Abstract
An analytical method for determination of multi-element composition of Vranec wines using microwave digestion for sample preparation and inductively coupled plasma mass spectrometry (ICP-MS) was optimized and validated. Best recoveries, ranging from 87 to 114 %, were obtained for all analyzed elements, using a volume of 5 mL wine and 5 mL HNO3 for sample microwave digestion. In total, 38 elements (Ag, Al, As, B, Ba, Be, Bi, Ca, Cd, Co, Cr, Cs, Cu, Fe, Ga, Ge, In, Li, Mg, Mn, Mo, Na, Ni, P, Pb, Pd, Rb, S, Sb, Se, Si, Sn, Sr, Te, Ti, Tl, V, Zn) were determined in wines. The calibration curves of all elements were linear with correlation coefficients (R 2) ranging from 0.9920 for 11B to 1.0000 for 53Cr and 88Sr. The accuracy of the method was checked with a standard addition method showing good repeatability and reproducibility (relative standard deviation, RSD <10 %). Vranec wines were fermented with Saccharomyces cerevisiae yeast strains commercial Clos, RC212, D254, and BDX, and six autochthonous Vinalco yeasts. The content of total elements in all Vranec wines ranged between 348 to 678 mg/L, observing lower amounts in wines fermented with the autochthonous Vinalco yeast. The content of harmful elements, such as Pb and Cu, was below the maximal allowed concentration in all wines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The multi-element composition of wine is a very important factor for its quality, stabilization, and nutritional value. In fact, the analysis of certain elements in wine is very important from nutritional point of view since wine contains essential elements required for the human organism, such as Ca, Co, Fe, K, Mg, Cu, Se, Zn, Ni, Cr, and Mn. On the other hand, wine could contain potentially toxic elements such as Pb, Cd, and As and therefore the determination of elements in wine is of special interest (Grindlay et al. 2011). The major elements in wine (Ca, K, Na, and Mg) are usually present at levels between 10 and 1000 mg/L, minor elements such as Al, Fe, Cu, Mn, Rb, Sr, and Zn are present in the range of 0.1–10 mg/L, and trace elements (Ba, Cd, Co, Cr, Li, Ni, Pb, and V) are ranged between 0.1 and 1000 g/L (Pohl 2007).
Mineral composition of wines is influenced by many factors, including the chemical and physical characteristics of the soil in which the vine is growing on, vine variety, capacity of the grapes to absorb mineral substances from the soil, climate changes, and viticultural practices, such as irrigation and application of fertilizers, pesticides, herbicides, and insecticides. Wines from vineyards located in industrial areas and near to road traffic contain higher levels of Cd and Pb because of vehicle exhaust fumes or other emissions to air, water, and soil (Pohl 2007). In addition, the elemental composition of wine depends on the applied winemaking practices during different steps of production. Thus, contamination could occur during the fermentation (addition of yeasts, maceration), storage, and aging (content of proteins, fining agents). Furthermore, longer contact of wine with the equipment produced from different materials (aluminum, brass, stainless steel) used for handling and storing of wine is a source of Al, Cd, Cr, Cu, Fe, and Zn (Castiñeira Gómez et al. 2004; Lara et al. 2005; Pohl 2007; Cheng and Liang 2012; Volpe et al. 2009; Tariba 2011; Hopfer et al. 2013).
During the technological process of winemaking, composition of elements is changing mainly due to the precipitation of K and Ca tartrates as well as precipitation of Al, Cr, Cu, Fe, Mn, Ni, Pb, and Zn (Rodriguez Mozaz et al 1999). In addition, the concentration of elements in wine could be modified by the presence of living or non-living Saccharomyces cerevisiae yeast lowering significantly the final content of some metals. Yeast consumes Ca, Cu, Fe, K, Mg, and Zn causing decrease of their content during fermentation (Volesky and May-Phillips 1995; Blackwell et al. 1995; Rodriguez Mozaz et al 1999; Nicolini and Larcher 2003).
Red wine is a very complex mixture of ethanol and different organic compounds such as carbohydrates, organic acids, volatiles, and bioactive compounds (anthocyanins, monomeric and polymeric flavan-3-ols, flavonols, and phenolic acids). Therefore, sample pre-treatments are necessary for its multi-element analysis. In fact, it is necessary to dilute or decompose the wine because of the possible matrix interferences. Elements Al, Cd, Cu, Fe, Ni, Pb, and Zn could be determined without dilution, while elements Ca, K, Mg, and Na are analyzed after dilution of wine sample with water (Frias et al 2001; Frias et al. 2003; Diaz et al. 2003). Decomposition could be performed by wet digestion on a hot plate or in a microwave oven using concentrated HNO3, HClO4, and H2SO4 or mixtures of these acids (Frias et al 2001; Frias et al. 2003; Diaz et al. 2003; Castiñeira Gómez et al. 2004; Álvarez et al. 2007; Ivanova-Petropulos et al. 2013, 2015b).
