Abstract
A high-performance thin-layer chromatographic (HPTLC) method was developed and validated according to the protocol on Validation of Analytical Procedures: methodology, Veterinary International Cooperation on Harmonization for simultaneous determination of enrofloxacin and ciprofloxacin in broiler chicken tissues viz. liver, kidney, muscle-breast, muscle-thigh, and skin. Chromatography was performed on thin-layer chromatography (TLC) silica gel 60F254, aluminum sheets (10 × 10 cm) by Camag Linomat-5 applicator. The isolation of enrofloxacin and ciprofloxacin was attained by two development stages, initially with diethyl ether followed by dichloromethane/methanol/aqueous ammonia/acetonitrile (2:3:2:3) as mobile phase. The developed TLC plates were exposed to hydrochloric acid fumes and fluorescent densitometric evaluation was performed at 366 nm using Camag TLC Scanner-3 with WinCAT 1.4.4 software. The RF value was 0.60 and 0.44 for enrofloxacin and ciprofloxacin, respectively. The detection limits were 2 and 3 ng/band for enrofloxacin and ciprofloxacin, respectively, and 5 ng/band as limit of quantification for both the compounds. Enrofloxacin and ciprofloxacin showed wide linear range from 2 to 110 ng/band and 3 to 110 ng/band with high correlation coefficient values of 0.99997 and 0.99990 for peak area, respectively. An exemplary precision was observed for individual compounds. The percent recovery for ciprofloxacin was 82.0–86.8% when compared to enrofloxacin 83.4–90.3%. The observed results were within the acceptable values as a function of the analyte concentration, suggested by the Association of Official Analytical Chemists peer-verified methods program. The present validated HPTLC method can be used as screening technique for antibiotics residue monitoring program in broiler chicken tissues to promulgate food safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The widespread use of fluoroquinolones as therapeutic and prophylactic agents, in food animals particularly in intensive poultry production, has become a matter of considerable concern in recent years due to the identification of resistant Campylobacter spp. (Humphrey et al. 2005), Escherichia coli (Khan et al. 2005), and Salmonella serovars (Zhao et al. 2010) in meat and possible transfer to humans via the food chain (Angulo et al. 2000; Tollefson and Karp 2004; Petrovic et al. 2009). This resistance has led to lowered therapeutic efficacy of these compounds in human infections (WHO 1998; Norstrom et al. 2006), suggestive of direct impact on public health (Wegner 1999; EMEA 2006).
The principal risk is associated with the drugs used both in human and veterinary medicine and with those that produce similar metabolites. Enrofloxacin, a fluoroquinolone developed exclusively for veterinary use is prescribed in poultry in large-scale for treatment of chronic respiratory disease, colibacillosis, salmonellosis, and fowl cholera (Anderson et al. 2003; Ellakany et al. 2007). After administration, enrofloxacin is metabolized in the liver via de-ethylation into pharmacologically active metabolite ciprofloxacin (Prescott et al. 2000). Ciprofloxacin is one of the most commonly used antimicrobial in human medicine worldwide. In cognizance to this, since 2005 the US Food and Drug Administration (FDA) has banned the use of enrofloxacin in poultry/food animals (Food and Administration 2005). The European Union (EU) has set the maximum residue limit (MRL) for sum of enrofloxacin and ciprofloxacin residues as 100 μg/Kg in muscle, skin and fat, 200 μg/Kg in liver and 300 μg/Kg in kidney (EMEA 2002); whereas, Japan has established much lower MRL (10 μg/Kg) in chicken muscle, liver, and kidney (Ministry of Health and Welfare, Japan 2005).
To monitor the levels of drug residues in animal products, analytical methods for their simultaneous determination are necessary (Herndndez-Arteseros et al. 2000). Efficient analytical methods are required to monitor the food supply to ensure that any antibiotic residues present are below the set tolerance level or MRL, thus promoting food safety and consumer confidence (Schneider 2004). This necessitates developing a fast, comprehensible and accurate quantification of residues of these antibacterials in food animals (Garcia Ovando et al. 2004). However, for MRL studies the analytical method for enrofloxacin and ciprofloxacin should analyze both drugs simultaneously (Garcia et al. 2005).
