Abstract
The essential oil yield and composition of the different growth stages of C. verum leaves were investigated. The results indicated that the density of the oil cells and the degree of oil accumulation were closely related to the essential oil yield. The leaves of the 2-year-old branches contained the highest density of oil cells (10.17 n/mm2), and the oils were largely accumulated, which coincided with the highest oil yield (5.81 %). The oils were less accumulated in leaves 1–4 collected from the annual branches and were mostly disintegrated in the leaves collected from the 4-year-old branches, resulting in lower oil yields (3.04 and 2.98 %, respectively). Eugenol was primarily found in the leaves collected from the 1-year- old branches, and the eugenol content decreased with the leaf growth. The results indicated clearly, that the leaves collected from the 1- and 2-year-old branches should be preferably chosen as the raw materials for the extraction of high-quality essential oils or eugenol. These results provide reference information for the rational utilization of cinnamon resources. Our research indicated that GC–MS and FTIR techniques, combined with microscopy, has been proved to be an effective strategy for assessment of essential oil quality for use in cinnamon plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cinnamomum verum J. Presl belongs to the Lauraceae family. This species occurs naturally in Sri Lanka and southern India and in the Tenasserim Hills of Myanmar (De Guzman and Siemonsma 1999). C. verum, C. cassia, C. burmanii and C. loureirii are the most important aromatic plant resources in international trade. C. verum is one of the world’s finest spices and is primarily exported as “cinnamon quills” (Wijesekera 1978). Sri Lanka is the traditional producer and exporter of C. verum, and the United States, Russia, Germany, the Netherlands and Japan import large quantities of C. verum oil every year (Ravindran et al. 2004). In China, C. verum was successfully introduced and is now widely cultivated in the Guangxi, Guangdong, Hainan and Taiwan provinces.
The chemical composition of C. verum is different from those of other cinnamon species. In addition, both the cinnamon bark and leaf contain important medicinal substances. The major constituent in the bark oil is cinnamic aldehyde. However, eugenol is a major constituent of leaf oil, and C. verum is also one of the main plant sources of eugenol (Mallavarapu et al. 1995). Currently, C. verum essential oil exhibits antioxidant and antibacterial activities (Moarefian et al. 2013). C. verum essential oil is commonly used as a raw material for treating gastrointestinal disturbance and loss of appetite (WHO 1999). In addition, C. verum oils have been widely used in various types of desserts, condiments, teas and flavoring agents (Jayaprakasha et al. 2007).
The essential oil yield and quality are affected by the different ages, habitats, species (Li et al. 2013a) and parts (Deng et al. 2014) as well as the different proportions of mature and juvenile leaves (Li and Madden 1995). In addition, the essential oil yield and trans-cinnamaldehyde content are significantly different at various development stages of the cassia leaves (Li et al. 2013b). Therefore, these internal and external factors directly influence the economic benefits and quality of the products. In addition, both the leaf size and shape are important factors for essential oil synthesis and accumulation of aromatic plants (Gang et al. 2001; Sangwan et al. 2001). Joy et al. (1998) analyzed the correlations between the fresh leaf yield, leaf oil yield and eugenol yield and determined that the eugenol content was not directly correlated with leaf oil yield and fresh leaf yield. The leaves of C. verum are the main raw material used for eugenol production. Therefore, it is important to investigate the variations of eugenol content and quality in the C. verum leaves to improve the quality and economic benefit of using C. verum leaves. To determine the effect of the growth stages of C. verum leaves on the yields and compositions of essential oils, GC–MS and FTIR spectra, combined with microscopy were used for the detection of the essential oil, and the results provided comprehensive information of the essential oil compositions during the leaf growth of C. verum. These analytical techniques will be useful for the quality assessment of C. verum. The current study provides valuable data for resource assessment and the rational utilization and harvest of C. verum leaves.
Materials and Methods
Materials
Fourteen-year-old C. verum trees have lush leaves, high oil yields and a high eugenol content (Li et al. 2013a). Therefore, we collected not mechanically wounded leaves from 14-year-old C. verum trees for this study. Leaves at different growth stages were collected and assessed based on the bud scale scars. The first leaf is the closest to the stem apex of a juvenile branch and so on until the seventh leaf. The age of the branches is determined by the number of bud scale scars plus 1. The leaves of the 5-year-old branches withered and fell on the 14-year-old C. verum trees. Therefore, the leaf samples were collected from branches between 1 and 4 years old. Fresh leaves (15–30 leaves) of per sample were randomly collected from 5 individual plants of C. verum for each analysis. The plants were identified by Fana Wei (Guangxi Institute of Botany, Guilin, China). For details, please refer to Table 1.
