Abstract
Constituents in soybean oil fried with chicken breast meat (CBM) were analyzed using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) according to the corresponding molecular weight (MW) distributions. The possible molecular formulas of the constituents deduced based on the parent triacylglycerols (TAGs) were investigated in three MW distributions. TAGs and oxygenated TAGs were found in region A (m/z853-1001). Diacylglycerols (DAGs), oxygenated DAGs, and oxidized TAG decomposition products were observed in region B (m/z600-853). Combination products between one TAG and one or two short-chain oxidative decomposition products (ODPs), one TAG and one DAG, two TAGs (dimers), one TAG dimer and one or more short-chain ODPs, and three TAGs (trimers) and so on were shown in region C (m/z1001-3000). Some even MWs were assigned to nitrogen- and sulfur-containing TAG derivatives due to the introduction of proteins contained in CBM. Furthermore, the possible reaction mechanisms which occurred during the deep-fat frying process were also discussed based on the deduced molecular structures of constituents. Depending on the obtained results, the composition profile of frying oil can be well elucidated by the MALDI-TOF-MS-based method for the quality monitoring of the frying oil or fried food.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a popular food processing method, frying produces fried food with the characteristics of crispy taste, good color, and pleasant odor. Meanwhile, significant changes in composition of the frying oil can occur under the conditions of high temperature, extra oxygen, and complex food components. Consequently, not only the service life of frying oil but also the final quality of fried food would be directly influenced by these chemical reactions and the corresponding products. Therefore, the characterization of the composition of frying oil is vital for both the evaluation of frying oil’s life span and the quality supervision of fried food.
However, a lot of products stemmed from triacylglycerols (TAGs) are generated by a series of chemical reactions during the deep-fat frying process (Choe and Min 2007; Zhang et al. 2012). Among these reactions, hydrolysis, thermal oxidation, thermal polymerization, and the Maillard reaction are proposed to describe the formation of the studied products. Thermal oxidation and the Maillard reaction are the two important reactions involved in the most components of fried food and frying oil (Zamora and Francisco 2005). Consequently, thermal oxidation of unsaturated TAGs occurs to form many types of homogeneous TAG oxidative decomposition products (ODPs) and extra oxygen-containing TAG polymers. Food components, such as reduced sugar and nitrogen-containing compounds, are always involved in the occurrence of the non-enzymatic browning reaction. Furthermore, some of the TAG decomposition products, such as the unsaturated aldehydes, can react with the nitrogen-containing compounds to form the aldimines, which would further decompose and polymerize (Buttery et al. 1977).
Based on the formation mechanisms mentioned above, three types of products that formed during the deep-fat frying process have been roughly classified by comparing their molecular weights (MWs) with those of the original TAGs (Paul and Mittal 1997; Zhang et al. 2012). The first type contains compounds whose MWs are smaller than those of the parent TAGs. The typical representatives are the hydrolysis products of TAGs, such as free fatty acids, diacylglycerols (DAGs), and monoacylglycerols (MAGs), and the ODPs of unsaturated TAGs, such as short-chain fatty acids, and volatile compounds and so on. Oxygenated TAGs, such as TAGs with hydroxyl, carbonyl, and epoxy groups, belong to the second type because their MWs are almost equal to those of the parent TAGs. Finally, the compounds with higher MWs are classified to the third type, which mainly consists of TAG polymers generated from the termination reaction of oxidation and the thermal polymerization. This indicates the importance of studying the products formed during the deep-fat frying process using their features of MW distributions.
Because of the complexity of products formed under the frying conditions, the conventional analytical techniques cannot fully measure all the products when the used frying oil or the fried food is analyzed. Even the chromatographic techniques are commonly selected for the determination of one or more types of products (Zhang et al. 2015a, b). Fortunately, along with the progress of analytical instrument and the increasing requirement of practical task, the so-called soft-ionization technique has been investigated to be applied in the mass spectrometric technique to analyze the biological matrix (Hillenkamp et al. 1991; Wilm 2011). One of the most advantages of this technique is the ability to directly show the MW of the targeted analyte under the condition of suitable ion source energy. Matrix-assisted laser desorption ionization (MALDI) coupled with time-of-flight (TOF) and mass spectrometry (MS) is the typical application of soft ionization for the substance analysis (Hillenkamp et al. 1991).
