 1 min and maintained for 1.50 min, with a total run time was of 26 min. The oven temperature was set at 65 °C and the injection volume was 5 μL. The flow rate was 0.4 ml/min.
The peaks were identified by comparing their retention times with those of the corresponding standards. Fluorimetric detection at 346 nm for excitation and 500 nm for emission was applied.
Results were calculated using Empower 3 Software (Waters Corporation).
Samples Used for the Matrix-Matched Calibration Curves
Using the developed method, pork bought in a Belgian supermarket was first lyophilized and then was evaluated as “blank” regarding its amine content. Pork samples were then used as blank samples and spiked quality control (QC) samples for the calibration curves.
For matrix-matched calibration curves, seven samples of rehydrated lyophilized pork spiked with the internal standard at a concentration of 5 mg kg−1 and with concentrations ranging from 0.75 to 33.75 mg kg−1 for spermidine and cadaverine; 1.25 to 56.25 mg kg−1 for spermine; 1.50 to 67.50 mg kg−1 for putrescine, methylamine, serotonin, and 2-phenylethylamine; 3.00 to 135.00 mg kg−1 for tryptamine; 3.50 to 157.50 mg kg−1 for tyramine; and 10.00 to 450.00 mg kg−1 for histamine were extracted simultaneously with the samples. The concentration range of the calibration curve was chosen to cover the range of concentrations observed for each biogenic amine analyzed in samples. The extracts of these seven samples were used to construct the calibration curves: the response (ratio between each amine and the internal standard peak areas) was plotted versus standard concentrations. Calibration points were injected before each series of samples and the extract spiked at a concentration corresponding to the central point of the calibration curve was injected one more time after all the samples.
Linear regression was used for all compounds except for histamine which required a quadratic regression. The correlation coefficients R2 associated with those curves were higher than 0.98. It was also established that only one point of the curve can deviate from the curve by more than 20% of the corresponding calculated value.
Performances of the Analytical Method
The use of Certified Reference Materials (CRM) is recommended to evaluate the performance of confirmatory methods used for organic residues and contaminants (Commission of the European Communities 2002). In our case, two reference materials (RM) made of incurred lyophilized tuna (T1530A and B) containing histamine at 2 different levels of concentrations (86.26 and 155.00 mg kg−1) were purchased from Test Veritas (Trieste, Italy). These reference materials were used to assess the performances of the developed method in accordance with the European (European Parliament and Council Directive No 2002/657/EC, 2002) and USFDA guidelines (US Department of Health and Human Services 2018). The RM were stored at − 20 °C until analysis and were analyzed with each series of samples as a control.
Beninese Pork Samples
A total of 12 grilled pork samples were obtained from local processors located in 5 different municipalities in the South of Benin.
Samples were selected according to the presentation of the meat (in skewers, in pieces, sliced, and stuffed) and the grilling technique (direct and indirect). The grilling technique used traditionally is direct grilling. Indeed, eight samples were prepared with this technique while 4 samples were prepared with the indirect grilling technique.
Around 150 g of muscle were bought for each sample on two different days.
Samples were stored frozen immediately after purchase. Then, samples were freeze-dried (Virtis) and stored in a desiccator until analysis.
Results and Discussion
Method Development
Extraction of biogenic amines in fish and fish products is well documented in the literature (Lapa-Guimarães and Pickova 2004; Kim et al. 2009; Tao et al. 2011; Latorre-Moratalla et al. 2009; Park et al. 2010). In most of these methods, biogenic amines are extracted with 0.1 N hydrochloric acid, 5% trichloroacetic acid, and 0.4 or 0.6 M perchloric acid. For meat extraction, only a few studies were published. Among them, Dadáková et al. (2009) suggest the use of 0.6 M perchloric acid. Different extraction conditions were tested: 0.1 N hydrochloric acid, 5% trichloroacetic acid, and 0.4, 0.5, and 0.6 M perchloric acid, with different volumes of extraction. Good extraction and the best chromatographic conditions were obtained with 2 × 2.5 mL of 0.4 N perchloric acid.