Sample Preparation and Essential Oil Extraction
The Fresh leaves were collected from the plants. Samples were clearly washed in distilled water and treated by air drying. The volatile oils of C. verum leaves were obtained by hydrodistillation in accordance with the method in Pharmacopoeia of the People’s Republic of China (China Pharmacopeia Commission 2010). Fifteen grams of C. verum leaf powders were precisely weighed and mixed with 200 mL of distilled water, and subsequently heated at 100°C for 5 h. Methylene dichloride was used to extract volatile compounds from the water phase three times. The Methylene dichloride fraction was dehydrated over anhydrous sodium sulphate and filtered. After most of the distillate was steamed at vapor bath in fuming cupboard, the concentrated solution was transfered in a dried test tube, yellow volatile oils were obtained and weighed, the essential oils were kept at 4°C and until further use.
GC–MS Analysis
GC–MS analysis was performed on a gas chromatograph 7890A (Agilent, American) interfaced with a 5975C Plus mass spectrometer (Agilent, American). A fused silica capillary Agilent Technology HP-5 ms (5 % phenyl methyl siloxane) column (30 m × 0.25 mm i.d., film thickness 0.1 μm) was used for the separation. The injector temperature was 150 °C, and the detector temperature was 250 °C. The initial temperature was kept at 100 °C for 4 min, and the temperature was gradually increased to 130 °C at a rate of 5 °C /min and was then held for 20 min at 130 °C. The linear velocity of the helium carrier gas was 1.2 mL.min−1 at a split ratio of 30:1; EI was used as the ion source, and the ion source temperature was 230 °C. The sector mass analyzer was set to scan from 30 to 550 amu, scan time, 1 s. Diluted samples (15 μg.mL−1) were prepared using methylene dichloride, and 0.6 μL samples were injected for analysis.
The compounds identification were confirmed by the comparison of the elution order of the compounds with their relative retention indices. The retention indices were calculated for all volatile constituents using a n-alkanes homologous series. In addition, their EI–mass spectra were compared with the National Institute of Standards and Technology (NIST05.LIB) library spectra and the literature (Adams 2001). Finally, the identity of the indicated phytochemicals was confirmed by comparison with available authentic samples.
FT-IR Analysis
An FTIR spectrometer (A Nicolet 5700 from Thermo Nicolet Corp.) was used that was equipped with a deuterated triglycine sulfate (DTGS) as a detector. IR spectra were recorded in the 400–4000 cm−1 range with a resolution of 4 cm−1. The room was kept at a controlled ambient temperature (25 °C) and relative humidity (30 %).
Precisely weighed, dry KBr powder (200 mg) was made into two pieces of transparent blank KBr tablets that were approximately 5 mm in diameter and approximately 1 mm in thickness. Volatile oils (2 μL) were coated on the KBr tablets to form thin liquid films for infrared spectrometry analysis. The sample measurements were replicated 5 times with 4 scans each for a total of 20 spectra, and then the average chart was taken as a last sample spectrum. The background air spectrum, water vapor and CO2 interference were subtracted from these spectra. After baseline correction and smoothing were performed using the OMNIC 8.0 software, the spectrum data were imported in Unscrambler 9.7 software to standardize the normal variations.
Tissue Clearing
The distribution of oil cells was observed using the method of tissue clearing (Platt-Aloia and Thomson 1992). The leaf blade was dissociated using a modified version of the tissue clearing method (Li et al. 2013b). The medium of the intact leaf was divided into 1 cm × 1 cm pieces. All of the samples were placed into a 5 % sodium hydroxide solution and maintained at 60 °C for 24–72 h followed by washing with distilled water. Next, the specimens were fixed in a hydrogen dioxide solution for 5–10 min to remove the color followed by washing with distilled water. The samples were subsequently stripped from the lower epidermis. After staining with Sudan III, the samples were observed using a Leica DMLB microscope. The density of the oil cells was examined and digitally recorded. The density (n/mm2) (n means number of oil cells) and the diameter (μm) of the oil cells were counted, measured and averaged from15 samples.
Statistical Analysis
Statistical analysis of the yield of essential oil, the chemical and enzyme activity were performed using SPSS 18.0 (SPSS Inc., Chicago, USA). The measured values were expressed as a mean ± standard deviation (SD), n = 3. The unpaired Student’s t-test at a probability of 5 % was used to compare the obtained data for differences due to different developmental stages for the leaves of C. verum.