Although the application of MALDI-TOF-MS for lipid analysis started at the end of 20th century, the study of lipid by this approach has been extensively carried out and reported (Fuchs et al. 2011; Schiller et al. 2004). Without complex sample preparations, such as isolation and purification, the scanning of mass spectrum can be conveniently achieved with the help of matrix when oil sample is investigated by MALDI-TOF-MS. Meanwhile, the peak strength of the signal is proportional to the content of the analyte in the oil sample (Schiller et al. 1999). Therefore, MALDI-TOF-MS has the advantages of fast determination, simple sample pretreatment, high resolution, and good stability. So far, all the known lipid classes, such as glycerides (i.e., TAG and DAGs), phospholipids, sterols, glycolipids, and sphingolipids, have been successfully investigated by this technique (Schiller et al. 2004). The specific theory, operation process, advantages, application fields, mass spectra analysis, and other information about application of this technique have well been reviewed (Fuchs et al. 2011; Fuchs and Schiller 2009).
Due to the strong oil absorption ability, fried food often contains high amounts of frying oil. Therefore, the monitoring of the composition of frying oil is very important to the quality guarantee of fried food. Considering the complex composition of the used frying oil and the advantages of MALDI-TOF-MS, constituents derived from TAGs in soybean oil fried with chicken breast meat were analyzed using this technique for the first time in this study.
Experimental
Materials and Chemicals
Refined soybean oil (first grade) and chilled chicken breast meat (CBM) were purchased from the local supermarket.
Methanol, chloroform, and hexane were bought from MREDA technology limited company (Beijing). 5-chloro-2-mercaptobenzothiazole was purchased from Alfa Aesar (Johnson Matthey Company, England). 2,5-Dihydroxybenzoic acid (DHB) and trifluoroacetic acid (TFA) were bought from Sigma-Aldrich. All the mentioned chemicals were chromatographically pure. Deionized water and ultrapure water was supplied by the lab.
Frying Procedure and Sampling
Fresh CBM was purchased in the morning and was then manually cut into uniform strips. Deep-fat frying with CBM was performed for 8 h/day over a period of seven consecutive days according to the practical food frying process in a domestic scale electrical fryer (HY-82EX, Guangzhou Huili Food Machinery Co., Ltd., China). Briefly, 5 L of soybean oil was placed into the fryer and heated to the set temperature (180 °C). The practical temperature of the frying system was measured at times, and no replenishment of fresh soybean oil was carried out during the whole deep-fat frying process. Food materials (approximately 100 g) were put in a stainless screen frame and were then fully immersed into the heated soybean oil. Frying time and the interval time among the batches of fried food were set to be 5 and 10 min, respectively. Oil samples were collected in triplicate at the second and eighth hour. Oil sample prepared at the second hour in the first day was denoted by 1–2 h. All the collected oil samples were maintained in a refrigerator at 4 °C prior to MALDI-TOF-MS analysis.
MALDI-TOF-MS Analysis
Dry drop method was used to perform the sample spotting. The sample preparation was carried out according to previous study with minor modification (Picariello et al. 2009). Briefly, both hexane and chloroform were used as the sample solvent. The oil sample concentration of 1 mg/mL was prepared. The matrix solution was prepared as follows: 10 mg of DHB was added into 1 mL of methanol contained with 0.1 % of TFA. Ultrapure water (1 mL) was then added and was totally mixed by a vortex mixer (HQ-60-IV, Beijing Beifang Tongzheng Biotechnology Development Co., Ltd.). After that, the oil sample solution and the matrix solution were mixed by 1:1 (v/v). 5-chloro-2-mercaptobenzothiazole (0.5 μL) was placed onto the stainless steel MALDI target using a 0.1–2.5-μL pipette (Eppendorf Research plus, Eppendorf, Germany). After the drying and stabilization of the thiazole spot, 0.8 μL of the oil sample matrix solution was dripped orderly to each spot.