For metal determination in wine, atomic absorption spectroscopy (AAS) is a technique of choice, suitable for direct determination of trace elements (Stafilov and Karadjova 2009). Flame AAS (FAAS) and graphite AAS (GAAS) are applied for measurements of alkalis (K, Li, Na, and Rb), alkaline earth metals, Cu, Fe, Mn, and Zn (Diaz et al. 2003), as well as trace elements (Rebolo et al. 2000; Esparza et al. 2004; Stafilov and Karadjova 2009; Ivanova-Petropulos et al. 2015b). Electrothermal atomic absorption spectroscopy (ETAAS) technique offers high sensitivity and selectivity for determination of low levels of metals. Nowadays, the most versatile techniques for wine multi-element analysis are inductively coupled plasma optical emission spectrometry (ICP-OES) and inductively coupled plasma mass spectrometry (ICP-MS) providing high detection power, and high selectivity and sensitivity (Muranyi and Kovacs 2000; Castiñeira Gómez et al. 2004; Sperkova and Suchanek 2005; Ivanova-Petropulos et al. 2013, 2015b).
Republic of Macedonia has a very long wine history. Different grape varieties are grown on Macedonian territory, and between them, Vranec is the most characteristic variety with the highest economic importance for the country. Preliminary studies on the phenolic and aromatic content of this wine variety produced under different vinification conditions have been performed (Ivanova et al. 2012; Ivanova-Petropulos et al. 2015a, 2015c; Ivanova Petropulos et al. 2014). Moreover, ICP-OES and ICP-MS techniques were applied for multi-elemental composition of different commercial Macedonian wines, including Vranec wines too, from different wine regions.
However, to the best of our knowledge, there has been no report on the characterization of the multi-element composition of Vranec wines fermented with different yeast strains, autochthonous and commercial. In fact, in Macedonia, there is only one autochthonous isolated yeast strain, Vinalco, which effect on phenolics extraction from grapes into Vranec, Chardonnay and Smederevka wines was studied (Ivanova et al. 2011a, 2011b; 2012), but until now, no research have been performed on its effect on the element content and composition in wines. Considering this, the objectives of the study were (1) to develop, optimize, and validate a microwave digestion method for wine sample pre-treatment, followed by ICP-MS determination of the elements, and (2) to assess the influence of different yeast preparations, Vinalco (Macedonian autochthonous yeast) and commercial yeasts from Lallemand, on the multi-element composition of Vranec wines.
Materials and Methods
Chemicals and Reagents
For all analytical procedures, ultrapure water was used (0.065 μS/cm), obtained from water purification system TKA Microlab, ASTM Type II water (Thermo Electron LED GmbH, Germany). Nitric acid (69.0 %, w/w, trace select, Sigma Aldrich, Munich, Germany) was used for wine digestion and for the conservation of standard solutions for construction of calibration curves. Multi-element certificate standard solution (Periodic table Mix 1 for ICP, 10 mg/L, Sigma Aldrich, Munich, Germany) contained 33 elements (Al, As, Ba, Be, Bi, B, Cd, Ca, Cs, Cr, Co, Cu, Ga, In, Fe, Pb, Li, Mg, Mn, Ni, P, K, Rb, Se, Si, Ag, Na, Sr, S, Te, Tl, V, and Zn). Single-element standards were used for the construction of the calibration curves for Ti, Ge, Sb, Sn, and Mo (10 ppm in 10 % HNO3 trace select, Sigma Aldrich, Munich, Germany). Tuning solution (ICP-MS Tuning Solution, contains 10 mg/L each of Li, Y, Ce, Tl, and Co in a matrix of 2 % HNO3, Agilent technologies, Santa Clara, CA, USA) was used for optimization of the ICP-MS instrument. Rhodium standard solution (1 mg/L, Sigma Aldrich, Munich, Germany) was used as an internal standard for correction of the drifts for external calibration curves.
Grapes
Grapes from V. vinifera L. varieties Vranec, cultivated in the Tikveš wine region (Republic of Macedonia), were harvested in September 2013, at optimal technological maturity (20.0°brix). Vranec grapes were collected from 15-year-old vineyards with area of 0.5 ha. The distance between the rows was 1.5 m and the distance between the vines was 1 m. Grapes were manually harvested early in the morning and placed in crates.