As LC eluent, a gradient of a mixture of acetonitrile/water 30/70 was used on an Acquity UPLC BEH C18 column. Optimization showed that the use of acetonitrile gave a higher sensitivity compared to methanol on this LC column. The 10 dansylated biogenic amines and the dansylated internal standard were separated in a run time of 26 min using the optimized UPLC-FLD parameters described in the “UPLC-Fluorescence Analysis” section. For all compounds, retention times were very stable. A good separation was achieved for the peaks of all compounds (Fig. 2).
Chromatograms of 10 amines as dansyl derivatives separated with a UPLC system and detected with fluorescence detection: a in a spiked “blank” pork sample, corresponding to the highest calibration curve point; b in a reference material, consisting lyophilized tuna muscle incurred with histamine, containing 86.26 mg kg−1 histamine
Performances of the Analytical Method
To evaluate the performances of the developed method, two available RM, consisting of lyophilized tuna muscle incurred with histamine at two different levels, were used. While no material consisting of meat or meat products is available for certifying the histamine or other biogenic amines content, the use of fish CRM was made.
Each of these materials was analyzed 10 times on different days (European Parliament and Council Directive No 2002/657/EC, 2002).
Selectivity and Specificity
Each compound was considered as positively identified in RM samples if the ratio between the chromatographic retention time of each analyte and that of the internal standard, i.e., the relative retention time (RRT) of each analyte, corresponded to that of the average retention time of the calibration solutions within a ± 2.5% tolerance, as set by the Commission Decision 2002/657/EC (European Parliament and Council Directive No 2002/657/EC, 2002).
Specificity was assessed by comparing chromatograms of blank meat samples with the CRM samples (Fig. 2).
Linearity
For calibration curves, the linear regression provided a good curve fitting, i.e., with low residuals, and demonstrated the linearity of the dose-response curve within the working range. Histamine content was, however, showing a non-linear behavior, leading to quadratic regression when calibration curves were established. For all biogenic amines, correlation coefficients R2 were higher than 0.99.
Limits of Detection and Quantification
The limit of quantification (LOQ) of each compound was set as the concentration of the lowest calibrator of the calibration curve (after checking that the signal to noise ratio was higher than 10 at that level) and corresponds to 0.75 mg kg−1 for spermidine and cadaverine; 1.25 mg kg−1 for spermine; 1.50 mg kg−1 for putrescine, methylamine, 2-phenylethylamine, and serotonin; 3.00 mg kg −1 for tryptamine; 3.50 mg kg −1 for tyramine; and 10.00 mg kg −1 for histamine. The limit of detection (LOD) was set at LOQ/2 after checking that the signal to noise ratio was higher than 3 at that level.
Precision and Accuracy
For the RM containing 86.26 mg kg−1 histamine, the average of the measured values (± standard deviation) (n = 10) was 85.12 ± 3.84 mg kg−1, corresponding to 98.7% of the expected value. For the RM containing 155.00 mg kg−1 histamine, the average of the measured values (± standard deviation) (n = 10) were 150.01 ± 10.1 mg kg−1, corresponding to 96.8% of the expected value.
Evaluation of Amines in Beninese Pork Samples
The UPLC-FLD method developed in this work was applied to evaluate the biogenic amines of twelve grilled pork samples purchased from local producers located in 5 different markets in the South of Benin. Samples were grilled, directly or indirectly, with wood or charcoal in different forms (in skewers, in pieces, sliced, and stuffed). The concentrations of biogenic amines measured in the samples are shown in Table 1. Concentrations are expressed as milligram of biogenic amine per kilogram wet weight muscle.