MALDI-TOF mass spectra of the oil samples were obtained by AB4700 Proteomics Analyzer (Applied Biosystems, Framingham, MA, USA) using 337-nm radiations from a nitrogen laser. After the natural drying of the sample matrix, the MALDI target was immediately placed into the vacuum chamber of the instrument. A laser intensity of 3592 and an accelerating voltage of 20 kV were used. The spectra were recorded in reflectron mode within an m/z of 600–3000. For enhancing the accuracy of the MWs of sample components, spectrum calibration of the standards (a mixture of low mass standard peptides) was carried out prior to the acquisition of the spectra of oil samples. Each oil sample was analyzed in triplicate.
Data Analysis
Mass spectra were elaborated using the Data Explorer 4.0 software (PerSeptive BioSystems). The relative intensity was expressed in the form of mean value of the three replicates. The possible constituents were calculated and deduced based on the mass-to-charge ratio signals and the main components contained in fresh soybean oil.
Results and Discussion
Solvent Selection
Generally, the property (i.e., polarity and volatility) of the sample solvent obviously impacts on the uniform distribution of the components of oil in the MALDI target. According to the previous publications (Kaufman and Wiesman 2007; Picariello et al. 2009), hexane and chloroform are the frequently used types of solvents used to dissolve the oil sample when lipid analysis is carried out by MALDI-TOF-MS. Therefore, these two solvents were comparatively used in this study due to the complexity of the frying oil composition.
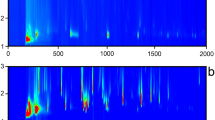
The MALDI-TOF mass spectra of fresh soybean oil dissolved in hexane and chloroform are shown in Fig. 1. According to the spectra, the MWs of sodiated molecules in the refined soybean oil mainly distribute in the range from 600 to 1100 Da. The apparent difference between the spectra is the relative abundances of the TAGs. Theoretically, refined soybean oil mainly consists of TAGs (Lay et al. 2006), which can be seen in Fig. 1a. This is attributed to the same polarity between hexane and TAGs. In other words, chloroform cannot effectively dissolve the TAGs to perform the reflection of TAG contents in the MALDI-TOF mass spectrum of vegetable oil. This phenomenon can also be observed in Supplementary information provided with this article. Furthermore, the MALDI-TOF mass spectra of oil samples of 6–8 and 7–8 h dissolved in chloroform were not observed during the test (shown in Supplementary information), indicating that most of the polar components of frying oil were lost due to the relatively higher volatility of chloroform. On the contrary, frying oil samples dissolved in hexane exhibited better MALDI-TOF mass spectra and reasonable MW distributions (shown in Supplementary information). Therefore, hexane is more suitable for the dissolution of frying oil under the selected conditions in this study when the constituents of frying oil are analyzed using MALDI-TOF-MS.
Distribution of the Frying Oil Constituents
According to the analysis mentioned above and the MALDI-TOF mass spectra of the studied frying oil samples shown in Supplementary information included with this article, the MW distributions of frying oil mainly center on three ranges of m/z, namely 600–853, 853–1001, and 1001–3000 Da. Because of the composition complexity of the frying oil and the characteristics of the adopted soft ionization technique, all the detected mass-to-charge ratios should represent the possible components of frying oil. On one hand, the edible oil mainly consists of TAGs and the unsaturated TAGs easily tend to be oxygenated under the conditions of high temperature and presence of oxygen. On the other hand, the MWs of the sodiated TAGs and their oxygenated monomers mainly distributed in the m/z region of 853–1001 in the MALDI-TOF mass spectrum (Chapagain and Wiesman 2009; Picariello et al. 2009). Therefore, this m/z region was assigned to region A for obtaining the efficient analysis of the frying oil components. Correspondingly, the lower and higher mass-to-charge ratios were assigned to region B and region C. The specific components in the different regions are discussed in the following text.
