Abstract
In this work, a simple, reliable and fast capillary electrophoresis method was developed and partially validated for simultaneous detection of 20 polyphenolic compounds (presumed to be found in propolis and plant extracts) in less than 27 min. The best results were obtained using 45 mM tetraborate buffer with 0.9 mM sodium dodecyl sulfate (pH = 9.35) as a background electrolyte. The polyphenolic compound order of elution was the following: resveratrol, pinostrobin, acacetin, chrysin, rutin, naringenin, isoquercitrin, umbelliferone, cinnamic acid, chlorogenic acid, galangin, sinapic acid, syringic acid, ferulic acid, kaempferol, luteolin, coumaric acid, quercetin, rosmarinic acid and caffeic acid. Linearity ranges used for compound quantification were satisfactory, presenting correlation coefficients between 0.997 and 0.999 for all 20 compounds. The method showed good performance characteristics: detection and quantification limits of 0.02 to 1.75 and 0.07 to 5.77 μg mL−1, respectively. The relative standard deviation values for repeatability did not exceed 4.86 % for intra-day assays and 5.07 % for inter-day assays. The recovery assays presented results between 87.4 and 114. 2 % for Origanum sample and between 85.0 and 111.0 % for propolis sample. The results obtained from the analysis of samples are in good correlation with literature data and bring new information about less studied samples such us aqueous Romanian propolis extracts and ethanolic Mentha aquatica extract.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The largely studied group of phytochemicals is polyphenol, an assembly of secondary metabolites with various chemical structures and functions, which are produced during the physiological plant growth process as a response to different forms of environmental conditions (Naczk and Shahidi 2004). Their biological activities have been widely studied during the last decades, providing strong evidence of their potential health benefits. Plants are mainly endorsed by their antioxidant properties, since they can act as free radical scavengers, electron or hydrogen donors and strong metal chelators, having neuroprotective effects and thus preventing the lipid peroxidation, DNA damage, etc. (Afanasev et al. 1989; Blokhina et al. 2003; Nazari et al. 2013; Taviano et al. 2013). As a consequence, radical scavenger compounds are nowadays gaining an increasing interest and the consumption of foods rich in antioxidants is greater than ever.
Medicinal plants, vegetables and fruits are the major source of natural antioxidants (Giada 2012). Besides that, propolis contains predominantly polyphenolic compounds including flavonoids and cinnamic acid derivatives which appear to be the main components responsible for its biological activities (Marcucci 1995). Propolis has a long history of being used in traditional medicine dating back to 300 BC (Ghisalberti 1979) and has been reported to have a broad spectrum of biological properties, namely anticancer, antioxidant, antiinflammatory, antibiotic and antifungal activities (Choudhari et al. 2013).
Clinical experiments have indicated that several polyphenolic compounds, such as phenolic acids (both hydroxybenzoic and hydroxycinnamic acids), flavonoids (catechin, quercetin, myricetin, kaempferol) and other polyphenols (epigallocatechins, resveratrol), could induce apoptosis in cancer cells (Knekt et al. 1997; Soleas et al. 2002; Darvesh and Bishayee 2013; Yu et al. 2014). Another aspect is that polyphenolic compounds may contribute to Alzheimer’s disease-modifying activity by reducing the generation of amyloid-β (Aβ) peptides that are critical for the early stage and progression of the disease (Wang et al. 2006). Ho et al. (2013) study indicated that quercetin-3-O-glucuronide derivatives (from red wine and some plants) accumulated in the brain are capable of interfering with the generation of Aβ peptides and may lower the relative risk for developing Alzheimer’s disease (AD) dementia.
High-performance liquid chromatography (HPLC) is the most selected method for separation and quantification of individual polyphenols in fruits, plants or beverages using detection systems based on spectrophotometry, fluorometry and/or mass spectrometry. Liquid chromatography–mass spectrometry (LC–MS) technique is considered to be the most efficient tool in the study of polyphenolic compounds from different natural sources (Ignat et al. 2011).
Capillary electrophoresis (CE) has proved to be a good alternative technique to HPLC for the investigation of various compounds due to its good resolution, versatility and simplicity, short analysis time and low consumption of chemicals and samples. UV–Vis absorption is obviously the most widely used detection technique (Hurtado-Fernández et al. 2010; Zhao et al. 2014), and even if nowadays, CE coupled to MS is getting increased attention. For obtaining a fine separation in CE, it is necessary to optimise several parameters, such as buffer (background electrolyte) type, concentration and pH, type and dimensions of capillary, temperature, voltage and injection mode, etc.
For quantification of polyphenolic compounds from natural sources, the UV–Vis detection mode is adequate due to the fact that these compounds are biologically useful in detectable quantities and not in trace concentrations. The major contribution of CE analysis compared to HPLC is the considerable low consumption of time, reagents and samples and a much simplified procedure.
This study introduces a simple, reliable and fast (under 27 min) CE method which was developed and partially validated for simultaneous separation of 20 polyphenolic compounds (thought to be found in propolis and plant extracts). According with previous research, only Franquet-Griell et al. (2012) had separated 20 polyphenols by capillary zone electrophoresis (CZE) from different sorts of Spanish wines. Our method was developed to identify different polyphenolic compounds and was successfully applied on propolis and plant extracts.