Wine Samples
Vranec wines (10 samples) were produced using electrical inox crusher/destemmer for grapes treatment. The churched grapes were supplied with SO2 (60–70 mg/L total concentration) in a form of 5 % sulphurous acid. Four wines were fermented with the S. cerevisiae yeast strains Clos, RC212, D254, and BDX (Lallemand, Bordeaux, France), and six wines with autochthonous Vinalco yeast (Bitola, Republic of Macedonia). Autochthonous Vinalco yeasts were selected by the factory for yeast and alcohol manufacture in Bitola, from grapes grown in the Tikveš region, Republic of Macedonia. Grape mash for all ten wines was macerated for 8–10 days at temperature of 23 ± 2 °C. Pumping over with delastage was performed once per day during the first 3 days of maceration, followed by pumping over two times a day. ICP-MS analyses of wines were performed after a 6-month period of stabilization.
Sample Preparation
To remove the appreciable amounts of deposits and colloidal suspensions present, the samples were filtered (cellulose acetate membrane, 0.45 μm pore size) and then acid-digested in a microwave oven (MARS 5, CEM corporation, Matthews, NC, USA). For sample preparation, 5 mL of wine was digested with 5 mL nitric acid (69.0 %, w/w), and samples were made up to a final volume of 25 mL with ultrapure deionized water. The operating conditions for the applied microwave digestion system are given in Table 1.
ICP-MS Analysis
The quadrupole inductively coupled plasma mass spectrometer (Q-ICP-MS) was used for all isotopic measurements (model 7500cx, Agilent technologies, Santa Clara, CA, USA). The instrument was tuned for standard robust plasma conditions, equipment with Micromist nebulizer. The Octopole Reaction System (ORS) was operated in “no-gas mode” (when no optional gas except argon was used) and in “helium mode” (when helium was used as optional gas). All relevant instrumental conditions are given in Table 2. Tuning was performed by optimizing the signal measured counts per ratio (CPS) for 7Li, 89Y, and 205Tl, from aqueous standard solution (Tune solution) that contained 10 ng/mL of Li, Y, Co, Ce, and Tl. For the ICP-MS analysis, the following 39 isotopes were recorded: 7Li, 9Be, 11B, 23Na, 24 Mg, 27Al, 28Si, 31P, 34S, 43Ca, 48Ti, 51 V, 53Cr, 55Mn, 56Fe/57Fe, 59Co, 60Ni, 63Cu, 66Zn, 69Ga, 72Ge, 75As, 77Se, 85Rb, 88Sr, 95Mo, 107Ag, 114Cd, 115In, 120Sn, 121Sb, 125Te, 137Ba, 205Tl, 206Pb/207Pb/208Pb, and 209 Bi.
Instrument drifts where corrected using rhodium as an internal standard, at concentration level of 10 μg/L, added to both calibration standards and wine samples, to normalize the instrument response. Rhodium was selected as an internal standard due to the very low background signal found for this element, and beacause it was not present in the wine. Addition of the internal standard to the samples was performed by a peristaltic pump.
A synthetic wine sample (12 % ethanol, 4 g/L tartaric acid, and pH 3.4) was prepared containing 5 % HNO3 and 10 μg/L of the multi-element certificate standard solution to give rise to multiple interferences across a range of common analytes and test the ability of He collision mode to remove all overlapping polyatomic species. Two sets of spectra were acquired to show the ability of the He collision mode to remove multiple interferences: one in “no gas” mode and the second with He added to the cell. Data correction or background subtraction was applied in He mode for the elements As, Bi, Ca, Co, Cs, Cu, Fe, Ga, Ge, Mg, Ni, P, Pd, S, Se, Si, Te, Ti, T, and Zn.
Validation of the Method
Limit of Quantification (LOQ)
Ten sample blanks were run to determine the instrument limits of detection (ILD) and limit of quantitation (LOQ), as suggested by the International Union of Pure and Applied Chemistry (IUPAC) (Hopfer et al. 2013). Background equivalent concentration (BEC) was calculated as an indicator for the calibration offset expressed as a concentration, due to the elemental contamination of the blank. The analysis of the blank solution for all target elements for 10 times with three repetitions at each measurement was conducted.
Recovery
Due to the lack of a sufficient wine certified reference material, the accuracy of the procedure was checked using synthetic wine (12 % ethanol, 4 g/L tartaric acid, pH 3.4), and red and white wine, spiked with two different standard additions: 10 μg/L (for trace elements) and 1 mg/L (for macro elements).
Repeatability and Reproducibility
The intra-day repeatability and inter-day reproducibility have been studied. One wine sample containing known amounts of the added elements was digested five subsequent times (in 1 day) applying the procedure described above, and the obtained solutions were analyzed by ICP-MS in order to study the intra-day repeatability. Furthermore, the wine samples were digested three times during three consecutive days, in order to study the inter-day reproducibility.