Hierarchical Clustering Analysis
Hierarchical clustering is a cluster analysis method that seeks to build a hierarchy of clusters (Buchgraber et al. 2004). Hierarchical cluster analysis and Euclidean distance were applied in this study. To establish the distance matrix for the chromatographic area and samples for the cluster analysis for the GC–MS, the relative absorbency was selected as a measurement for the cluster analysis in the FTIR. The cluster analysis and dendrogram were performed with SPSS version 18.0 software (SPSS Inc., Chicago, USA).
Results
Essential Oil Yields
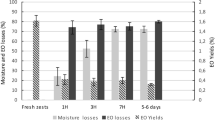
We investigated the yield of essential oils in leaves of different ages. As shown in Fig. 1, C. verum leaves contained a high oil yield, and there were apparent differences in the oil content among C. verum leaves of different ages. The highest oil yield was obtained from the leaves from the 2-year-old branches (5.81 %) followed by leaves 5–7 collected from the 1-year-old branches (4.95 %). The leaves collected from the 3-year-old branches only contained 3.10 % oil. Leaves 1–4 collected from the 1-year-old branches had 3.04 % oil, and the leaves collected from the 4-year-old branches contained the lowest yield with only 2.98 % oil.
Yields (% w/w) of essential oil from different developmental stages for the leaves of C. verum. The columns with different lowercase letters are significantly different (P<0.05). (1: The 1–4 leaves of the 1-year-old branches; 2: The 5–7 leaves of the 1-year-old branches; 3: The leaves of the 2-year-old branches; 4: The leaves of the 3-year-old branches; 5: The leaves of the 4-year-old branches)
To study the correlation of the essential oil yields with the growth stages of the C. verum leaf, we used a modified method in which the leaves were stained with Sudan III after clearing the tissue, and then, the essential oils were stained bright yellow or red color and observed under a light microscope. The oil droplets had not only accumulated in the oil cells (Fig. 2, 13–16) but also in common mesophyll cells (Fig. 2, 17). Our observations revealed that the yield of the essential oils was correlated with the density of the oil cells and the degree of oil accumulation. The first leaves from the 1-year-old branches had a low density of oil cells (i.e., 4.64 n/mm2) (Fig. 3), and oils exhibited a lower accumulated in the main vein (Fig. 2, 1) and mesophyll cells (Fig. 2, 7). Only a few oils were stained yellow or red (Fig. 2, 7). The density of oil cells increased to 7.26, 8.62 and 9.29 n/mm2 (Fig. 3) in leaves 2–4, and the oil accumulation also began to increase (Fig. 2, 8). In general, a lower density of oil cells resulted in a lower oil yield (3.04 %) in leaves 1–4 collected from the annual branches. As the leaves developed, oils formed and accumulated, and numerous oils were stained red and yellow in the main vein and mesophyll cells of the six leaves from the 1-year-old branches (Fig. 2, 3 and 9), which resulted in a sharp increase in the essential oil yield (4.95 %). In the mature leaves collected from the 2-year-old branches, the highest number of oil cells was found in the main vein where the oils were stained red (Fig. 2, 4), and the density of oil cells in the leaf reached a maximum at 10.17n/mm2 (Fig. 3), which coincided with the highest oil yield (5.81 %). The oils in the leaves collected from the 3- and 4-year-old branches began to degrade, and the oils were stained reddish brown (Fig. 2, 5 and 6; 11 and 12). The essential oil yields significantly decreased (3.10 and 2.98 %, respectively) even though there was a high density of oil cells (Fig. 3).