Literature on simultaneous determination of enrofloxacin and ciprofloxacin were always with some shortcomings such as time-consuming sample preparation and expensive methods using solid phase extraction cartridges. Although few studies have reported the separation of quinolones by thin-layer chromatography (TLC) (Wang et al. 2001; Thangadurai et al. 2002; Choma 2003; Dorofeev et al. 2004; Krzek et al. 2005; Bober 2008; Saleh et al. 2013), fluoroquinolones are still insufficiently characterized with respect to chromatographic behavior, in particular, in the case of TLC (Dorofeev et al. 2004). Hence, there is a need for developing simple, sensitive, and cost effective analytical protocol for selective separation and simultaneous estimation of enrofloxacin and its primary metabolite ciprofloxacin residues in edible animal tissues, to ensure that the residues present are below the MRL, thus promoting food safety and consumer confidence, ultimately global food security.
Materials and Methods
HPTLC Instrumentation and Chromatographic Conditions
Reagents and Reference Standards
All solvents/chemicals used were of analytical grade reagent of MERCK, and high pure distilled water (TKA-GenPure, Germany) was used throughout the experiment. Certified Reference Material standards of enrofloxacin and ciprofloxacin with purity of 99.99% were obtained as gratis from M/s. Neospark Drugs and Chemicals Private Limited, Hyderabad, India.
Stock and Working Standards Solution
Enrofloxacin and ciprofloxacin stock standards of 1 mg/mL concentration were prepared in 0.1 M HCl separately. Mixed working standards of enrofloxacin and ciprofloxacin were prepared by diluting appropriate volume of stock standards with ACN, 1% KCl in 0.02 M KOH (7:3) to get 100, 10, 5, and 1 ng/μL concentration each.
HPTLC System
The HPTLC system (Camag, Switzerland) used was composed of an application device Linomat-5 applicator, a densitometer TLC scanner-3 equipped with WinCATs 1.4.4 version software and a Camag twin trough glass tank.
Sample Preparation
Liver, kidney, muscle, and skin samples were extracted separately as described by Anadon et al. (1995) and Verdon et al. (2005). Tissue samples (4 g) were homogenized (Potter-Elvehjem glass Teflon homogenizer) with 16 mL of 5% aqueous trichloroacetic acid and centrifuged at 5000 rpm (Remi research centrifuge-R-24, 4/2000). The resultant aqueous supernatant was collected separately by filtering through Whatman filter paper No.1 and subjected to liquid-liquid extraction by adding 32 mL of dichloromethane in a separating funnel. The organic fraction-I was collected separately and left out aqueous fraction was once again extracted with 16 mL of dichloromethane. The organic fraction-II obtained was added to the fraction-I. The resultant organic extract thus obtained was passed through sodium sulfate bed and evaporated in a rotary vacuum evaporator and further concentrated in an amber colored sample glass vial on a hot plate under fume hood. The dried extract was reconstituted with 200 μL of dichloromethane and subjected to enrofloxacin and ciprofloxacin quantification by HPTLC.
Separation and Quantification
Chromatography was performed on silica gel TLC aluminum sheets (60F254, 10 × 10 cm). Appropriate concentrations of working standards (1–110 ng) were applied to the plate as 8 mm length bands using the Camag 100 μL syringe with the applicator having high pure N2 flow @ 150 nL/s. The bands were positioned 8 mm from the bottom and 11 mm from the side of the plate, on each plate with seven applications. The space between two bands was 3.66 mm. The application parameters were identical for all the analyses performed.
