Abstract
A sensitive voltammetric method for the determination of tert-butylhydroquinone (TBHQ), a widely used synthetic phenolic antioxidant in oils and fats, using multiwalled carbon nanotube modified gold electrode (MWCNT/GE) was developed. In 0.10 M phosphate buffer solution (PBS) of pH 2, TBHQ gave redox peaks at E pa = 258 mV and E pc = 228 mV on MWCNT/GE. Diffusion-controlled electrooxidation of TBHQ was found to be perfectly reversible with the involvement of two electrons and two protons. The anodic peak currents varied linearly with concentrations of TBHQ in the range 4.0 × 10−6 to 1.00 × 10−4 M. The limit of detection achieved for the developed sensor was 3.20 × 10−8 M (5.31 ng mL−1). Developed sensor was used for the determination of TBHQ in commercially available coconut oil. The results obtained from the developed method were in good agreement with the standard method (HPLC-UV).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antioxidants are substances used to preserve food by retarding deterioration, rancidity or discolouration due to oxidation (Food and Drug Administration). tert-Butylhydroquinone (TBHQ) is one of the commonly used synthetic antioxidants in food. Due to high thermal stability and anti-lipid peroxidation activity shown by TBHQ in comparison to other antioxidants, it is widely used in fats and oils (Shahidi 2000; Gharavi et al. 2007).
In 1972, TBHQ was first approved as a food additive in the USA (Robards and Dilli 1987). In contrast to the beneficial effects of TBHQ, there is a possibility of a number of problems in a complex matrix like food. Studies demonstrate that excess use of TBHQ, added to foodstuff, may cause a loss of sustenance, produce toxic substances that harm human health (Guan et al. 2006) and even carcinogenicity (Saad et al. 2007). The acceptable daily intake (ADI) recommended by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 2007) is 0–1.40 mg of additive per kilogram body weight for TBHQ, while the Scientific Committee for Food (SCF) established a value of 0.50 mg/kg (Andrea et al. 2010). Plastics and wrapping materials frequently contain antioxidants capable of migrating into the enclosed food (Crompton 1979). Obviously, the concern for “total” or “free” antioxidant is therefore an important question.
Several analytical methods are available for the determination of TBHQ. These include high-performance liquid chromatography (Li et al. 2009; Tagliabue et al. 2004), gas chromatography (Guo et al. 2006; Gonzalez et al. 1998) and electrokinetic capillary chromatography (Guan et al. 2006). Though the above-mentioned methods are appropriate, time-consuming analyte extraction and sample cleanup is required. Electrochemical methods such as voltammetry are alternatives. Its wide application is attributed with relatively cheap and portable instrumentation, very good sensitivity with wide linear concentration range, rapid analysis (in seconds) and on site analysis without sample cleanup. Determination of TBHQ by polarographic (Cortes et al. 1994; De la Fuente et al. 1999) and voltammetric methods (Araujo et al. 2011; Goulart et al. 2014) using different electrodes are reported.
Carbon nanotubes have been at the fore front of novel nanoscale investigations owing to their unique structure-dependent electronic and mechanical properties (Balasubramanian and Burghard 2008; Jos et al. 2012). The performance of multiwalled carbon nanotube (MWCNT) modified electrodes has been found to be superior to the performance of conventional carbon electrodes in terms of electron transfer rate, reversibility, conductivity and adsorptivity (Britto et al. 1996; Zhao et al. 2002).
Voltammetric determination of TBHQ using modified gold electrode is not yet reported. In continuation to the sensors for food additives reported from our group (Thomas et al. 2012; Rasheed et al. 2014; Vikraman et al. 2013; Chandran et al. 2014), the present investigations involve modification of gold electrode with MWCNT. It aims at the development of a quantitative electroanalytical method for the determination of TBHQ at multiwalled carbon nanotube modified gold electrode (MWCNT/GE), based on cyclic voltammetry (CV) and square wave voltammetry (SWV). It also aims at the application of proposed method for TBHQ determination in coconut oil.
Materials and Methods
Reagents
All reagents and solvents used for the investigations were of analytical grade and Millipore water was used throughout the studies. MWCNT, Nafion, alumina and TBHQ were purchased from Sigma-Aldrich Co., USA. Ethanol, sodium dihydrogen orthophosphate and disodium hydrogen orthophosphate were purchased from Merck, Germany. Except MWCNT, other chemicals were used as received. All other common reagents were obtained from S.D. Fine-Chem Ltd., Mumbai, India.