Frying oil Constituents in Region A
As shown in Table S2, oil sample of 7–8 h presents the largest number of MWs. In other words, the largest number of components has been detected by the adopted method in the oil sample of 7–8 h. Therefore, components in oil sample of 7–8 h are analyzed in this study.
As mentioned above, the sodiated TAGs are found around the m/z of 900. According to previous studies (Chapagain et al. 2009; Picariello et al. 2009), the deduced component profile of frying oil is listed in Table 1. It should be noted that the isomerides of the possible component also existed from the same MW. The common TAGs, such as PLP, POP, PLnL, PLL, PLO, POO, PLL, LLnLn, LLnL, LLL, LLO, OLO, OOO, and SOO and so on, contained in soybean oil were mostly detected in relatively lower concentrations in the fried oil. This could be attributed to that the original TAGs contained in fresh soybean oil had been involved in many reactions after deep-fat frying treatment; therefore, their amounts were reduced. Several MWs of 899.7, 913.7, 915.7, 917.7, 919.7, 935.7, 941.6, 955.6, 969.6, 985.6, and 1001.6 Da and so on presented in relatively higher amounts compared to those of the original TAGs are significantly found in the deep-fat fried soybean oil. Based on the differences in MWs between these components and the original TAGs, difference value of 16 Da and its multiples are observed. Therefore, these components are deduced as mono-, di-, and poly-oxygenated TAGs according to the thermal instability of these unsaturated TAGs. Remarkable quantity of these oxygenated TAGs were also observed in the thermal-treated olive and sunflower oils (Schiller et al. 2002; Picariello et al. 2009). Extra oxygen was abstracted by the unsaturated TAGs during the oxygenation under the conditions of high temperature and presence of oxygen. The possible binding forms of the extra oxygen might be the hydroxyl, carbonyl, epoxy, and peroxyl groups presenting in the position of carbon-carbon double bonds in aliphatic chains of TAGs (Giuffrida et al. 2004). From the prospective of the relative intensities, these oxygenated TAGs reflected the complexity of the constituents of frying oil and the intensive chemical reactions that occurred during the deep-fat frying process.
Generally, the MWs of the sodiated TAGs are in the form of odd number. However, MWs in the even number mode, such as 854.7, 876.7, 882.8, 896.7, 906.8, 916.7, 946.7, and 970.6 and so on were observed during the deep-fat frying process with CBM. On one hand, these even MWs might be the isotopic peaks of the adjacent components based on the nature of the atoms. However, these even MWs were mostly observed in relatively high intensity. On the other hand, given that the high amount of protein contained in CBM (Zhang Zhang et al. 2015a, b), the nitrogen-containing compounds might be generated and dissolved in the frying oil. Thus, these even numbers are probably the MWs of the nitrogen-containing TAG derivatives.
Frying oil Constituents in Region B
As indicated above, lower MWs distributed in region B were assigned to the DAGs, oxygenated DAGs, DAG combination compounds, and oxidized TAG decomposition compounds in light of the possible chemical reactions that occurred in TAGs. According to the data shown in Table 2, sodiated DAGs and DAG cations were deduced to characterize the possible DAGs presented in the frying oil (Picariello et al. 2009). DAG cations were generated by the loss of an RCOO−Na+ from the original TAGs. The sodiated DAGs were the result of the hydrolysis products of the original TAGs due to the influence of water and high temperature. In addition, the relative intensities of the oxygenated DAGs, such as 96.9 % of OO + 16O (m/z659) and 61.6 % of OO + 416O (m/z685), indicated that these compounds were one type of the dominating constituents contained in the oil sample of 7–8 h. This is in accordance to the high amount of oleic acid contained in soybean oil and the relatively oxidation stability of oleic acid compared to those of the linoleic and linolenic acids (Porter et al. 1995).