The spotted TLC plates were subjected to two development stages. For tissue sample clean-up, earlier studies have used solid-phase extraction (SPE) cartridge, an expensive procedure or an extraction by hexane which consumes high volumes of solvent. One of the main aims of the present study was to develop cost effective manner without utilizing SPE cartridges but through one developing step in diethyl ether directly on plate. Diethyl ether removed the fatty interference from the matrix and there by reduced the background noise. Further, the volume of solvent consumed is very minimal. In the first stage, plates were developed up to 90 mm distance in a Camag twin trough glass tank (20 × 10 cm) which was pre-saturated with 10 mL of diethyl ether, subsequently the developed plates were dried by hair drier. In the second stage, the dried plates were developed in a Camag twin trough glass tank (10 × 10 cm) which was pre-saturated with 10 mL of mobile phase dichloromethane, methanol, 25% aqueous ammonia, acetonitrile (2:3:2:3, v/v/v/v) for 30 min; the solvent front position was 70 mm. The developed and dried TLC plates were exposed to HCl fumes for 2 min by placing over the exit of a glass chamber, which was pre-saturated with HCl fumes, under fume hood and then subjected to quantification by Camag Scanner-3.
The HPTLC bands corresponding to sample and standard were quantified at 366 nm using a Camag HPTLC scanner-3 equipped with Camag WinCats software and a mercury lamp source, slit width 6 × 0.45 mm, remission-fluorescence scan mode and a scanning speed at 20 mm/s. The WinCats software controlling the densitometer produces a calibration plot by linear regression relating standard concentration to the peak area and enrofloxacin and ciprofloxacin in samples were automatically interpolated from the calibration curve. The HPTLC runs were performed under laboratory conditions of 25 ± 5 °C throughout the experiment.
HPTLC Method Validation
The present HPTLC method was validated according to Veterinary International Cooperation on Harmonization (VICH) and Codex Alimentarius guidelines for residue control program (VICH Guideline on Validation of Analytical Procedures 1998; FAO/WHO 1995).
Linearity and Calibration Curve
Appropriate concentration of aliquots of mixed working standards were prepared and applied on the TLC plate in the range of 2–11 μL to give a series of bands covering the range 2–110 ng/band. The peak area obtained for each concentration level was recorded and calibration curves were prepared by plotting integrated areas on Y-axis versus concentrations on the X-axis.
Precision
Intra- and Inter-Day Precision
The precision of the method in terms of inter-day variation was determined by analyzing the mixed working standard solutions in the calibration range of 2–110 ng for enrofloxacin and 3–110 ng for ciprofloxacin, three times on the same day. Inter-day precision was assessed on three different days over a period of 1 week.
Recovery
To 4 g of blank sample (breast muscle, thigh muscle, liver, kidney, and skin), appropriate volume of aliquots of working standard was spiked to obtain 10, 25, 50, 100, 200, 300, and 500 ppb concentration of enrofloxacin and ciprofloxacin each. The spiked tissue samples were subjected to liquid-liquid extraction and quantification.
Limit of Detection and Quantification
The chromatogram peaks originated from known constituents were well resolved and did not interfere with other constituents of the mobile phase used/matrix analyzed, thus assessing the limit of detection (LOD) and limit of quantification (LOQ). Accordingly, a signal to noise (S/N) ratio of 3 has been used for determining the LOD, whereas, an S/N ratio of 10 has been used to determine the LOQ.
Results and Discussion
Optimization of Chromatographic Separation of Enrofloxacin and Ciprofloxacin
Hunt for the mobile phases of choice for TLC/HPTLC separation of fluoroquinolones revealed that multicomponent organic mobile phases were used by various researchers (Thangadurai et al. 2002; Choma 2003; Dorofeev et al. 2004; Krzek et al. 2005). In the present study different mobile phases were tested for their effect on separation of enrofloxacin and ciprofloxacin as follows: mobile phase-I Methanol/25% aqueous ammonia/ethyl acetate/acetonitrile (1:1:2:1, v/v/v/v), mobile phase-II Chloroform/methanol/25% ammonia (43:43:14, v/v/v), mobile phase-III Dichloromethane/methanol/2-propanol/25% aqueous ammonia (2.5:2.5:5:2, v/v/v/v), and mobile phase-IV Dichloromethane/methanol/25% aqueous ammonia/acetonitrile (4:4:2:1, v/v/v/v) (Table 1).