It can be observed that tryptamine and 2-phenylethylamine were not detected in all samples while methylamine and putrescine were detected but at concentrations lower than their limit of quantification, which was of 0.6 and 0.8 mg/kg, respectively. Serotonin, spermidine, and spermine were detected in all samples with concentrations ranging from 0.5 to 1.0 mg/kg, 1.4 to 6.3 mg/kg, and 7.9 to 21.8 mg/kg. According to Ntzimani et al. (2008), spermine and spermidine are two biogenic amines naturally formed in fresh pork. Tyramine was measured in one sample of pork grilled in skewers (1.3 mg/kg) and in one stuffed sample (3.8 mg/kg). Cadaverine was only detected in two samples, one grilled in pieces and the other stuffed, with 4.8 and 16.9 mg/kg, respectively. Histamine was only detected in the sample grilled in skewers with a high concentration of 81.5 mg/kg.
The BAI, biogenic amines index, was calculated for each sample by making the sum of the concentrations of putrescine, cadaverine, tyramine, and histamine (Table 1). According to Cheng et al. (2016), when the BAI values are lower than 5 mg/kg, between 5 and 20 mg/kg, between 20 and 50 mg/kg, or higher than 50 mg/kg, the pork samples are considered as fresh, acceptable, low quality, or spoiled, respectively. In our study, ten pork samples can be considered as fresh with values lower than 5 mg/kg, while one sample of stuffed pork is considered as acceptable (16.9 mg/kg) and one sample in skewers is considered as spoiled (82.8 mg/kg).
To our knowledge, no study shows the biogenic amine content measured in cooked pork samples. Anyway, since those compounds are reported as heat stable (Tapingkae et al. 2010) and cooking or prolonged exposure to heat will not destroy them (Naila et al. 2010) (Shalaby 1996; Duflos 2009; Gonzaga et al. 2009), the comparison of their content in fresh or cooked meat can be done.
In the literature, Santiyanont et al. (2019) evaluated the biogenic amine content in fermented pork from Thailand. For meat before fermentation, they observed on three different batch levels from not detected (nd) to 21.24 mg/kg for putrescine, from nd to 12.26 mg/kg for cadaverine, from nd to 14.33 mg/kg for histamine, no detection of tyramine, from nd to 2.57 for spermidine, and from 15.76 to 21.91 mg/kg for spermine. Similarly, Ngapo and Vachon (2017) studied the conservation of chilled Canadian pork and measured overall concentrations of putrescine of 5.69 mg/kg, cadaverine of 0.70 mg/kg, tyramine of 0.41 mg/kg, spermidine 1.77 mg/kg, and spermine 36.17 mg/kg.
The measured concentrations of biogenic amines in our study are in concordance with what is shown in the studies from Santiyanont et al. (2019) and Ngapo and Vachon (2017), even if the concentrations measured in Beninese samples are slightly higher for some compounds. This could be explained by bad hygienic conditions when storing meat before cooking, during cooking, or due to the use of some fermented sauce, marinade, or other ingredients that are put on the meat before cooking. It means that the Beninese population could be exposed to sometimes high biogenic amines content, leading to allergies or other more serious health problems.
Because the biogenic amines are not heat sensitive, it was not possible to establish a link between the cooking conditions applied to the different meat samples and the concentrations of biogenic amines measured.
Conclusion
The quantification of biogenic amines is a good indicator of the spoilage level of food matrices. In pork, the sum of putrescine, tyramine, cadaverine, and histamine, calculated as biogenic amines index, is often used to evaluate the freshness and quality of meat. The analytical method presented in this work brings something new compared to what is commonly found in the literature since this method allows quantifying, in the same run, ten biogenic amines in cooked pork samples using Ultra-Performance Liquid Chromatography combined with fluorescence detection. The developed method was used to evaluate the biogenic amine concentration in grilled pork samples, purchased in Beninese markets.
The BAI, biogenic amines index, was calculated for each sample. In our study, ten pork samples can be considered as fresh with values lower than 5 mg/kg, while one sample of stuffed pork is considered as acceptable (16.9 mg/kg) and one sample in skewers is considered as spoiled (82.8 mg/kg). No link between the biogenic amine concentrations and the cooking conditions was observed. Because the biogenic amines are not heat sensitive, the measured concentrations of biogenic amines in our study could be explained by bad hygienic conditions during meat storage or cooking. It means that the Beninese population is exposed to sometimes high biogenic amines content, leading to allergies or other more serious health problems.