The MWs from 693 to 727 Da were assigned to the combination products between the DAGs and the short-chain TAG ODPs. Given that the possible intermediates generated during the thermal oxidation, the combination form was probably the covalent bond formed between a DAG-derived radical and a short-chain TAG-derived radical. Additionally, in view of the thermal oxidation which occurred during the deep-fat frying process with CBM, the produced low-molecular-weight ODPs could be dissolved in the frying oil and be reacted with the oxygenated TAGs or DAGs via the radical reaction.
The last important type of constituents distributed in region B is the oxidized TAG decomposition compounds. Based on the possible chemical formulas of these products, loss of a short carbon chain from the original TAGs were observed. Core aldehydes, the representative oxidized TAG decomposition compounds with one or more aldehyde groups in the aliphatic chains, have been mostly characterized as a potential hazard in the fried food (Sjövall et al. 2002). It is well known that the homolytic β-scission of alkoxy radicals generated during the oxidation of unsaturated TAGs can easily occur to form the TAG fragment radicals. Therefore, the core aldehydes are then formed when the TAG fragment radicals (i.e., TAG-O·) react with a hydroxyl radical. According to this aliphatic chain-shortened reaction, compounds with one or more hydroxyl, carboxyl, and (or) peroxyl groups presenting in the decomposition position of the TAG fragments were other type of oxidized TAG decomposition compounds (Suomela et al. 2011).
In addition, ten nitrogen-containing compounds were also observed in this region. According to the possible chemical formulas of the nitrogen-containing DAGs or oxidized TAG decomposition compounds, the components to react with nitrogen-containing compounds were probably the unsaturated TAGs or DAGs and the oxidized TAGs or DAGs.
Frying Oil Constituents in Region C
A total of 97 constituents were found in region C and their possible chemical formulas were listed in Tables 3 and 4. It can be observed that the constituents with higher MWs compared to the original TAGs were much more complex than those in the other two regions not only from the aspect of number of compounds but also from the aspect of possible chemical structures. Combination products between one TAG and one short-chain ODP, combination products between one TAG and two short-chain ODPs, combination products between one TAG and one DAG, TAG dimers, combination products between one TAG dimer and one or more short-chain ODPs, and TAG trimers and so on were deduced based on the MWs and the possible compounds contained in soybean oil.
Constituents with MWs from 1045 to 1097 Da and from 1033 to 1083 Da reported in the thermally stressed (180 °C) sunflower and virgin olive oils have been assigned to the TAG combination products (Picariello et al. 2009). The TAG dimers were characterized based on the MWs from 1754 to 1832 Da and from 1780 to 1824 Da in these two heated vegetable oils. However, constituents with MWs from 1101 to 1731 Da and from 1850 to 2694 Da were observed in the CBM-fried soybean oil for the first time in this study. High amounts of linolenic acid and repeated CBM frying treatment resulted in the large number and the complex structure of products. According to the characterization of chain-branched addition products in previous papers (Byrdwell and Neff 1998), the chemical formulas of these complex constituents were analyzed. Similar to the formation mechanism of the DAG combination compounds, free radical reaction was probably the reason for the formation of these chain-branched addition products. It is generally known that free radical chain reaction is the major reaction that occurred in the unsaturated TAGs under the frying conditions (Choe et al. 2007). As the accumulation of the components of fried food and the frying products, the radicals such as alkoxy radicals, alkyl radicals, allyl radicals, hydroxyl radical, and oxygen species and so on could be reacted with each other to form the stable compounds. Therefore, covalent bonds of C–C, C–O–C, and C–O–O–C might be the connection ways among the TAGs and the DAGs or the short-chain TAG oxidation decomposition products to form the TAG combination products and polymerized TAGs (Byrdwell and Neff 2002).
The studied TAG combination products and TAG polymers were also the main constituents contained in CBM fried oil sample of 7–8 h in terms of their significantly relative intensities, such as 70.1 % for C54:6 + 16O + C8H16 (m/z1029), 65.4 % for C54:4 + 16O + C10H16O (m/z1073), and 29.1 % for C54 + C54 + 216O: n = 5 (m/z1824) and so on. The relative intensities of the detected TAG combination products and TAG polymers well explained the major constituents contained in the frying oil and also reflected the complexity of the reactions that have taken place during the deep-fat frying process. The formation and accumulation of these products with high molecular weight were generally regarded as the quality index of the frying oil and the potential harmful factors of the fried food for the consumer’s health (Caldwell et al. 2011). However, the specific structure and the formation mechanism of this type of products are still unknown and needed to be further studied for the insurance of the safety of fried food.