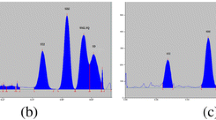
An increase in the content of high-polarity components in the mobile phase led to an increase in the mobility of enrofloxacin and ciprofloxacin, all bands on the chromatogram had RF above 0.8 and some occurred near the solvent front. An increase in the content of low-polarity components decreased the mobility of enrofloxacin and ciprofloxacin, showed RF below 0.2 with error limit of ± 0.03 for both enrofloxacin and ciprofloxacin (Table 1). With mobile phase-IV, various ratios of organic solvents were tested, consequently, Dichloromethane/methanol/25% aqueous ammonia/acetonitrile, in the ratio of 2:3:2:3 (v/v/v/v) was found to be optimum for the efficient separation of enrofloxacin and ciprofloxacin with RF value 0.60 and 0.44 respectively with error limit of ± 0.02 for both enrofloxacin and ciprofloxacin (Figs. 1, 2, and 3). In the present study, the background noise was controlled by two ways. First, introduction of first stage development in diethyl ether which removed the fatty interference from the matrix; secondly, exposure to HCl fumes prior to scanning enhanced the fluorescence quenching and ultimately better sensitivity of our compounds as well as masked the noises if any. Therefore, there was no background noise disturbing the analytes peak.
Both the bands could be visualized under short and long wave length UV light (254 and 366 nm), appeared violet in 254 nm and fluorescent blue in 366 nm. The separated bands on TLC plates were also visualized by spraying 0.5% ferric chloride in 0.5 M hydrochloric acid and appeared yellow, as reported earlier by Thangadurai et al. (2002).
Generally, it is difficult to separate pairs of drugs that differ only slightly in their structure, like ciprofloxacin and enrofloxacin (only in C2H5 group) (Choma 2003). Surprisingly, this behavior seems not to concern the pair of enrofloxacin and its metabolite ciprofloxacin, as it was separated very well in the present mobile system. The optimum mobile phase composition was selected taking into account the following facts. Organic mobile phases containing ammonia possess pH values above 10, so all piperazinyl fluoroquinolones are in the anionic form. Basic pH controlled by addition of ammonia prevents appearance of the double bands on the plate resulting from different ionic forms of the drugs. Additionally, ammonia hinders adsorption of fluoroquinolones on silanol groups and metal impurities of silica gel (Choma 2003). Dorofeev et al. (2004) optimized the mobile phase to contain aqueous ammonia and methanol 20% each, where methanol increased the HPTLC system selectivity.
In the present HPTLC method, the observed RF value 0.60 and 0.44 for enrofloxacin and ciprofloxacin, respectively, is in close agreement with Wang et al. (2001) who reported 0.60 and 0.40 for enrofloxacin and ciprofloxacin, respectively. Whereas, Choma (2003) reported RF value of 0.60 and 0.33 for enrofloxacin and ciprofloxacin, respectively (Table 2).
HPTLC Method Validation
Linearity and Calibration Curve
The linearity in terms of slope, Y-intercept and correlation coefficient values was excellent for both the compounds (Table 3; Figs. 4 and 5) with the developed HPTLC method. Enrofloxacin and ciprofloxacin showed wide linear range 2–110 ng/band and 3–110 ng/band with high correlation coefficient values 0.99997 and 0.99990 for peak area, respectively, signifying the suitability of the method for analysis. Further, the low relative standard deviations (RSD) guarantee the correctness of the applied procedure and reliable results.