The variation in the development of essential oils in the oil cells of C. verum leaves (Sudan III staining) 1–6. Distribution of essential oils in main vein cells of C. verum leaves. 1. 1 leaf of the 1-year-old branches; 2. 4 leaves of the 1-year-old branches; 3. 6 leaves of the 1-year-old branches; 4. The leaves of the 2-year-old branches; 5. The leaves of the 3-year-old branches; 6. The leaves of the 4-year-old branches. 7–12. Distribution of essential oils in the mesophyll cells of C. verum leaves. 7. 1 leaf from the 1-year-old branches; 8. 4 leaves from the 1-year-old branches; 9. 6 leaves from the 1-year-old branches; 10. The leaves from the 2-year-old branches; 11. The leaves from the 3-year-old branches; 12. The leaves from the 4-year-old branches. 13–14. Oil cells in the mesophyll; 15–16. Oil cells in the main vein; 17. Essential oils in the common mesophyll cells. The arrows indicate oil droplets
The density of oil cells at different developmental stages for the leaves of C. verum. (1–1: The 1 leaves of the 1-year-old branches; 1–2: The 2 leaves of the 1-year-old branches; 1–3: The 3 leaves of the 1-year-old branches; 1–4: The 4 leaves of the 1-year-old branches; 2–1: The 5 leaves of the 1-year-old branches; 2–2: The 6 and 7 leaves of the 1-year-old branches; 3: The leaves of the 2-year-old branches; 4: The leaves of the 3-year-old branches; 5: The leaves of the 4-year-old branches)
GC–MS Fingerprint of C. verum Leaves at Different Growth Stages
To reveal the variations in the chemical composition of the oils during C. verum leaf growth, we analyzed the oil compositions of the C. verum leaves using GC–MS. The identified components are listed in Table 2, the GC–MS analysis of the oils in leaves 1–4 collected from the 1-year-old branches, leaves 5–7 collected from the 1-year-old branches and the leaves collected from the 2-, 3- and 4-year-old branches allowed the identification of 26, 29, 27, 29 and 28 components, respectively, representing 97.05-99.50 % of the total oil. Our results revealed obvious differences in the oil constituents of C. verum leaves with different ages. The results indicated that the content of eugenol gradually decreased with the development of the leaves. Leaves 1–4 collected from the annual branches contained the highest eugenol content (93.69 %), while the leaves collected from the 4-year-old branches contained the lowest content of eugenol (89.98 %) (Table 2). In addition, C. verum leaf oils also contained relatively high amounts of trans-cinnamaldehyde. The highest trans-cinnamaldehyde content was observed for the leaves from the 2-year-old branches followed by leaves 5–7 from the 1-year-old branches and the leaves from the 3-year-old branches. Leaves 1–4 from the annual branches contained the lowest content of trans-cinnamaldehyde (i.e., only 1.68 %) (Table 2). In addition, the C. verum leaf oils contained high amounts of benzenepropanal (1.03-1.65 %), trans-caryophyllene (0.40-0.81 %), clovene (0.41-0.90 %) and hexadecanoic acid (0.31-0.55 %). In addition, the content of spathulenol, clovene and benzyl benzoate increased with the development of the leaves, while the content of gurjunene decreased with the development of the leaves. A small amount of cinnamyl acetate and cinnamic acid was detected in the leaves collected from the 1-year-old branches. Thereafter, their content decreased to a very small amount or was not detected for older leaves.
To more directly observe the differences in the essential oil components of C. verum leaves at different growth stages, the oil components were analyzed using a hierarchical cluster for the five samples. As shown in Fig. 4, sample No. 1 (leaves 1–4 from the 1-year-old branches) was distinguished as a cluster because it was obviously different from the other four samples. Sample No. 2 (leaves 5–7 from the 1-year-old branches) and sample Nos. 3 and 4 (the leaves from the 2- and 3-year-old branches) were classified as a cluster due to their small distance coefficient, which indicated that the oil compositions of these three samples were the most similar.
FT-IR Fingerprints of the Leaves of C. verum at Different Growth Stages
The FT-IR spectrum shows an overall overlap of the characteristic absorption peaks for all of the functional groups (Cai et al. 2006). To further determine the accumulation of the volatile chemical compositions of the C. verum leaves, the five samples were studied using IR characteristic fingerprints and the results are shown in Fig. 5a. The IR characteristic fingerprint peaks for all of the samples are primarily in the range of 1800–600 cm−1 (Fig. 5a). A typical spectrum for the C. verum leaves is shown in Fig. 5b, and several characteristic peaks were observed in this spectrum including peaks at 1604, 1511 and 1432 cm−1, which correspond to the stretching vibration of the benzene ring. The peak at 1367 cm−1 is due to the C-H bending vibrations. The more intense peaks at 1271 and 1233 cm−1 correspond to the bending vibrations of the phenol OH group and the stretching vibrations of C-O, which is due to high levels of eugenol in the volatile oil of the C. verum leaves. The peaks at 1150 and 1035 cm−1 are attributed to the stretching vibrations of C-O. The peaks at 913 and 816 cm−1 are attributed to the aromatic ring, and the peaks at 748 and 646 cm−1 are due to the benzene ring = CH vibrations. As shown in Fig. 5a, the spectra for the five samples in the range of 1800–600 cm−1 are quite similar. However, the volatile oil compounds of the five samples are complex and diverse.
It is difficult to identify the differences in the components of the samples because the chemical composition fingerprints overlap significantly. To more directly determine the differences in the components of the C. verum leaves, the data from the IR spectra of the five samples were selected for hierarchical cluster analysis. As shown in Fig. 6, sample No. 1 (leaves 1–4 from the 1-year-old branches) was placed in its own category because it was significantly different from the other four samples. Sample Nos. 2–4 (leaves 5–7 from the 1-year-old branches and the leaves from the 2- and 3-year-old branches) were distinguished as a cluster due to their small distance coefficient. These results further confirmed the results obtained from the GC-MS hierarchical cluster analysis.