As shown in Table 4, a large number of even MWs were also observed in this mass-to-charge ratio range. Except for the combination compounds between a TAG and a DAG, and TAG polymers, other lower even mass-to-charge ratios might be the MWs of the nitrogen-containing TAG derivatives. As previously indicated (Pokorný 1998; Pokorný and Kołakowska 2011), proteins and their decomposition products, peptides and amino acids, could be transferred into the frying oil to influence the changes of TAGs when the meat was fried. Therefore, many amino acid-containing compounds probably reacted with the unsaturated TAGs during the frying process with CBM in soybean oil. Condensation reaction between the aldehyde group-containing TAGs and the amino compounds might be the major reaction for the generation of these nitrogen-containing TAG derivatives with the aldimine structure or the imine linkage. In addition to the original amino compounds, the intermediate products of the Maillard reaction could also be the precursors of these nitrogen-containing TAG derivatives.
It is interesting that a difference value of 32 Da between the signal clusters, such as m/z1029.6 and m/z1061.6, m/z1057.6 and m/z1089.7, and m/z1073.6 and m/z1105.6 and so on was found among the homogeneous constituents whose MWs were distributed from 1029 to 1337 Da. Combining the feature of the components contained in CBM, the 32 Da might be attributed to the introduction of sulfur-containing compounds to the frying system during the CBM-frying process. According to the previous study, the amounts of cysteine and methionine in the chicken meat were 12.9 and 26.0 mg/g, respectively (Gilbert et al. 2011). These two amino acids could anticipate the reactions with the TAGs to form the sulfur-containing TAG derivatives during the frying process of chicken meat (Pokorný et al. 2011).
The nitrogen- or sulfur-containing TAG derivatives contained in the frying oil are derived from the interactions between the nitrogen- or sulfur-containing compounds in food and the TAGs or TAG derivatives (Zamora et al. 2005). This type of component might be not only related to the flavor but also to the safety of the fried food or by the form of precursor substances for the flavor substances (such as alkylpyrazines and alkyltrithiolanes) (Jayasena et al. 2013) and hazardous substances (such as heterocyclic amines) (Trafialek and Kolanowski 2014). Therefore, this type of component is very important for the frying oil because the fried food can absorb the frying oil during the deep-fat frying process.
According to the chemical structures of the deduced possible constituents of frying oil, the reaction types which occurred in TAGs were hydrolysis, oxidation (decomposition and polymerization), thermal polymerization, condensation reaction, and the Maillard reaction (shown in Fig. 2). Thereinto, the oxidation, also known as the free radical reaction, was the major reaction type according to previous studies (Choe et al. 2007). Consequently, although the appearance of oil was kept in the frying oil, the composition profile was much more complex compared to the original soybean oil. In other words, the frying oil was changed to a complex mixture by the modification of chemical components after the deep-fat frying process.
It is worth noting that all the above deduced compounds were at the basis of the structures and the MWs of the original TAGs. Thus, these TAG-derived compounds were just the possible constituents of the frying oil. For example, the MWs of the sulfur-containing TAG derivatives might also be the MWs of TAG combination compounds (shown in Tables 3 and 4). Furthermore, theoretically, the m/z range of 600–3000 Da set in this study cannot reflect the whole non-volatile constituents formed during the deep-fat frying process. However, the mass-to-charge signals in the range of 600–3000 Da are enough to reflect the information of the non-volatile compounds and the possible reaction types which occurred during the deep-fat frying process. The divided MW regions facilitated the analysis of the complex constituents of frying oil in terms of the changes of the original TAGs other than the preseparation of the heat-treated vegetable oils according to the polarity of the possible components (Picariello et al. 2009).