Calibration Curves
For quantitative analysis of the elements in digested wine samples, external calibration curves were built at different concentration levels: 0.5, 1, 3, 5, 10, 30, 50 μg/L for the trace elements (Li, Be, Al, Ti, V, Cr, Co, Ni, Ga, Ge, As, Se, Mo, Pd, Ag, Cd, In, Sn, Sb, Te, Cs, Ba, Tl, Pb, and Bi); 100, 300, and 500 μg/L for the elements Cu, Zn, Rb, Sr, Mn, and Fe; and 1, 3, 5, and 10 mg/L for the macro elements Na, P, S, Ca, Mg, K, and Si.
Statistical Analysis
Statistical treatments, including mean, standard deviation, relative standard deviation, t test, and one-way ANOVA were performed using the XLSTAT Software, Version 2012.6.09, Copyright Addinsoft 1995-2012, applied to the multi-element data set in order to extract the important information and to represent the pattern of similarity or differences between the studied wines in order to make a conclusion about the possible classification.
Results and Discussion
Optimization of the Method
The ICP-MS system was optimized under typical tuning conditions for high and variable sample matrices (plasma conditions optimized for 0.65 % CeO/Ce) using multi-element standard solution. No attempt was made to optimize any parameter for the targeted removal of any specific interference. A flow of 5.5 mL/min He gas (only) was added to the cell for the collision mode measurements. Normal background components of the argon plasma gas and aqueous sample solution (Ar, O, H), together with the additional components of the synthetic sample matrix (HNO3 and ethanol) lead to formation of several high-intensity background peaks in the no-gas mode spectrum, notably 40Ar16O, 40Ar 38Ar, 40Ar18O, and 40Ar2, from the plasma. Also, 40Ar18OH, 40Ar12C, 36Ar16OH, 40Ar12CH, 40Ar12C, 40Ar13C, 38Ar12C14N, and 40Ar14N were qualitatively determined as polyatomic interferences from the matrix. Their higher-intensity background peaks show why several interfered elements (56Fe, 52Cr, 53Cr, 59Co, 58Ni, 60Ni, 64Zn, 77Se, 78Se, and 80Se) were traditionally measured in helium mode. Despite the optional helium gas for the polyatomic interferences removal, for some of the isotopes, very low sensitivity (spike recoveries <80 %) was obtained (52Cr, 58Ni, 78Se, and 80Se).
In order to develop a convenient and accurate sample preparation method followed with ICP-MS method for multi-element analysis of wine samples, several investigations were performed. In this study, we decided to use digestion microwave method for wine preparation in order to (i) eliminate molecular interferences and plasma disorders caused by the organic content of the samples, (ii) to avoid the presence of colloidal suspensions and particulates on the nebulisation system, and (iii) to equalize matrix influences. Moreover, the main aim of the method development was to find the minimum aliquot of wine and acid that will allow successful digestion of the sample, presenting best recoveries for the elements. Thus, different volumes of nitric acid (3, 5, and 10 mL) were added to different volumes of wine sample (1, 3, and 5 mL). Additionally, a volume of 10 mL of wine was previously evaporated on a electrical heater at 70 °C to final volume of 1 mL, followed by additions of nitric acid (3 and 5 mL). Best results were obtained when a volume of 5 mL nitric acid was added to the volume of 5 mL wine sample and then digested.
For total microwave digestion of the wine sample, different temperatures were tested, 150, 180, and 220 °C, observing best digestion at 180 °C. Finally, the applied power effect (400, 800, and 1600 W) was also examined in each digestion step.
In order to confirm the best chosen conditions for wine digestion, synthetic sample, red wine, and white wine were digested and recoveries were calculated (Table 3). The digestion seemed visually completed in all of the combinations, but the spiked recoveries showed significant differences for total element content (p < 0.05). Thus, the average recoveries of total elements for the synthetic wine sample when volumes of 1 and 3 mL were digested with 3, 5, and 10 mL HNO3 were 68.6, 64.4, and 72.5 %, and 77.6, 81.8, and 66.9 %, respectively. Similar results were obtained for digestion of the red and white wine sample: for volumes of 1 and 3 mL wine digested with 3, 5, and 10 mL HNO3. Thus, average recoveries for the total elements content were 77.8, 72.3, and 66.5 %, and 62.4, 85.9, and 78.2 %, respectively, for red wine and 66.4, 86.4, and 83.4 %, and 78.2, 76.9, and 89,5 %, respectively, for white wine. The best recoveries (R > 90 %, on average for total elements) were obtained when volume of 5 mL sample (synthetic, red and white wine) was digested with 5 mL HNO3. The recoveries for the individual isotopes of the 36 analyzed elements determined with three repetitions for each element are presented in Table 3. Values ranged from 85 % for Se to 117 % for Ti in synthetic wine, 86 % for S to 118 % for Ba in red wine, and 83 % for Ca to 116 % for Sn in the white wine sample.