Instruments
Electrochemical measurements were performed on an electrochemical analyser (CH instruments, USA) with a conventional three-electrode system. A gold electrode with suitable modifications as working electrode, Ag/AgCl as reference electrode and a platinum wire as the auxiliary electrode constituted the three-electrode system. A Metrohm pH meter was used to carry out the pH measurements. SEM images were recorded using JOEL 6390 LV. An ultrasonicator (Oscar Ultrasonics Pvt. Ltd., Mumbai) and HPLC-UV (LC-20AT, SPD-20A, Shimadzu) were also used.
Fabrication of MWCNT Modified GE
The GE was mechanically polished with aqueous slurries of alumina (1 μm) on a flat pad prior to modification. Polished GE was rinsed ultrasonically with water and absolute ethanol to remove residual alumina particles from the surface, then with a piranha solution (H2O2:H2SO4 = 1:3 v/v) for 10 min when required. The mechanical cleaning followed an electrochemical cleaning process which included the performance of cyclic voltammetry from 0 to 1500 mV in 0.50 M sulphuric acid solution at a scan rate of 100 mV s−1 until a stable cyclic voltammogram was obtained.
MWCNT was refluxed in conc. HNO3 for 48 h to cause segmentation and carboxylation (Tsang et al. 1994; Issac and Kumar 2009). Five milligrams of the acid-treated MWCNT was dispersed in 13 % (v/v) Nafion–water solution (2.30 mL) to give a 0.22 % black homogenous suspension. Five microliters of the MWCNT suspension was dropped on the clean GE surface, and the solvent was evaporated to get the modified GE.
Analytical Procedure
A suitable amount of the stock solution of TBHQ (1 × 10−3 M) in methanol was transferred to the electrochemical cell and diluted to 10 mL with the supporting electrolyte. The cyclic voltammograms and square wave voltammograms were recorded from −400 to 1000 mV at a scan rate of 100 mV s−1 with the three-electrode system. All experiments were carried out at room temperature (~25 °C).
Treatment of Coconut Oil Sample
Five grams of the commercially available coconut oil sample was placed in a 100-mL Erlenmeyer flask (with a screw cap), and pure methanol (10 mL) was added. The mixture was then shaken vigorously for 30 min, transferred to a 25-mL centrifuge tube and centrifuged at 3000 rpm for 10 min. After a settling time of 2 min, the extracts were transferred to a sample bottle. A 100 μL aliquot of this sample was analysed by the voltammetric procedure.
HPLC-UV as the Standard Method
The chromatographic conditions for the determination of TBHQ using methodologies previously proposed by Lin et al. (2013) was used with some modifications. An HPLC instrument equipped with UV–Vis detection at 280 nm was used. The mobile phase was a combination of (A) methanol and (B) 1 % orthophosphoric acid. A gradient was developed as follows: the solution A (v/v) ratio was varied from 40 to 80 % during 0–15 min, 100 % during 15–20 min. The flow rate was kept at 1 mL min−1, and under these conditions, the retention times was 13.99 min for TBHQ. Each sample solution (20 μL) was injected, and the concentrations were calculated on the basis of peak area.
Results and Discussion
Electrocatalytic Action of MWCNT on the Electrochemical Behaviour of TBHQ
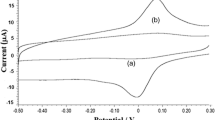
Electrochemical behaviour of TBHQ (5 × 10−5 M) was studied at bare GE and MWCNT/GE using cyclic voltammetry (CV) and square wave voltammetry (SWV), in 0.10 M PBS at a scan rate of 100 mV s−1. The results are compared in Fig. 1. Using CV, bare GE could hardly sense the redox nature of TBHQ (E pa = 466 mV, i pa = 0.66 μA), but under similar experimental conditions, TBHQ gave redox peaks at E pa = 258 mV and E pc = 228 mV (i pa = 6.45 μA and i pc = 10.55 μA) on MWCNT/GE. This shows the electrocatalytic role played by MWCNT towards the reversible oxidation of TBHQ. A difference of about 30 mV was obtained between the peak potentials. According to equation, \( \varDelta {E}_{\mathrm{p}}={E}_{\mathrm{p}\mathrm{a}}-{E}_{\mathrm{p}\mathrm{c}}=\frac{59\; mV}{n} \) (Anson et al. 1983; Gan et al. 2012), the number of electrons involved in the reversible reaction was found to be 2.