Conclusion
Many types of products which originated from parent TAGs in the deep-fat frying oil were clearly characterized by the signal clusters in the MALDI-TOF mass spectrum for the first time. The constituents of fried food can also participate in the complex reactions to form the nitrogen- and sulfur-containing TAG derivatives. Some similarities, such as the functional group, structure, and property and others, can be concluded among these types of products. Therefore, the possible products formed during the deep-fat frying process can be classified to analyze using the MALDI-TOF-MS technique. With the help of the assigned constituents, hydrolysis, oxidation (including decomposition and polymerization), thermal polymerization, condensation reaction, and the Maillard reaction were the possible reactions that occurred during the frying process with CBM in soybean oil. Moreover, the free radical reaction, namely the thermal oxidation, was the major reaction based on the feature of the components contained in the soybean oil.
Based on the obtained results, it can be concluded that as a fast analysis technique with simple procedure of sample preparation, MALDI-TOF-MS is an efficient technique for investigating of the non-volatile constituents contained in the complex frying oil. And on this basis, the quality of the frying oil and the final fried food could be fast and accurately evaluated using the MALDI-TOF-MS-based method. However, the characterization of the definite types or molecule structures of the TAG-derived products formed during food frying process needs the application of appropriate sample separation techniques.
References
Buttery RG, Ling LC, Teranishi R, Mon TR (1977) Roasted lamb fat: basic volatile components. J Agric Food Chem 25:1227–1229
Byrdwell WC, Neff WE (1998) Non-volatile products of triolein produced at frying temperatures characterized using liquid chromatography with online mass spectrometric detection. J Chromatogr A 852:417–432
Byrdwell WC, Neff WE (2002) Dual parallel electrospray ionization and atmospheric pressure chemical ionization mass spectrometry (MS), MS/MS and MS/MS/MS for the analysis of triacylglycerols and triacylglycerol oxidation products. Rapid Commun Mass Spectrom 16:300–319
Caldwell JD, Cooke BS, Greer MK (2011) High performance liquid chromatography-size exclusion chromatography for rapid analysis of total polar compounds in used frying oils. J Am Oil Chem Soc 88:1669–1674
Chapagain BP, Wiesman Z (2009) MALDI-TOF/MS fingerprinting of triacylglycerols (TAGs) in olive oils produced in the Israeli Negev desert. J Agric Food Chem 57:1135–1142
Choe E, Min DB (2007) Chemistry of deep-fat frying oils. J Food Sci 72:R77–R86
Fuchs B, Schiller J (2009) Application of MALDI-TOF mass spectrometry in lipidomics. Eur J Lipid Sci Technol 111:83–98
Fuchs B, Bresler K, Schiller J (2011) Oxidative changes of lipids monitored by MALDI MS. Chem Phys Lipids 164:782–795
Gilbert JA, Bendsen NT, Tremblay A, Astrup A (2011) Effect of proteins from different sources on body composition. Nutr Metab Cardiovasc Dis 21:B16–B31
Giuffrida F, Destaillats F, Skibsted LH, Dionisi F (2004) Structural analysis of hydroperoxy- and epoxy-triacylglycerols by liquid chromatography mass spectrometry. Chem Phys Lipids 131:41–49
Hillenkamp F, Karas M, Beavis RC, Chait BT (1991) Matrix-assisted laser desorption/ionization mass spectrometry of biopolymers. Anal Chem 63:1193A–1203A
Jayasena DD, Ahn DU, Nam KC, Jo C (2013) Flavour chemistry of chicken meat: a review. Asian Australas J Anim Sci 26:732–742
Kaufman M, Wiesman Z (2007) Pomegranate oil analysis with emphasis on MALDI-TOF/MS triacylglycerol fingerprinting. J Agric Food Chem 55:10405–10413
Lay JO Jr, Liyanage R, Durham B, Brooks J (2006) Rapid characterization of edible oils by direct matrix-assisted laser desorption-ionization time-of-flight mass spectrometry analysis using triacylglycerols. Rapid Commun Mass Spectrom 20:952–958