When volume of 10 mL wine was evaporated to volume of 1 mL and then digested with 3, 5, and 10 mL HNO3, the recoveries ranged from 53 % for S to 81 % for N, and average recovery of 69.3 % for total elements content (data not shown).
Validation of the Method
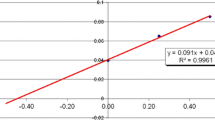
The linearity data, including slope, intercept, and correlation coefficient, are presented in Table 4. As it can be seen from the table, the linearity is satisfactory in all cases with correlation coefficients (R 2 > 0.99) ranging from 0.9963 for B to 1.0000 for As, Co, Mo, Na, Ni, Rb, and Sr.
The estimated instrument detection limit based on calibration linearity (external calibration), linear range, limit of detection (LOD), and limit of quantification (LOQ), as well as the background estimated concentration (BEC), was calculated for all elements in three consecutive days. Results are presented in Table 4. The lowest instrument detection limit (0.12 ng/L) was obtained with satisfactory sensitivity for 59Co in helium mode (R 2 = 1.0000).
The accuracy of the procedure was checked using the standard addition method. One Vranec wine sample was spiked with appropriate volumes of the multi-element standard solution: standard addition 1 with concentration of 10 μg/L, for the trace elements, and standard addition 2 with concentration of 1000 μg/L, for the macro elements. The satisfactory results for the recovery ranged between 83 and 120 % (Table 5) and confirmed that the method is accurate and convenient for quantitative analysis of elements in red and white wines. Precision of the method was defined as a relative standard deviation (RSD) calculated as a percentage using the standard deviation divided by the mean of replicated samples (Table 5). The values for the relative standard deviation (RSD) ranged from 0.55 % for Mg to 10.2 % for Ba.
Additionally, to confirm the accuracy of the method and to check the repeatability, five replicated measurements on an actual white and red wine samples were performed within 1 day. Every digested sample was injected three times into the ICP-MS system. The relative standard deviations (RSD) of the five replicate samples for each element are presented in Table 6. Satisfactory values for the RSD ranging from 1.04 % for Na to 10.9 % were found for red wine and RSD values for white wines were 1.16 % for Li to 11.8 % for Ni.
Reproducibility was also checked with replicate samples analyzed in three different days (3 replicates × 3 injections × 3 days), and the RSD for each element was calculated (Table 7). The RSD values ranged from 0.32 % for Si (day 2) to 15.1 % for Se (day 3) for red wines, and for the white wine, the RSD ranged from 0.19 % for Cu (day 2) to 8.73 % for Cu (day 2). In order to confirm the inter-day reproducibility, t test was used to evaluate the similarities or differences in the content of elements during three consecutive days. The p level was reported with t test, representing the probability of error. Satisfactory p values (p > 0.05) were obtained for day 1/day 2, day 1/day 3, and day 2/day 3 comparisons for both red and white wine samples, indicating acceptable reproducibility of the applied method (Table 7).
Method Application and Elemental Characterization of Vranec Wines
Table 8 shows the content of 38 elements (Ag, Al, As, B, Ba, Be, Bi, Ca, Cd, Co, Cr, Cs, Cu, Fe, Ga, Ge, In, Li, Mg, Mn, Mo, Na, Ni, P, Pb, Pd, Rb, S, Sb, Se, Si, Sn, Sr, Te, Ti, Tl, V, Zn) determined in Vranec wines fermented with different yeasts (autochthonous yeast, Vinalco, and four commercial yeasts, Clos, RC212, D254, BDX, from Lallemand).
For all wines, the total content of elements ranged from 348 to 678 mg/L (mean 499 mg/L). Elements B, Ca, Mg, Na, P, S, and Si were dominant in all wines regardless the yeast strain used for fermentation. In fact, the group of major elements (B, Ca, Mg, Na, P, S, and Si) represented the highest proportion in all wines ranging from 49 to 95.6 mg/L, followed by the minor elements (Al, Ba, Cu, Fe, Mn, Rb, Sr, and Zn) present in a range of 0.42 to 0.97 mg/L and trace elements (Ag, As, Be, Bi, Cd, Co, Cr, Cs, Ga, Ge, In, Li, Mo, Ni, Pb, Pd, Sb) ranging between 2.12 to 6.53 μg/L, comparable to those reported in the literature (Pohl 2007). Boron is an essential element for plants which is easily mobilized from the soil into the plant. Similarly, P is an essential plant element, which is often added to the soil with fertilizers, while, Mg, Mn, and Si are mainly influenced the soil mineral content (Hopfer, et al. 2015). All wines presented high values of P ranging from 96 to 211 mg/L confirming the high nutritional value of Vranec wines. Moreover, results for all determined elements are in accordance to previous data about multi-element composition of Macedonian wines (Ivanova-Petropulos et al. 2013). In addition, analyzed wines presented higher content of Ca and Mg, but lower amount of Na compared to Vranec wines and other red wines produced in Serbia (Mitic et al. 2014).