Comparative Analysis of the IR Characteristic Peaks and Eugenol Content
Because eugenol is the major component in C. verum oil, pure eugenol standard samples (Sigma-Aldrich, Co., USA) were compared with the oils of the C. verum leaf samples in this study (Fig. 5c). By comparing the IR spectra of the eugenol standards with the samples, the peaks at 1604, 1511, 1432, 1367, 1271, 1035, 913, 816 and 748 cm−1 corresponded to peaks in the 5 samples (Figs. 5b and c). The peak height and width represents the absorption strength of the components. The primary absorption peaks for eugenol, which were the highest and widest, were located at 1604, 1511, 1432, 1367, 1271 and 1233 cm−1. The peaks at 1604, 1511 and 1432 cm−1 correspond to the stretching vibrations of the benzene ring, The peaks of 1271 and 1233 cm−1 correspond to the bending vibrations of the phenol OH group and the stretching vibrations of C-O, respectively. Because the absorption peaks in the original spectra overlap with various other chemical components, it is difficult to analyze the differences in the eugenol content using these peaks. Therefore, the peaks at 1604, 1511, 1432, 1367, 1271 and 1233 cm−1 were analyzed by curve fitting using Gaussian bands, and ten peaks were fitted (Figs. 7a and b). According to the Lambert-Beer Law, Lorenz-Fonfria and Padros (2004) used curve fitting to analyze the area percentage (A%). Typically, this relative content can be used to analyze each functional group in the samples. To compare the differences in relative eugenol content, we studied the relative content of eugenol based on the relative absorption peak areas at 1513, 1463, 1429 and 1269 cm−1 for the five samples. The results indicated that sample No. 1 (leaves 1–4 from the 1-year-old branches) exhibited the highest relative content at peaks of 1513, 1463 and 1429 cm−1 (33.32 %) and 1269 cm−1 (25.15 %). Therefore, sample No. 1 contained a higher eugenol content. The relative content of eugenol decreased with the growth of the leaves. Sample No. 5 (the leaves from the 4-year-old branches) had the lowest relative content of eugenol (Table 3). The results obtained were identical to those obtained by GC–MS.
Discussion
Variation Characteristics of Essential oil Yields from C. verum Leaves
In general, leaf age and traits have a significant influence on the oil content (James and Bell 1995). The important characteristics of essential oil production and accumulation are highly influenced by the physiology of the plant and therefore depend on its developmental stage (Singh et al. 1989; Sangwan et al. 2001). Volatile compounds primarily accumulate in mature leaves. After this point, the accumulation of essential oils will cease, and the content of essential oils decreases due to evaporation or degradation of the oils (List et al. 1995; Fischer et al. 2011). Therefore, the oil yield of adult leaves is consistently higher than that of juvenile leaves (Li and Madden 1995; Li et al. 1996). Indeed, young and old leaves contained lower essential oil yields in cinnamon compared with the mature leaves of cinnamon (Ravindran et al. 2004; Li et al. 2013b). New or “flush” leaves contain no oil. Young leaves have a low oil content, and mature leaves have the highest cinnamon oil content (Lawrence and Farbman 1984). Recently, our results revealed that the oil yields were closely associated with the oil cell ontogeny and the developmental stage of cassia leaves. In particular, the distribution density of oil cells and the degree of accumulation of the oils have a significant influence on the oil yield and quality (Li et al. 2013b). The present study revealed that C. verum leaves had a higher essential oils yield, and substantial differences in the yield of essential oils at differently growth stage leaves of C. verum were observed. The juvenile leaves (leaves 1–4 from the 1-year-old branches) exhibited a low density of oil cells, and oils were less accumulated in the main vein and mesophyll cells, which had a markedly low oil yield. The density of the oil cells continuously increased in the mature leaves (leaves 5–7 from the 1-year-old branches and the leaves from the 2-year-old branches), and the oils were largely accumulated and contained the highest oil yield in leaves 5–7 from the 1-year-old branches and the leaves from 2-year-old branches. The leaves from the 3- and 4-year-old branches were in the aging stage where the content of essential oils rapidly decreased due to evaporation or degradation of the oils (List et al. 1995). These observations were in agreement with the results reported by Li et al. (2013b). A conservative balanced relationship between the developmental stages of leaves and oil accumulation in cinnamon has been demonstrated.
In addition, the oil yields of C. verum leaves (2.98-5.81 %) are higher than those of C. cassia leaves (0.54-2.12 %) (Li et al. 2013b), which may be due to the high density of oil cells and the common mesophyll cells participating in the synthesis of the essential oils.