SWV was also employed to explore the electrochemical behaviour of TBHQ (Fig. 1). In the case of bare gold electrode, TBHQ (5 × 10−5 M) gave an oxidation peak at E pa = 432 mV with a small current (0.85 μA). It was observed that the current decreases with multiple cycles. This is due to adsorption of oxidative products on the surface of the electrode. Higher conductivity, more active sites, larger surface area and topological defects on the MWCNT surface (Xu et al. 2003) helped in enhancing the sensitivity of the modified electrode, MWCNT/GE. This resulted in lowering the peak potential of oxidation of TBHQ to 220 mV with an enhanced current (24.47 μA) at MWCNT/GE.
Evidences for Electrode Modification—Surface Area of MWCNT/GE
The Randles–Sevcik equation (Randles 1948) was used to calculate the surface area of bare GE and MWCNT/GE. CVs were recorded with GE and MWCNT/GE at different scan rates by measuring microscopic areas of the electrodes with a probe of 2 × 10−3 M K3[Fe(CN)6]. For a reversible system, the anodic peak current i p is linear to the square root of scan rate, ν 1/2 as follows
where n is the number of electrons transferred (n = 1), D is the diffusion coefficient D = 7.6 × 10−6 cm2 s−1, A is the surface area of the electrode, C is the concentration of K3[Fe(CN)6] and ν refers to the scan rate.
The plot of i p vs. ν 1/2 follows the linear regression equation i p = 2.28 ν 1/2 − 2.37 and i p = 7.51 ν 1/2 − 5.41 for the bare and modified electrodes, respectively. Thus from the slope of the plot, effective surface areas of bare GE and MWCNT/GE were calculated to be 0.5604 and 1.8398 cm2, respectively. The 3.28-fold increase in the surface area of MWCNT-modified GE compared to bare GE enhanced the response of the electroactive species. SEM images of bare GE and MWCNT/GE given in Fig. 2 give further evidences for surface modification.
Performance Characteristics of the Developed Sensor
Choice of the Supporting Electrolyte and the Effect of pH
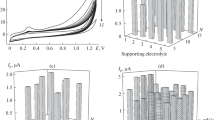
In order to choose the most suitable supporting electrolyte for the voltammetric determination of the antioxidant TBHQ, its electrochemical behaviour was compared in various supporting electrolytes of 0.10 M PBS, citrate buffer solution, acetate buffer solution, hydrochloric acid, sulphuric acid, sodium hydroxide, potassium chloride and potassium nitrate. Of all the electrolytes tested, PBS gave better shaped reversible voltammetric peaks with relatively high sensitivity. Thus, PBS was chosen as the suitable medium for TBHQ determination.
The Effect of pH
Figures 3 and 4 show the relationships between the peak potentials and peak currents with pH values, respectively At a constant scan rate, peak potentials shifted towards negative values with increasing pH, in the range of 2–8, suggest the involvement of protons in the redox reaction of TBHQ (Ghoreishi et al. 2012). However, when the pH exceeded 8, the electrode response became unstable.
From Fig. 3, it is clear that E pa shows a linear relation with pH (R 2 = 0.993) with a slope of −58.71. The slope is in agreement with the theoretical value (59 mV/pH), indicating that the oxidation of TBHQ occurred with the involvement of equal number of electrons and protons (De la Fuente et al. 1999).
From pH 2 to 6, the anodic and cathodic peak currents decreased gradually (Fig. 4), and the reversibility of the reaction became worse. It is apparent that both the electron transfer kinetics and the redox potential for this oxidation are pH dependent. This can be attributed to the involvement of protons in the electron transfer reactions; the two-electron oxidation requires the removal of two protons, and the two-electron reduction requires the addition of two protons. Therefore, lowering the pH (increasing the proton concentration) facilitates the reduction, but hinders the oxidation, and hence, the peaks in the CV are shifted to more positive potentials. The well-shaped peaks and larger current was the reason to select pH 2 for the following studies.
Effect of MWCNT Film Thickness
Influence of thickness of the film formed by MWCNT–Nafion suspension on the redox peaks of TBHQ was studied using CV by changing the dropping volume of MWCNT–Nafion suspension (2.17 × 10−6 g μL−1) on the bare electrode from 1 to 6 μL. From 1 to 5 μL, peak currents increased significantly, decreased on changing the volume to 6 μL, insulating nature of Nafion towards electrons (Nafion acts as a cation exchange resin) (Gryger et al. 2002) and hampering of electron transfer by excess amount of MWCNT (Lonappan et al. 2011) may be the reason. Thus, 5 μL of MWCNT–Nafion suspension was fixed for further studies.