Considerable amounts of S were found in all Vranec wines (79.2 to 315 mg/L). In fact, S is mainly present due to the SO2 which is usually an added agent into the grape must to protect the enzymatic and non-enzymatic oxidation of phenolics, sugars, and amino acids that could cause browning of the wine. The addition of SO2 is traditionally considered as an efficient method to protect and preserve the wine at different stages of its elaboration.
Regarding the harmful elements, the content of As, Cd, Cu, Fe, Pb, and Zn was below the maximal allowed concentration in all wines. Thus, As ranged from 0.57 to 1.23 μg/L (mean value 0.88 μg/L), Cd from 0.2 to 1.22 μg/L (mean value 0.36 μg/L), Cu was present in range of 0.015 to 0.041 mg/L (mean value 0.06 mg/L), concentration of Fe ranged from 0.56 to 2.73 mg/L (mean value 1.22 mg/L), and levels for Pb and Zn ranged between 3.06 to 13.6 μg/L and 0.05 to 0.44 mg/L, respectively (mean values 6.87 μg/L and 0.19 mg/L). Maximal acceptable limits for these toxic elements are as follows: As 0.2 mg/L, Cd 10 μg/L, Cu 1 mg/L, Pb 0.15 mg/L, and Zn 5 mg/L. Comparing the obtained results with red wines from the region, Macedonian wines presented lower content of Cu, Fe, and Zn, but slightly higher amount of Mn (Mitic, et al. 2014).
Influence of yeast strains was noticed on the content of elements. On average, Vranec wines fermented with Lallemand yeasts contained higher content of total elements (14.3 mg/L) compared to wines fermented with Vinalco yeast (12.5 mg/L) (p < 0.05). The average levels of major elements B, Ca, Mg, and Na (4.27, 70.8, 119, and 12.7 mg/L, respectively) in wines fermented with Lallemand yeasts were significantly higher (p < 0.05) than in the wines fermented with Vinalco yeasts (3.79, 74.6, 104, and 5.46 mg/L, respectively), while the content of P and Si was similar between the wines. The content of S was significantly higher (p < 0.05) in the wines fermented with the commercial Lallemand yeasts (168 mg/L on average) compared to wines produced in presence of autochthonous Vinalco yeasts (120 mg/L on average). In fact, yeasts themselves produce SO2 during fermentation of grape juice influencing the content of sulphurous. Most of the S. cerevisiae yeast strains produce 10–20 mg/L SO2 during fermentation that can increase the total amount of bound SO2 causing too high levels with regard to the legal limits.
Concerning the harmful elements, slightly higher content of As and Cd was noticed for wines fermented with Vinalco yeast (1.01 and 0.43 μg/L on average, respectively) (p < 0.05) than in wines fermented with Lallemand yeasts (0.7 and 0.27 μg/L on average, respectively). Content of Cu, Fe, and Ni was similar in all wines, while higher proportion (p < 0.05) of Pb (mean value 7.32 μg/L) and Zn (mean value 0.27 mg/L) was present in Vranec wines fermented with Lallemand yeasts.
In general, yeast strains presented influence on metal ions in Vranec wines, showing that different yeasts could cause changes in the mineral composition of wines, probably because yeast strains possess different potential for accumulating a range of metal cations as well as they have different metabolic needs. According to the literature (Blackwell et al. 1995), large amounts of metals can remain associated with the cell wall. As regard this, a biosorption effect of heavy metals by S. cerevisiae was observed by Volesky and May-Phillips (1995) and Nicolini and Larcher (2003). In these studies, yeast strains were shown to affect the final content of a few elements, including Co, Cu, Mg, Na, Pb, Sr, and Zn. Probably, this was related to the different genetic capabilities of the strains to produce H2S as well as different metabolic needs, e.g., for Zn and Co, beside biosorption on cell walls.
Conclusion
Fast and accurate method for sample preparation followed with ICP-MS for multi-element analysis of wine was optimized and developed. The method presented satisfactory linearly, LOD, LOQ, accuracy, repeatability, and reproducibility for total 38 elements (Ag, Al, As, B, Ba, Be, Bi, Ca, Cd, Co, Cr, Cs, Cu, Fe, Ga, Ge, In, Li, Mg, Mn, Mo, Na, Ni, P, Pb, Pd, Rb, S, Sb, Se, Si, Sn, Sr, Te, Ti, Tl, V, Zn). It was then used for determination of the element content in Vranec wines fermented with S. cerevisiae yeast strains commercial Clos, RC212, D254, and BDX and six autochthonous Vinalco yeasts. Vranec wines fermented with the autochthonous Vinalco yeasts presented lower content of total elements compared to the wines fermented with commercial yeasts.