Formation and Variation of Compositions in Essential Oils from C. verum Leaves
Plants synthesize various secondary metabolites via photosynthesis during the growth process and also synthesize different compounds in different organs or at different growth stages (Jayaprakasha and Jagan Mohan Rao 2011; Kaneria et al. 2012). Some reports indicated that the variation in the essential oil composition is related to the age of the leaves (Zygadlo et al. 1994; Gershenzon et al. 2000; Dudai et al. 2001; Santos-Gomes et al. 2005). Argyropoulou et al. (2007) reported that the different growth stages showed significant differences in the percentages of the geranial, neral and limonene from leaves of Lippia citriodora H.B.K. (Verbenaceae). Amaral et al. (2015) observed that the linalool content in young leaves was consistently higher than that of old leaves of Nectandra megapotamica (Spreng.) Mez. The bark and leaf are the main medicinal parts of C. verum. Cinnamaldehyde is the primary component of cinnamon bark oils (52.0-83.0 %), and eugenol is the primary component of cinnamon leaf oils (65.0-96.0 %) (Paul and Sahoo 1993; Mallavarapu et al. 1995; Sahoo et al. 2000). The differences between the primary compositions of cinnamon bark and leaves result in the unique value of the commodity. It has been confirmed that the leaves are the first location in the synthesis of eugenol (Neish 1960), and the barks contribute very little to the biosynthesis of eugenol (Ravindran et al. 2004). Eugenol is one of the main phenylpropenes. In the biosynthetic pathway of the EO, certain compounds are derived from each other (Koeduka et al. 2006). It is documented that eugenol, the precursor of methyleugenol, was predominant in younger leaves, where as methyleugenol predominated in the older leaves (Dudai et al. 2002). Fischer et al. (2011) demonstrated that eugenol levels were higher in younger leaves (53 %), and methyleugenol levels predominated in older leaves (68 %). Linalool was higher in younger leaves than in mature leaves. Hence, this suggested that eugenol converted into methyleugenol and linalool decreased as leaf mature. Moreover, it is demonstrated that the essential oil composition in an individual leaf is mostly affected by the leaf position on the stem (Fischer et al. 2011). However, eugenol synthesis and its variation at different growth stages in the leaves of C. verum remain unknown. Insight into these processes has important practical significance for obtaining high quality oils. Our research indicated that the eugenol content from C. verum leaves differed greatly at different growth stages. The content of eugenol decreased with the development of the leaves. Based on the variations in eugenol content, it appears that the eugenol was primarily formed in the juvenile leaves (leaves 1–4 from the 1-year-old branches) of C. verum, and then, the eugenol synthesis decreased with the growth stages. These results are in agreement with previous reports that eugenol gradually decreased with progress of development stage of the leaf (Dey and Choudhuri. 1983; Fischer et al. 2011). Our study demonstrated that eugenol was primarily synthesized in leaves 1–4 collected from the 1-year-old branches. Furthermore, eugenol may be evaporated or degraded in the leaves of branches that are more than 3 years old. Further studies on the genes and key enzymes regulating the biosynthesis of eugenol could provide insight into the biosynthesis of eugenol and provide valuable information for the artificial regulation of eugenol biosynthesis.
Conclusions
The use of GC–MS and FT-IR, combined with microscopy to analyze the volatile oil compounds of C. verum leaves, can effectively evaluate the 5 samples. The content of the primary components of essential oils directly influences the oil quality grades and commodity values, while cinnamon leaf oil is graded according to the eugenol content (Ravindran et al. 2004). Based on analysis of the variations in oil yields and compositions, leaves 5–7 collected from the 1-year-old branches and the leaves collected from the 2-year-old branches should be chosen as the raw material for extraction of high quality C. verum oils or eugenol. The highest eugenol content was observed for leaves 1–4 collected from the 1-year-old branches, which could be used as the raw materials for extraction of elite eugenol. Our results revealed that C. verum contains higher oil yields (2.98-5.81 %) and eugenol contents (89.98-93.69 %) and indicated that the Guangxi province is a suitable cultivation site for C. verum development and utilization. Based on leaves 1–4 collected from the 1-year-old branches, which are the predominant site of eugenol synthesis, we propose that strengthening the cultivation and management of young leaves will facilitate the synthesis of eugenol. The results provide reference information for the rational utilization of cinnamon resources and the harvest of cinnamon leaves. In addition, these results have important practical significance for improving the quality grades and economic benefit of cinnamon leaves, and the techniques used in this study can provide a comprehensive evaluation for cinnamon quality.