Paul S, Mittal GS (1997) Regulating the use of degraded oil/fat in deep-fat/oil food frying. Crit Rev Food Sci Nutr 37:635–662
Picariello G, Paduano A, Sacchi R, Addeo F (2009) MALDI-TOF mass spectrometry profiling of polar and nonpolar fractions in heated vegetable oils. J Agric Food Chem 57:5391–5400
Pokorný J (1998) Substrate influence on the frying process. Grasas Aceites 49:265–270
Pokorný J, Kołakowska A (2011) Lipid-protein and lipid-saccharide interactions. In: Sikorski ZE, Kołakowska A (eds) Chemical, biological, and functional aspects of food lipids, 2nd edn. CRC press, Florida, pp 455–472
Porter NA, Caldwell SE, Mills KA (1995) Mechanisms of free radical oxidation of unsaturated lipids. Lipids 30:277–290
Schiller J, Arnhold J, Benard S, Muller M, Reichl S, Arnold K (1999) Lipid analysis by matrix-assisted laser desorption and ionization mass spectrometry: a methodological approach. Anal Biochem 267:46–56
Schiller J, Süß R, Petković M, Arnold K (2002) Thermal stressing of unsaturated vegetable oils: effects analysed by MALDI-TOF mass spectrometry, 1H and 31P NMR spectroscopy. Eur Food Res Technol 215:282–286
Schiller J, Süß R, Arnhold J, Fuchs B, Leßig J, Müller M, Petković M, Spalteholz H, Zschörnig O, Arnold K (2004) Matrix-assisted laser desorption and ionization time-of-flight (MALDI-TOF) mass spectrometry in lipid and phospholipid research. Prog Lipid Res 43:449–488
Sjövall O, Kuksis A, Kallio H (2002) Formation of triacylglycerol core aldehydes during rapid oxidation of corn and sunflower oils with tert-butyl hydroperoxide/Fe2+. Lipids 37:81–94
Suomela JP, Leskinen H, Kallio H (2011) Analysis of isomeric forms of oxidized triacylglycerols using ultra-high-performance liquid chromatography and tandem mass spectrometry. J Agric Food Chem 59:8095–8100
Trafialek J, Kolanowski W (2014) Dietary exposure to meat-related carcinogenic substances: is there a way to estimate the risk? Int J Food Sci Nutr 65:774–780
Wilm M (2011) Principles of electrospray ionization. Mol Cell Proteomics 10:M111–M9407
Zamora R, Hidalgo FJ (2005) Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit Rev Food Sci Nutr 45:49–59
Zhang Q, Saleh ASM, Chen J, Shen Q (2012) Chemical alterations taken place during deep-fat frying based on certain reaction products: a review. Chem Phys Lipids 165:662–681
Zhang Q, Qin W, Li ML, Shen Q, Saleh ASM (2015a) Application of chromatographic techniques in the detection and identification of constituents formed during food frying: a review. Compr Rev Food Sci Food Saf 14:601–633
Zhang Q, Qin W, Lin DR, Shen Q, Saleh ASM (2015b) The changes in the volatile aldehydes formed during the deep-fat frying process. J Food Sci Technol 52:7683–7696
Acknowledgments
The authors would like to thank Dr. Shutao Sun who is working in the Institute of Microbiology Chinese Academy of Science for her kind help in completing the whole test and the use of the analysis software.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Qing Zhang declares that he has no conflict of interest. Shang Lin declares that he has no conflict of interest. Jie Li declares that he has no conflict of interest. Yuntao Liu declares that he has no conflict of interest. Wen Qin declares that she has no conflict of interest. Qun Shen declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM_Fig 1
(DOC 345 kb)
ESM_Table 1
(DOC 599 kb)
ESM_Table 2
(DOC 592 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Lin, S., Li, J. et al. Application of Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry for the Analysis of Compounds in Deep-Fat Frying Oil. Food Anal. Methods 9, 2352–2363 (2016). https://doi.org/10.1007/s12161-016-0413-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0413-x