Effect of Potential Scan Rate
The effect of the potential scan rates on the peak currents of the antioxidant TBHQ (5 × 10−5 M), at the MWCNT/GE was also investigated in 0.1 M PBS (pH 2.0). Resulting peak currents are plotted against the scan rate (Fig. 5) which demonstrates that the redox peak currents of TBHQ was proportional to the square root of the potential scan rate in the range of 20–140 mV s−1 with linear regression equations (E pa = 1.45 ν 1/2 − 1.68 and E pc = −1.00 ν 1/2 + 2.93) (R 2 > 0.99). This indicated that the redox reaction of TBHQ at the surface of MWCNT/GE was controlled by diffusion mechanism (De la Fuente et al. 1999; Bond 1980).
Also, the shift of peak potential (Fig. 5) as a function of the scan rate indicated that with increasing scan rate, the anodic and cathodic peak potentials for TBHQ shifted in positive and negative directions as shown; this suggests that the electron transfer rate was not fast at higher scan rate, and the electrochemical reactions gradually became less reversible (Wang et al. 2011).
Mechanism of the Electrochemical Redox Reaction of TBHQ
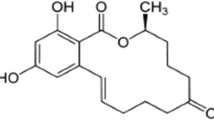
From the earlier sections, it can be seen that electrochemical redox reaction of TBHQ is perfectly reversible with two-electron transfer. From the plot of pH with anodic peak potential, it is clear that equal number of electrons and protons are involved in the redox reaction of TBHQ. Thus, the possible mechanism of electrochemical redox reaction of TBHQ is given in Fig. 6. TBHQ undergoes oxidation by the removal of two electrons and two protons to form tert-butylquinone and vice versa.
Linear Range and Limit of Detection
Linear range and limit of detection were determined under optimised experimental conditions using SWV in 0.10 M PBS (pH 2) (Fig. 7). The anodic peak currents showed linear relationship with concentrations of TBHQ in the range 4 × 10−6 to 1 × 10−4 M (R 2 = 0.99). The linear regression equation was formulated as i p = 3.30 × 10−5 C + 7.52. The limit of detection achieved for the developed sensor was 3.20 × 10−8 M (5.31 ng mL−1). The limit of detection of TBHQ for the proposed sensor is compared with that of the reported works (Table 1).
Interferences
Various common interfering substances such as ascorbic acid, citric acid, EDTA and common metal salts were evaluated at the MWCNT/GE under optimal experimental conditions to investigate method selectivity. The tolerance limit for the interfering species was the maximum concentration that gave a relative error of ±5 % at a concentration of 5 × 10−5 M TBHQ. The signal change produced by the interferents in the response of TBHQ is tabulated in Table 2.
Studies showed that up to twofold excess of ascorbic acid concentration had no effect on the peak currents of the analyte, but interferes when present in higher concentration. EDTA, citric acid, K+, Ca2+, Zn2+, Cl−, SO4 2−, NO3 −, CO3 2− and CH3COO− did not interfere until a 100-fold increase in the concentration level (There were no obvious electrochemical responses for these species in the potential window between −400 and 1000 mV except the peak potential (804 mV) of EDTA which was different from the analyte) .
The possibility of interference by other antioxidants such as butylated hydroxyl anisole (BHA) and propyl gallate (PG) on the determination of TBHQ was studied under similar conditions. PG gave an anodic peak at 468 mV. It was seen that TBHQ was suffering from an interference of 52.29 %, by PG but BHA do not interfere though it shows an anodic peak at 380 mV.
Repeatability and Reproducibility
Electrochemical response of TBHQ (5 × 10−5 M or 8.30 μg mL−1) was determined with six modified electrodes (MWCNT/GE) prepared by the same fabrication method. The relative standard deviation (RSD) of the peak currents was calculated to be 3.8 %. This indicated that the reproducibility of the developed sensor was satisfactory. The modified electrode was repeatedly used 10 times (CV/SWV was performed at least five times in the buffer solution after each use to regenerate the electrode surface) to perform the experiment in solution containing 8.30 μg mL−1 of TBHQ. The RSD of these replicates obtained was 2.4 %.
Analytical Application
To validate the developed method, proposed sensor was used for the determination of TBHQ in commercially available coconut oil. Each spiked sample solution was subjected to three parallel determinations as per the procedure discussed earlier. Experimental results are shown in Table 3. The results obtained with the developed sensor were compared with that obtained from the HPLC-UV method. The results obtained from the standard method were in good agreement with the developed method and establishes the utility of the present method.