Precision
Intra- and Inter-Day Precision
The results showed that % RSD for intra-day precision is lower than that of inter-day precision for both enrofloxacin and ciprofloxacin (Table 4). The lower % RSD indicated that the method has good precision. The observed % RSD values are within the acceptable precision values as a function of the analyte concentration, suggested by the AOAC peer-verified methods program ( 1993).
Recovery
Recovery of enrofloxacin and ciprofloxacin in spiked edible tissue samples of broiler chicken such as breast muscle, thigh muscle, liver, kidney and skin are summarized in Table 5. Low RSD values indicate that the method is precise. The recovery of enrofloxacin and ciprofloxacin showed highest in liver (90.3 and 86.8%) and lowest in skin (82.5 and 81.7%), respectively. The recovery of enrofloxacin and ciprofloxacin were found to be in the following order; liver > kidney > thigh muscle > breast muscle > skin and liver > thigh muscle > breast muscle = kidney > skin, respectively.
The recovery data obtained in the present study is within the acceptable recovery data as a function of analyte concentration, suggested by the AOAC peer-verified methods program ( 1993). The low recovery of ciprofloxacin when compared to enrofloxacin could be attributed to covalent binding property of ciprofloxacin to proteins and speculated that it could be lost during deprotenization (Gorla et al. 1997).
Limit of Detection and Quantification
The sensitivity of the TLC can be enhanced using derivatization as a less expensive way (Juhel-Gaugain and Abjean 1998) or by combining the method with mass spectrometry (Wilson 1999) or, bioautography (Choma et al. 2005). In drug residue analysis, the application of fluorescence detection is recommended because of its higher selectivity and sensitivity. Owing to the fluorescent nature of the piperazinol fluoroquinolones, they are mainly detected by fluorescent detector. Fluorescence strongly depends on the pH of the medium and the highest fluorescence is obtained at a pH value from 2.5 to 4.5 (Herndndez-Arteseros et al. 2000; Posyniak et al. 1999; Ramos et al. 2003). This analytical character of the fluoroquinolone was very well exploited in the present study. After development in the mobile phase, the TLC plates were exposed to HCl fumes prior to scanning, which resulted in enhanced sensitivity of the enrofloxacin and ciprofloxacin by fluorescence detection. Thereby, the LOD obtained in the present experiment for ciprofloxacin (3 ng/band) was found to be lower than the earlier findings of Krzek et al. (2005) who reported 10 ng as LOD for ciprofloxacin. Whereas, LOQ of 5 ng/band observed in the present study for both the compounds is comparable to the earlier reports of Wang et al. (2001), who showed LOQ of 1 ng/spot for enrofloxacin and 0.5 ng/spot for ciprofloxacin.
Conclusions
The HPTLC method developed in the present study is sensitive, simple, rapid, convenient, inexpensive (without the necessity of imported cartridges), and ideally tailored to the reality of developing countries. The obtained validation results recommend that it can be used for residue study purposes and routine screening tests in broiler chicken tissues.