References
Álvarez M, Moreno IM, Jos MJ, Cameám AM, Gustavo González A (2007) Study of mineral profile of Montilla-Moriles “fino” wines using inductively coupled plasma atomic emission spectrometry methods. J Food Compos Anal 20:391–395. doi:10.1016/j.jfca.2006.07.010
Blackwell KJ, Singleton I, Tobin JM (1995) Metal cation uptake by yeast: a review. Appl Microbiol Biotechnol 43:579–585. doi:10.1007/BF00164757
Castiñeira Gómez MDM, Brandt R, Jakubowski N, Andersson JT (2004) Classification of German white wines with certified brand of origin by multielement quantitation and pattern recognition techniques. J Agric Food Chem 52:2953–2974. doi:10.1021/jf035120f
Cheng J, Liang C (2012) The variation of mineral profiles from grape juice to monovarietal Cabernet Sauvignon wine in the vinification process. J Food Process Preserv 36:262–266. doi:10.1111/j.1745-4549.2011.00586.x
Diaz C, Conde JE, Estevez D, Perez Olivero SJ, Perez Trujillo JP (2003) Application of multivariate analysis and artificial neural networks for the differentiation of red wines from the canary islands according to the island of origin. J Agric Food Chem 51:4303–4307. doi:10.1021/jf0343581
Esparza I, Salinas I, Caballero I, Santamaria C, Calvo I, Garcia Mina JM, Fernandez JM (2004) Evolution of metal and polyphenol content over a 1-year period of vinification: sample fractionation and correlation between metals and anthocyanins. Anal Chim Acta 524:215–224. doi:10.1016/j.aca.2004.06.074
Frias S, Perez Trujillo JP, Pena EM, Conde JE (2001) Classification and differentiation of bottled sweet wines of Canary Islands (Spain) by their metallic content. Eur Food Res Technol 213:145–149. doi:10.1007/s002170100344
Frias S, Conde JE, Rodriguez Bencomo JJ, Garcia Montelongo F, Perez Trujillo JP (2003) Classification of commercial wines from the Canary Islands (Spain) by chemometric techniques using metallic contents. Talanta 59:335–344. doi:10.1016/S0039-9140(02)00524-6
Grindlay G, Mora J, Gras L, De Loos-Vollebregt MTC (2011) Atomic spectrometry methods for wine analysis: a critical evaluation and discussion of recent applications. Anal Chim Acta 691:18–32. doi:10.1016/j.aca.2011.02.050
Hopfer H, Nelson J, Mitchell AE, Heymann H, Ebeler SE (2013) Profiling the trace metal composition of wine as a function of storage temperature and packaging type. J Anal At Spectrom 28:1288–1291. doi:10.1039/C3JA50098E
Hopfer H, Nelson J, Collins TS, Heymann H, Ebeler SE (2015) The combined impact of vineyard origin and processing winery on the elemental profile of red wines. Food Chem 172:486–496. doi:10.1016/j.foodchem.2014.09.113
Ivanova Petropulos V, Bogeva E, Stafilov T, Stefova M, Siegmund B, Pabi N, Lankmayr E (2014) Study of the influence of maceration time and oenological practices on the aroma profile of Vranec wines. Food Chem 165:506–514. doi:10.1016/j.foodchem.2014.05.144
Ivanova V, Dörnyei Á, Márk L, Vojnoski B, Stafilov T, Stefova M, Kilár F (2011a) Polyphenolic content of Vranec wines produced by different vinification conditions. Food Chem 124:316–325. doi:10.1016/j.foodchem.2010.06.039
Ivanova V, Vojnoski B, Stefova M (2011b) Effect of the winemaking practices and aging on phenolic content of Smederevka and Chardonnay wines. Food Bioprocess Technol 4:1512–1518. doi:10.1007/s11947-011-0566-y
Ivanova V, Vojnoski B, Stefova M (2012) Effect of winemaking treatment and wine aging on phenolic content in Vranec wines. J Food Sci Technol 49:161–172. doi:10.1007/s13197-011-0279-2
Ivanova-Petropulos V, Wiltsche H, Stafilov T, Stefova M, Motter H, Lankmayr E (2013) Multielement analysis of Macedonian wines by inductively coupled plasma–mass spectrometry (ICP-MS) and inductively coupled plasma–optical emission spectrometry (ICP-OES) for their classification. Maced J Chem Chem Eng 32:265–281
Ivanova-Petropulos V, Hermosín-Gutiérrez I, Boros B, Stefova M, Stafilov T, Vojnoski B, Dörnyei Á, Kilár F (2015a) Phenolic compounds and antioxidant activity of Macedonian red wines. J Food Compos Anal 41:1–14. doi:10.1016/j.jfca.2015.01.002