References
Adams RP (2001) Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Allured Publishing Corporation, Carol Stream, IL, USA
Amaral LP, Tondol JSM, Schindler B, Silva DT, Pinheiro CG, Longhi SJ, Mallmann CA, Heinzmann BM (2015) Seasonal influence on the essential oil production of Nectandra megapotamica (Spreng.) Mez. Braz Arch Biol Technol 58:12–21
Argyropoulou C, Daferera D, Tarantilis PA, Fasseas C, Polissiou M (2007) Chemical composition of the essential oil from leaves of Lippia citriodora H.B.K. (Verbenaceae) at two developmental stages. Biochem Syst Ecol 35:831–837
Buchgraber M, Ulberth F, Anklam E (2004) Cluster analysis for the systematic grouping of genuine cocoa butter and cocoa butter equivalent samples based on triglyceride patterns. J Agric Food Chem 52:3855–3860
Cai JB, Lin P, Zhu XL, Su QD (2006) Comparative analysis of clary sage (S. sclarea L.) oil volatiles by GC–FTIR and GC–MS. Food Chem 99:401–407
China Pharmacopeia Commission (2010) Pharmacopoeia of the People’s Republic of China 2010, vol 1. Chinese medical science and technology press, Beijing, China, pp 63–127
De Guzman CC, Siemonsma JS (1999) Plant resources of South East Asia. 13. Spices. Backhuy’s Publishers, Leiden
Deng XJ, Liao QF, Xu XJ, Yao MC, Zhou YT, Lin MN, Zhang PT, Xie ZY (2014) Analysis of Essential Oils from Cassia Bark and Cassia Twig Samples by GC-MS Combined with Multivariate Data Analysis. Food Anal Methods 7:1840–1847
Dey BB, Choudhuri MA (1983) Effect of leaf development stage on changes in essential oil of Ocimum sanctum L. Biochem Physiol Pflanz 178:331–335
Dudai N, Chaimovitsh D, Reuveni R, Larkov O, Putievsky E (2002) Breeding of sweet basil (Ocimum basilicum) resistant to Fusarium wilt caused by Fusariun oxysporum f. sp. basilicum. J Herbs Spices Med Plants 9:45–51
Dudai N, Larkov O, Ravid U, Putievsky E, Lewinsohn E (2001) Developmental control of monoterpene content and composition in Micromeria fruticosa (L.) Druce. Ann Bot 88:349–354
Fischer R, Nitzan N, Chaimovitsh D, Rubin B, Dudai N (2011) Variation in essential oil composition within individual leaves of Sweet Basil (Ocimum basilicum L.) is more affected by leaf position than by leaf age. Agr Food Chem 59:4913–4922
Gang DR, Wang JH, Dudareva N, Nam KH, Simon JE, Lewinsohn E, Pichersky E (2001) An investigation of the storage and biosynthesis of phenylpropenes in basil. Plant Physiol 125:539–555
Gershenzon J, McConkey ME, Croteau RB (2000) Regulation of monoterpene accumulation in leaves of peppermint. Plant Physiol 122:205–213
James SA, Bell DT (1995) Morphology and anatomy of leaves of Eucalyptus camaldulensis clones: variation between geographically separated locations. Aust J Bot 43:415–433
Jayaprakasha GK, Jagan Mohan Rao L (2011) Chemistry, Biogenesis, and Biological Activities of Cinnamomum zeylanicum. Crit Rev Food Sci Nutr 51:547–562
Jayaprakasha GK, Negi PS, Jena BS, Jagan Mohan Rao L (2007) Antioxidant and antimutagenic activities of Cinnamomum zeylanicum fruit extracts. J Food Compos Anal 20:330–336
Joy PP, Thomas J, Mathew S, Ibrahim KK (1998) Growth, leaf oil yield and quality investigations in Cinnamon (Cinnamomum verum). Journal of Medicinal and Aromatic Plant Sciences 28:401–406
Kaneria MJ, Bapodara MB, Chanda SV (2012) Effect of extraction techniques and solvents on antioxidant activity of Pomegranate (Punica granatum L.) leaf and stem. Food Anal Methods 5:396–404
Koeduka T, Fridman E, Gang D, Vasso D, Jackson B, Kish C, Orlova I, Spassova S, Lewis N, Noel J, Baiga T, Dudareva D, Pichersky E (2006) Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proc Natl Acad Sci U S A 103:10128–10133
Lawrence B, Farbman M (1984) Feasiblbility study for Cinnamon processing in the Seychelles. A Report to the U.S. Agency for International Development Nairobi, Kenya pp.1–90
Li H, Madden JL (1995) Analysis of leaf oils from a Eucalyptus species trial. Biochem Syst Ecol 23:167–177