Conclusions
A method was developed for the determination of TBHQ using a carbon nanotube modified gold electrode (MWCNT/GE). The electrochemical behaviour of TBHQ at MWCNT/GE was investigated, and it was found that on modification, the anodic peak current was shifted by 208 mV negatively. This lowering of potential indicated the electrocatalytic effect of MWCNT.
TBHQ gave best responses in 0.10 M PBS (pH 2) when 5 μL of (2.17 × 10−3 g mL−1) MWCNT–Nafion suspension was used as the modifier. Under optimised conditions, a linear concentration range of 4.00 × 10−6 to 1.00 × 10−4 M TBHQ, and a detection limit of 3.20 × 10−8 M were obtained.
The developed sensor showed good reproducibility (RSD = 3.80 %) and repeatability (RSD = 2.40 %). Satisfactory quantitative results were obtained for the determination of TBHQ in coconut oil sample, and the obtained results were found to be in agreement with those from the standard HPLC method.
References
Andrea C, Castanheira I, Cruz JM, Paseiro P, Silva A (2010) Analytical strategies to evaluate antioxidants in food. Trends Food Sci Technol 21:229–246
Anson F, Huang WZ, Gao XX (1983) Electrochemistry and electroanalytical chemistry. Beijing University Press, Beijing
Araujo TA, Barbosa AMJ, Viana LH, Ferreira VS (2011) Electroanalytical determination of TBHQ, a synthetic antioxidant, in soybean biodiesel samples. Fuel 90:707–712
Balasubramanian K, Burghard M (2008) Electrochemically functionalized carbon nanotubes for device applications. J Math Chem 18:3071–3083
Bond AM (1980) Modern polarographic methods in analytical chemistry. Marcel Dekker, New York
Britto PJ, Santhanam KSV, Ajayan PM (1996) Carbon nanotube electrode for oxidation of dopamine. Bioelectrochem Bioenerg 41:121–125
Caramit RP, Andrade AGF, Souza JBG, Araujo TA, Viana LH, Trindade MAG, Ferreira VS (2013) A new voltammetric method for the simultaneous determination of the antioxidants TBHQ and BHA in biodiesel using multi walled carbon nanotube screen printed electrodes. Fuel 105:306–313
Chandran S, Lonappan LA, Thomas D, Jos T, Kumar KG (2014) Development of an electrochemical sensor for the determination of amaranth: a synthetic dye in soft drinks. Food Anal Methods 7:741–746
Cortes AG, Armisen P, Ruiz MA, Sedeno PY, Pingarron JM (1994) Electroanalytical study of the antioxidant tert-butylhydroquinone in an oil in water emulsified medium. Electroanalysis 6:1014–1019
Crompton TR (1979) Additive migration from plastics into food. Pergamon Press, London
De la Fuente C, Acuna JA, Vazquez MD, Tascon LM, Batanero PS (1999) Voltammetric determination of the phenolic antioxidants 3-tert-butyl-4-hydroxyanisole and tert-butylhydroquinone at a polypyrrole electrode modified with a nickel phthalocyanine complex. Talanta 49:441–452
Gan T, Sun J, Cao S, Gao F, Zhang Y, Yang Y (2012) One step electrochemical approach for the preparation of graphene wrapped-phosphotungstic acid hybrid and its application for simultaneous determination of sunset yellow and tartrazine. Electrochim Acta 74:151–157
Gharavi N, Haggarty S, Ayman OS (2007) Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr Drug Metab 8:1–7
Ghoreishi SM, Behpour M, Golestaneh M (2012) Simultaneous determination of sunset yellow and tartrazine in soft drinks using gold nanoparticles carbon paste electrode. Food Chem 132:637–641
Gonzalez M, Ballesteros E, Gallego M, Varcarcel M (1998) Continuous flow determination of natural and synthetic antioxidants in foods by gas chromatography. Anal Chim Acta 359:47–55
Goulart LA, Teixeira ARL, Ramalho DA, Terezo AJ, Castilho M (2014) Development of an analytical method for the determination of tert-butylhydroquinone in soybean biodiesel. Fuel 115:126–131
Gryger T, Marken F, Schroder U, Scholz F (2002) Electrochemical analysis of solids. Collect Czechoslov Chem Commun 67:163–208