References
Anadon A, Martinez-Larranaga MR, Diaz MJ, Bringas P, Martinez MA, Fernandez-Cruz ML, Fernandez MC, Fernandez R (1995) Pharmacokinetics and residues of enrofloxacin in chickens. Am J Vet Res 56:501–506
Anderson AD, Nelson JM, Rossiter S, Angulo FJ (2003) Public health consequences of use of antimicrobial agents in food animals in the United States. Microb Drug Resist 9:373–379
Angulo FJ, Johnson KR, Tuxe RV, Cohen ML (2000) Origins and consequences of antimicrobial-resistant nontyphoidal salmonella: implications for the use of fluoroquinolones in food animals. Microb Drug Resist 6:77–83
AOAC Peer Verified Methods Program (1993) Manual on policies and procedures; Arlington
Bober K (2008) Determination of selected quinolones and fluoroquinolones by use of TLC. Anal Lett 41:1909–1913
Choma IM (2003) TLC separation of fluoroquinolones: searching for better selectivity. J Liq Chromatogr Relat Technol 26:2673–2685
Choma IM, Choma A, Komaniecka I, Pilorz K, Staszczuk K (2005) Semiquantitative estimation of enrofloxacin and ciprofloxacin by thin-layer chromatography-direct bioautography. J Liq Chromatogr Relat Technol 27:2071–2085
Dorofeev VL, Konovalov AA, Kochin VY, Arzamastsev AP (2004) TLC analysis of drugs of the fluoroquinolone group. Pharmaceut Chem J 38:510–512
Ellakany HF, Abu El-Azm IM, Bekhit AA, Shehawy MM (2007) Studies on the effects of enrofloxacin overdose on different health parameters in broiler chickens. In: BS Vet Med J., 5th Scientific Conference. pp 176–186
EMEA (2002) The European Agency for the Evaluation of Medicinal Products, Veterinary Medicines and Inspections. Committee for Veterinary Medicinal Products, Enrofloxacin (Extension to all food producing species), Summary report (5), EMEA/MRL/820/02-FINAL
EMEA (2006) Use of fluoroquinolones in food producing animals: precautions for use in the SPC regarding prudent use guidance. Committee for medicinal products for veterinary use. (EMEA/CVMP/416168/2006)
FAO/WHO (1995) Codex Alimentarius, residues of veterinary drugs in foods, volume 3, 2nd edition, section 3, part II. FAO, Rome
Garcia MA, Solans C, Calvo A, Hernandez E, Rey R, Bregante MA, Puig M (2005) Determination of enrofloxacin and its primary metabolite ciprofloxacin in pig tissues. application to residue studies. Biomed Chromatogr 19:27–31
Garcia Ovando H, Gorla N, Weyers A, Ugnia L, Magnoli A (2004) Simultaneous quantification of ciprofloxacin, enrofloxacin and balofloxacin in broiler chicken muscle. Arch Med Vet 36:93–98
Gorla N, Chiostri E, Ugnia L, Weyers A, Giacomelli N, Davicino R, Garcia Ovando H (1997) HPLC residues of enrofloxacin and ciprofloxacin in eggs of laying hens. Int J Antimicrob Agents 8:253–256
Herndndez-Arteseros JA, Boronat I, Compan R, Prat MD (2000) Liquid chromatographic separation of fluoroquinolone antibacterials used as veterinary drugs. Chromatographia 52:295–300
Humphrey TJ, Jorgensen F, Frost JA, Wadda H, Domingue G, Elviss NC, Griggs DJ, Piddock LJ (2005) Prevalence and subtypes of ciprofloxacin resistant Campylobacter spp. in commercial poultry flocks before, during and after treatment with fluoroquinolones. Antimicrob Agents Chemother 49:690–698
Juhel-Gaugain M, Abjean JP (1998) Screening of quinolone residues in pig muscle by planar chromatography. Chromatographia 47:101–104
Khan AA, Nawaz MS, Summage West C, Khan SA, Lin J (2005) Isolation and molecular characterization of fluoroquinolones resistant Escherichia coli from poultry litter. Poult Sci 84:61–66
Krzek J, Hubicka U, Szczepanczyk J (2005) High-performance thin-layer chromatography with densitometry for the determination of ciprofloxacin and impurities in drugs optimized chromatographic conditions. J AOAC Int 88:1530–1536
Ministry of Health and Welfare, Japan (2005) Specifications and standards for food, food additives. Notification no. 499. Ministry of Health and Welfare, Tokyo
Norstrom M, Hofshagen M, Stavnes T, Schau J, Lassen J, Kruse K (2006) Antimicrobial resistance in Campylobacter jejuni from humans and broilers in Norway. Epidemiol Infect 134:127–130