Li H, Madden JL, Potts BM (1996) Variation in volatile leaf oils of the Tasmanian Eucalyptus species II subgenus Symphyomyrtus. Biochem Syst Ecol 24:547–569
Li YQ, Kong DX, Huang RS, Huang HL, Xu CG, Wu H (2013a) Variations in essential oil yields and compositions of Cinnamomum cassia leaves at different developmental stages. Ind Crop Prod 47:92–101
Li YQ, Kong DX, Wu H (2013b) Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind Crop Prod 41:269–278
List S, Brown PH, Walsh KB (1995) Functional anatomy of the oil glands of Melaleuca alternifolia (Myrtaceae). Aust J Bot 43:629–641
Lorenz-Fonfria VA, Padros E (2004) Curve-fitting of Fourier manipulated spectra comprising apodization, smoothing, derivation and deconvolution. Spectrochim Acta Part A-Mol Biomol Spectrosc 60:2703–2710
Mallavarapu GR, Ramesh S, Chandrasekhara RS, Rajeswara Rao BR, Kaul PN, Bhattacharya AK (1995) Investigation of the essential oil of cinnamon leaf grown at Bangalore and Hyderabad. Flavour Fragr J 10:239–242
Moarefian M, Barzegar M, Sattari M (2013) Cinnamomum zeylanicum essential oil as a natural antioxidant and antibactrial in cooked sausage. J Food Biochem 37:62–67
Neish AC (1960) Biosynthetic pathways of aromatic compounds. Ann Rev Plant Physiol II:55–80
Paul SC, Sahoo S (1993) Selection of elite cinnamon plants for quality bark production. J Econ Taxon Bot 17:353–355
Platt-Aloia KA, Thomson WW (1992) Idioblast oil cells of avocado: distribution, isolation, ultrastructure, histochemistry, and biochemistry. Int J Plant Sci 153:301–310
Ravindran PN, Nirmal Babu K, Shylaia M (2004) Cinnamon and Cassia (The genus Cinnamomum). The Chemical Rubber Company Press, Boca Raton, USA, pp 1–37
Sahoo S, Paul SC, Patra P (2000) Quality cinnamon production in India. Med Aromat Plant Sci 22:361–365
Sangwan NS, Farooqi AHA, Shabih F, Sangwan RS (2001) Regulation of essential oil production in plants. Plant Growth Regul 34:3–21
Santos-Gomes PC, Fernandes-Ferreira M, Vicente AMS (2005) Composition of the essential oils from flowers and leaves of Vervain (Aloysia triphylla (L’Herit.) Britton) grown in Portugal. J Essent Oil Res 17:73–78
Singh N, Luthra R, Sangwan RS, Thakur RS (1989) Metabolism of monoterpenoids in aromatic plants. Curr Res Med Aromat Plants 11:174–197
WHO (1999) Cortex Cinnamomi. In: WHO Monographs on Selected Medicinal Plants. World Health Organization, Geneva, pp.95–1040
Wijesekera ROB (1978) The chemistry and technology of Cinnamon. CRC Crit Rev Food Sci Nutr CRC Critical Review in Food Science and Nutrition 10:1–30
Zygadlo JA, Lamarque AL, Maestri DM, Guzman CA, Lucini EI, Grosso NR, Ariza-Espinar L (1994) Volatile constituents of Aloysia triphylla (L’Herit.) Britton. J Essent Oil Res 6:407–409
Acknowledgments
We wish to thank Mr. Lei Fan for their technical assistance. The authors wish to thank Guiqing Li for their assistance in collecting the cinnamon samples. This study was supported by the PhD Start-up Fund of Natural Science Foundation of Guangdong Province (2014A030310433), China and the Science and Technology Innovation Fund Project on Forestry of the Guangdong Province (2012KJCX015-06).
Conflict of Interest
Yanqun Li declares that he has no conflict of interest. Dexin Kong declares that she has no conflict of interest. Xiaoman Lin declares that she has no conflict of interest. Zhaohong Xie declares that he has no conflict of interest. Mei Bai declares that he has no conflict of interest. Shushi Huang declares that he has no conflict of interest. Hai Nian declares that he has no conflict of interest. Hong Wu declares that he has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Kong, D., Lin, X. et al. Quality Evaluation for Essential Oil of Cinnamomum verum Leaves at Different Growth Stages Based on GC–MS, FTIR and Microscopy. Food Anal. Methods 9, 202–212 (2016). https://doi.org/10.1007/s12161-015-0187-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0187-6