Ivanova-Petropulos V, Jakabová S, Nedelkovski D, Pavlík V, Balážová Ž, Hegedűs O (2015b) Determination of Pb and Cd in Macedonian wines by electrothermal atomic absorption spectrometry (ETAAS). Food Anal Methods. doi:10.1007/s12161-014-0062-x
Ivanova-Petropulos V, Ricci A, Nedelkovski D, Dimovska V, Parpinnelo GP, Versari A (2015c) Targeted analysis of bioactive phenolic compounds and antioxidant activity of Macedonian red wines. Food Chem 171:412–440. doi:10.1016/j.foodchem.2014.09.014
Lara R, Cerutti S, Salonia JA, Olsina RA, Martinez LD (2005) Trace element determination of Argentine wines using ETAAS and USN-ICP-OES. Food Chem Toxicol 43:293–297. doi:10.1016/j.fct.2004.10.004
Mitic MN, Kostic DA, Pavlovic AN, Tosic SB, Stojanovic BT, PAUNOVIC DD (2014) Determination of metals in white and red wines using ICP-OES method. Oxid Commun 37:1074–1082
Muranyi Z, Kovacs Z (2000) Statistical evalution of aroma and metal content in Tokay wines. Microchem J 67:91–96. doi:10.1016/S0026-265X(00)00103-X
Nicolini G, Larcher R (2003) Evidence of changes in the micro-element composition of wine due to the yeast strain. Riv Vitic Enol 56:45–48
Pohl P (2007) What do metals tell us about wine? Trac-Trend Anal Chem 26:941–949. doi:10.1016/j.trac.2007.07.005
Rebolo S, Pena RM, Latorre MJ, Garcia S, Botana AM, Herrero C (2000) Characterisation of Galician (NW Spain) Ribeira Sacra wines using pattern recognition analysis. Anal Chim Acta 417:211–220. doi:10.1016/S0003-2670(00)00929-6
Rodriguez Mozaz S, Garcia Sotro A, Garrido Segovia J, Ancin Azpilicueta C (1999) Influence of decantation of viura must on the cation content. Evolution during wine fermentation and stabilization. Food Res Int 32:683–689. doi:10.1016/S0963-9969(99)00147-7
Sperkova J, Suchanek M (2005) Multivariate classification of wines from different Bohemian regions (Czech Republic). Food Chem 93:659–663. doi:10.1016/j.foodchem.2004.10.044
Stafilov T, Karadjova I (2009) Atomic absorption spectrometry in wine analysis. Maced J Chem Chem En 28:17–31
Tariba B (2011) Metals in wine—impact on wine quality and health outcomes. Biol Trace Elem Res 144:143–156. doi:10.1007/s12011-011-9052-7
Volesky B, May-Phillips HA (1995) Biosorption of heavy metals bySaccharomyces cerevisiae. Appl Microbiol Biotechnol 42:797–806. doi:10.1007/BF00171964
Volpe MG, La Cara F, Volpe F, De Mattia A, Petitto F, Di Stasio M, Serino V (2009) Heavy metal uptake in the enological food chain. Food Chem 117:553–560. doi:10.1016/j.foodchem.2009.04.033
Acknowledgments
Financial support provided by the Research Fund of the University “Goce Delčev” – Štip, R. Macedonia for the project titled “Polyphenolic and aroma profile of Vranec wines fermented with isolated yeasts from Tikveš wine area” is gratefully acknowledged.
Conflict of Interest
Violeta Ivanova-Petropulos declares that she has no conflict of interest. Biljana Balabanova declares that she has no conflict of interest. Sasa Mitrev declares that he has no conflict of interest. Dusko Nedelkovski declares that he has no conflict of interest. Violeta Dimovska declares that she has no conflict of interest. Rubin Gulaboski declares that he has no conflict of interest. This article does not contain any studies with human or animal subjects.
Compliance with Ethics Requirements
This is an original research article that has neither been published previously nor considered presently for publication elsewhere. All authors named in the manuscript are entitled to the authorship and have approved the final version of the submitted manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Violeta Ivanova-Petropulos and Biljana Balabanova are the first authors.
Rights and permissions
About this article
Cite this article
Ivanova-Petropulos, V., Balabanova, B., Mitrev, S. et al. Optimization and Validation of a Microwave Digestion Method for Multi-element Characterization of Vranec Wines. Food Anal. Methods 9, 48–60 (2016). https://doi.org/10.1007/s12161-015-0173-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0173-z