References
Alvarez MA, Moreno-Arribas MV (2014) The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci Tech 39:146–155
Aquiles Lazaro C, Conte-Júnior CA, Canto AC, Guerra Monteiro ML, Costa-Lima B, Gomes da Cruz A, Teixeira Marsico E, Maia Franco R (2015) Biogenic amines as bacterial quality indicators in different poultry meat species. LWT Food Sci Technol 60:15–21
Ayssiwede SB, Mankor A, Missohou A, Abiola FA (2009) Commercialisation et consommation de la viande de porc au Bénin. Revue Africaine de Santé et de Productions animales 7:105–112
Baliño-Zuazo L, Barranco A (2016) A novel liquid chromatography–mass spectrometric method for the simultaneous determination of trimethylamine, dimethylamine and methylamine in fishery products. Food Chem 196:1207–1214
Bilgin B, Genccelep H (2015) Determination of biogenic amines in fish products. Food Sci Biotechnol 24:1907–1913
Cheng W, Sun DW, Cheng JH (2016) Pork biogenic amines index (BAI) determination based on chemometric analysis of hyperspectral imaging data. LWT Food Sci Technol 73:13–19
Commission of the European Communities (2002) Commission decision 2002/657/EC implementing council directive 96/23/EC, concerning the performance of analytical methods and the interpretation of results. EC Commission, Brussels
Commission of the European Communities (2005) Commission regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs
Dadáková E, Krˇízˇek M, Pelikánová T (2009) Determination of biogenic amines in foods using ultra-performance liquid chromatography (UPLC). Food Chem 116:365–370
De Mey E, De Klerck K, De Maere H, Dewulf L, Derdelinckx G, Peeters MC, Fraeye I, Vander Heyden Y, Paelinck H (2014) The occurrence of N-nitrosamines, residual nitrite and biogenic amines in commercial dry fermented sausages and evaluation of their occasional relation. Meat Sci 96:821–828
del Rio B, Redruello B, Linares DM, Ladero V, Fernandez M, Cruz Martin M, Ruas-Madiedo P, Alvarez MA (2017) The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem 218:249–255
Duflos G (2009) Histamine risk in fishery products. Bull Acad Vet Fr 162:241–246
Gonzaga VE, Lescano AG, AA H’a, Salmn-Mulanovich G, Blazes DL (2009) Histamine levels in fish from markets in Lima, Peru. J Food Prot 72:1112–1115
Kim MK, Mah JH, Hwang HJ (2009) Biogenic amine formation and bacterial contribution in fish, squid and shellfish. Food Chem 116:87–95
Lapa-Guimarães J, Pickova J (2004) New solvent systems for thin-layer chromatographic determination of nine biogenic amines in fish and squid. J Chromatogr A 1045:223–232
Latorre-Moratalla ML, Bosch-Fusté J, Lavizzari T, Bover-Cid S, Veciana-Nogués MT, Vidal-Carou MC (2009) Validation of an ultra high pressure liquid chromatographic method for the determination of biologically active amines in food. J Chromatogr A 1216:7715–7720
Linares DM, del Rio B, Redruello B, Ladero V, Cruz Martin M, Fernandez M, Ruas-Madiedo P, Alvarez MA (2016) Comparative analysis of thein vitro cytotoxicity of the dietary biogenic amines tyramine and histamine. Food Chem 197:658–663
Mohammed GI, Bashammakh AS, Alsibaai AA, Alwael H, El-Shahawi MS (2016) A critical overview on the chemistry, clean-up and recent advances in analysis of biogenic amines in foodstuffs. TrAC - Trends Anal Chem 78:84–94
Naila A, Flint S, Fletcher G, Bremer P, Meerdink G (2010) Control of biogenic amines in food-existing and emerging approaches. J Food Sci 75:R139–RR50
Ngapo TM, Vachon L (2017) Biogenic amine concentrations and evolution in “chilled” Canadian pork for the Japanese market. Food Chem 233:500–506