Guan Y, Chu Q, Fu L, Wu T, Ye J (2006) Determination of phenolic antioxidants by micellar electrokinetic capillary chromatography with electrochemical detection. Food Chem 94:157–162
Guo L, Xie MY, Yan AP, Wan YQ, Wu YM (2006) Simultaneous determination of five synthetic antioxidants in edible vegetable oil by GC–MS. Anal Bioanal Chem 386:1881–1887
Issac S, Kumar KG (2009) Voltammetric determination of sulfamethoxazole at a multiwalled carbon nanotube modified glassy carbon sensor and its application studies. Drug Test Anal 1:350–354
Jos T, Issac S, Joseph R, Rajith L, Kumar KG (2012) Electrocatalysis and determination of pyridine-2-aldoxime methochloride using carbon nanotube modified gold electrode. Micro Nano Lett 7:854–858
Li XQ, Ji C, Sun YY, Li YM, Chu XG (2009) Analysis of synthetic antioxidants and preservatives in edible vegetable oil by HPLC/TOF-MS. Food Chem 113:692–700
Lin X, Ni Y, Kokot S (2013) Glassy carbon electrodes modified with gold nanoparticles for the simultaneous determination of three food antioxidants. Anal Chim Acta 765:54–62
Lonappan L, Issac S, Joseph R, Thomas D, Kumar KG (2011) Electrochemical studies of TAM using multiwalled carbon nanotube modified glassy carbon sensor. Micro Nano Lett 6:867–870
Randles JEB (1948) Cathode ray polarograph. Trans Faraday Soc 44:322–327
Rasheed Z, Vikraman AE, Thomas D, Jagan JS, Kumar KG (2014) Carbon nanotube based sensor for the determination of butylated hydroxyanisole in food samples. Food Anal Methods. doi:10.1007/s12161-014-9894-7
Robards K, Dilli S (1987) Analytical chemistry of synthetic food antioxidants. Analyst 112:933–943
Saad B, Sing YY, Nawi MA, Hashim N, Ali ASM, Saleh MI (2007) Determination of synthetic phenolic antioxidants in food items using reversed-phase HPLC. Food Chem 105:389–394
Shahidi F (2000) Antioxidants in food and food antioxidants. Nahrung 44:158–163
Summary of evaluations performed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 1956–2007). [http://jecfa.ilsi.org/index.htm, www.efsa.europa.eu, www.fao.org]
Tagliabue S, Gasparoli A, Bella DL, Bondioli P (2004) Quali-quantitative determination of synthetic antioxidants in biodiesel. Riv Ital Sostanze Grasse 80:37–40
Thomas D, Rajith L, Lonappan L, Issac S, Kumar KG (2012) Sensitive determination of nitrite in food samples. Food Anal Methods 5:752–758
Tsang SC, Chen YK, Harris PJE, Green MLH (1994) A simple chemical method of opening and filling carbon nanotubes. Nature 372:159–161
Vikraman AE, Rasheed Z, Rajith L, Lonappan LA, Kumar KG (2013) MWCNT-modified gold electrode sensor for the determination of propyl gallate in vegetable oils. Food Anal Methods 6:775–780
Wang MY, Zhang DE, Tong ZW, Xu XY, Yang XJ (2011) Voltammetric behavior and the determination of quercetin at a flowerlike Co3O4 nanoparticles modified glassy carbon electrode. J Appl Electrochem 41:189–196
Xu JZ, Zhu JJ, Wu Q, Hu Z, Zhen HY (2003) An amperometric biosensor based on the coimmobilisation of horseradish peroxidase and methylene blue on a carbon nanotubes modified electrode. Electroanalysis 15:219–224
Zhao Q, Gan Z, Zhuang Q (2002) Electrochemical sensors based on carbon nanotubes. Electroanalysis 14:1609–1613
Acknowledgments
The authors are grateful to the Defence Research and Development Organisation (DRDO), the Government of India for the financial assistance in the form of a research project to carry out this work. Krishnapillai Girish Kumar has received research grant from DRDO, Government of India.
Conflict of interest
Ambily Thomas declares that she has no conflict of interest. Anuja Elevathoor Vikraman declares that she has no conflict of interest. Divya Thomas declares that she has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thomas, A., Vikraman, A.E., Thomas, D. et al. Voltammetric Sensor for the Determination of TBHQ in Coconut Oil. Food Anal. Methods 8, 2028–2034 (2015). https://doi.org/10.1007/s12161-015-0092-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0092-z