Petrovic J, Baltic M, Stefanovic S, Milanov D, Ratajac R (2009) Determination of flumequine residues in broiler chickens with HPLC and screening method. Acta Vet (Beograd) 59:547–555
Posyniak A, Zmudzki J, Semeniuk S, Niedzielska J, Eblis R (1999) Determination of fluoroquinolone residues in animal tissues by liquid chromatography. Biomed Chromatogr 13:279–285
Prescott JF, Baggot JD, Walker RD (2000) Fluoroquinolones. In: Prescott JF, Baggot JD, Walker RD (eds) Antimicrobial therapy in veterinary medicine, 3rd edn. Iowa State University Press, Iowa, pp 315–339
Ramos M, Aranda A, Garcia E, Reuvers T, Hooghuis H (2003) Simple and sensitive determination of five quinolones in food by liquid chromatography with fluorescence detection. J Chromatogr B 789:373–381
Saleh GA, Askal HF, Refaat IH, Abdel-aal FAM (2013) Review on recent separation methods for determination of some fluoroquinolones. J Liq Chromatogr Relat Technol 36:1401–1420
Schneider MJ (2004) Rapid fluorescence screening assay for enrofloxacin and tetracyclines in chicken muscle. J Agric Food Chem 52:7809–7813
Thangadurai S, Shukla SK, Anjaneyulu Y (2002) Separation and detection of certain beta lactam and fluoroquinolones antibiotic drugs by thin layer chromatography. Anal Sci 18:97–100
Tollefson L, Karp BE (2004) Human health impact from antimicrobial use in food animals. Med Mal Infect 34:514–521
U.S. Food and Drug Administration (2005) Enrofloxacin for poultry: final decision of withdrawal of new animal drug application following formal evidentiary public hearing availability. Fed Regist 70:44105
Verdon E, Couedor P, Roudaut B, Sanders P (2005) Multiresidue method for simultaneous determination of ten quinolones antibacterials residues in multimatrix/multispecies animal tissues by liquid chromatography with fluorescence detection: single laboratory validation study. J AOAC Int 88:1179–1192
VICH Guideline on Validation of Analytical Procedures (1998) Methodology, Veterinary International Cooperation on Harmonization, EMEA/CVMP/590/98-Final, London, UK
Wang PL, Chen L, Fan YF (2001) Selective separation and simultaneous determination of trace levels of five types of fluorinated quinolone drugs by thin layer chromatography/fluorescence densitometry. J AOAC Int 84:684–688
Wegner HC (1999) The consequences for food safety of the use of fluoroquinolones in food animals. N Engl J Med 340:1525–1532
WHO (1998) Use of quinolones in food animals and potential impact on human health. Report of a WHO meeting, Geneva, Switzerland, 2–5 June, 1998. WHO/EMC/2DI/98
Wilson ID (1999) The state of the art in thin-layer chromatography-mass spectrometry: a critical appraisal. J Chromatogr A 856:429–442
Zhao L, Dong YH, Wang H (2010) Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci Total Environ 408:1069–1075
Acknowledgements
The Authors are highly thankful to the Drugs and Pharmaceutical Research Programme, Department of Science and Technology (DST), Government of India, New Delhi, for the financial assistance in conducting the experiment as part of the DST scheme entitled “A National Facility for Pharmacovigilance on Drug Residue and other Toxic Xenobiotics including Genetically Manipulated Organisms in Veterinary Products” at Pharmacovigilance Laboratory for Animal Feed and Food Safety, Directorate of Centre for Animal Health Studies, Tamil Nadu Veterinary and Animal Sciences University, Chennai-600 051.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Veerapandian Sureshkumar declares that he has no conflict of interest.
Ghadevaru Sarathchandra declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Additional information
Part of Ph.D. thesis submitted to Tamil Nadu Veterinary and Animal Sciences University, Tamil Nadu State, India.
Rights and permissions
About this article
Cite this article
Sureshkumar, V., Sarathchandra, G. A HPTLC-Fluorescent Densitometry Assay for Simultaneous Detection of Enrofloxacin and Ciprofloxacin in Broiler Chicken Tissues. Food Anal. Methods 11, 1076–1085 (2018). https://doi.org/10.1007/s12161-017-1077-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-1077-x