Ntzimani AG, Paleologos EK, Savvaidis IN, Kontominas MG (2008) Formation of biogenic amines and relation to microbial flora and sensory changes in smoked Turkey breast fillets stored under various packaging conditions at 4°C. Food Microbiol 25:509–517
Papageorgiou M, Lambropoulou D, Morrison C, Kłodzinska E, Namiesnik J, Płotka-Wasylka J (2018) Literature update of analytical methods for biogenic amines determination in food and beverages. Trends Anal Chem 98:128–142
Park JS, Lee CH, Kwon EY, Lee HJ, Kim JY, Kim SH (2010) Monitoring the contents of biogenic amines in fish and fish products consumed in Korea. Food Control 21:1219–1226
RASFF (2019) (http://ec.europa.eu/food/food/rapidalert/rasff_portal_database_en.htm, accessed 02/22/2019)
Sagratini G, Fernandez-Franzon M, De Berardinis F, Font G, Vittori S, Manes J (2012) Simultaneous determination of eight underivatised biogenic amines in fish by solid phase extraction and liquid chromatography-tandem mass spectrometry. Food Chem 132:537–543. https://doi.org/10.1016/j.foodchem.2011.10.054
Santiyanont P, Chantarasakha K, Tepkasikul P, Srimarut Y, Mhuantong W, Tangphatsornruang S, Zo YG, Chokesajjawatee N (2019) Dynamics of biogenic amines and bacterial communities in a Thai fermented pork product Nham. Food Res Int 119:110–118
Shalaby AR (1996) Significance of biogenic amines to food safety and human health. Food Res Int 29:675–690
Silla Santos MH (1996) Biogenic amines: their importance in foods. Int J Food Microbiol 29:213–231
Simon Sarkadi L (2019) Amino acids and biogenic amines as food quality factors. Pure Appl Chem 91:289–300
Tao Z, Sato M, Zhang H, Yamaguchi T, Nakano T (2011) A survey of histamine content in seafood sold in markets of nine countries. Food Control 22:430–432
Tapingkae W, Tanasupawat S, Parkin KL, Benjakul S, Visessanguan W (2010) Degradation of histamine by extremely halophilic archaea isolated from high salt-fermented fishery products. Enzyme Microbial Technol 46:92–99
Tittarelli F, Perpetuini G, Di Gianvito P, Tofalo R (2019) Biogenic amines producing and degrading bacteria: a snapshot from raw ewes’ cheese. LWT-Food Sci Technol 101:1–9
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM) (2018) Bioanalytical method validation. In: Guidance for industry
Yongsawatdigul J, Choi YJ, Udomporn S (2004) Biogenic amines formation in fish sauce prepared from fresh and temperature-abused Indian anchovy (Stolephorus indicus). J Food Sci 69:FCT312–FCT319
Funding
This study was performed with the financial support of ARES-CCD (Academy of Research and Higher Education—Committee for Development Cooperation, Belgium) (Project Qualisani).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Caroline Douny declares that she has no conflict of interest. Soumaya Benmedjadi declares that she has no conflict of interest. François Brose declares that he has no conflict of interest. O.H. Iko Afé declares that he has no conflict of interest. Ahmed Igout declares that he has no conflict of interest. D.J. Hounhouigan declares that he has no conflict of interest. Victor Bienvenu Anihouvi declares that he has no conflict of interest. Marie-Louise Scippo declares that she has no conflict of interest.
Human Participants and Animal Studies
This article does not contain any studies with human or animal subjects.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Douny, C., Benmedjadi, S., Brose, F. et al. Development of an Analytical Method for the Simultaneous Measurement of 10 Biogenic Amines in Meat: Application to Beninese Grilled Pork Samples. Food Anal. Methods 12, 2392–2400 (2019). https://doi.org/10.1007/s12161-019-01587-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01587-4