Experimental
Reagents
Folin–Ciocalteu reagent was purchased from Sigma-Aldrich Chemie, Steinheim, Germany, and AlCl3 from ICN Biomedicals, Inc., OH, USA. The caffeic acid (Sigma, C0625), quercetin (Sigma, Q4951), kaempferol (BioChemika, 60010), rutin (Sigma, R5143), luteolin (Fluka, 72511), ferulic acid (Aldrich, 128708), chlorogenic acid (Aldrich, C3878), gallic acid (Fluka, 48630), rosmarinic acid (Sigma, R4033), sinapic acid (Sigma, D7927), resveratrol (Sigma, R5010), p-coumaric acid (Fluka, 28200), pinostrobin (Fluka, 80614), syringic acid (Fluka, 86230), naringenin (BioChemika, 71155), galangin (Aldrich, 282200), acacetin (Sigma, A8206), chrysin (Aldrich, C8010-5), isoquercitrin (Roth, 7586.1), umbelliferone (Aldrich, H24003) and cinnamic acid (Fluka, 96340) stock solutions, 1 mg mL−1, were prepared by dissolving in methanol the appropriate amount of substance.
Sodium tetraborate and sodium phosphate were purchased from Sigma (Germany) and sodium dodecyl sulfate from Fluka (Switzerland).
Ultrapure water and 0.1 and 1 N sodium hydroxide solutions were purchased from Agilent Technologies (Germany). Solvents (Merck, Germany) and solutions were filtered on 0.2-μm membranes (Millipore, Bedford, MA, USA) and degassed prior to use. Stock solutions for each standard were stored at +4 °C. Working solutions were prepared daily by diluting the stock solutions in background electrolyte (BGE).
Equipments and Methods
A Thermo Fisher spectrophotometer Evolution 260 Bio was used for all spectrometric analyses. The content in total phenolics of samples was quantified using the Folin–Ciocalteu reagent solution (Woisky and Salatino 1998; Popova et al. 2004). Samples or standard solutions of gallic acid or methanol as blank (0.2 mL) were transferred to a 10-mL volumetric flask, containing 1.5 mL water, 0.8 mL Folin–Ciocalteu reagent and 1.2 mL 20 % sodium carbonate solution (w/v). The volume was adjusted to 10 mL with ultrapure water. The absorbance was measured at 760 nm after 2 h of incubation at room temperature. Total phenolic content was estimated using a calibration curve of standard gallic acid: y = 0.0116x + 0.0392; R 2 = 0.999 in concentration range 10–100 μg mL−1. The total flavonoid content was established with the method employing AlCl3 to form a complex, which was spectrophotometrically measured at 425 nm (Popova et al. 2004). To 0.2 mL of sample or standard solution of quercetin or methanol as blank, 4 mL methanol and 0.2 mL 5 % AlCl3 (w/v) were added and the volume was adjusted to 10 mL with water in a volumetric flask. After 30 min, the absorbance was measured at 425 nm. Total flavonoid content was estimated using a calibration curve of standard quercetin: y = 0.0082x − 0.0112; R 2 = 0.999. Every assay was carried out in triplicate.
Electrophoretic separation was achieved using an Agilent CE instrument with diode array detector (DAD) (ChemStation software) and CE standard bare fused silica capillary (Agilent Technologies, Germany) with an internal diameter of 50 μm and an effective length of 72 cm. Prior to use, the capillary was washed successively with basic solutions: 10 min with 1 N NaOH, 10 min with 0.1 N NaOH followed by ultrapure water for 10 min and buffer for 20 min. The capillary was flushed between runs with 0.1 M NaOH for 1 min, H2O for 1 min and background electrolyte for 2 min. The electrolyte was refreshed after three consecutive runs.
Sample injection was performed using the hydrodynamic mode (35 mbar/12 s), while the capillary was maintained at a constant temperature of 30 °C.
The simultaneous separation of polyphenolic compounds was obtained using 45 mM tetraborate buffer with 0.9 mM sodium dodecyl sulfate (SDS) (pH = 9.35 adjusted with 1 M HCl) as a background electrolyte. BGE was filtered on 0.2-μm membranes (Millipore, Bedford, MA, USA) and degassed before use. The applied voltage was 30 kV; direct UV absorption detection was carried out from 200 to 360 nm, and the quantification of samples was performed at 280 nm.
Sample Preparation
The propolis sample was collected in 2012 from Dambovita County, Romania. The sample was homogenized and frozen at −18 °C, and an aliquot (100 g) was grounded to powder by hand in a porcelain mortar. Ten-gram frozen propolis was mixed with 100 mL of different solutions (distilled water; glycine buffer 0.1 M, pH = 2.5; acetate buffer 0.1 M, pH = 5; phosphate buffer 0.1 M, pH = 7.4; and carbonate buffer 0.1 M, pH = 9). The suspensions were maintained for 15 min under stirring at 70 °C and were then cooled at room temperature. The mixtures were left for maceration for 10 days at room temperature and then were filtered through Whatman no. 1 filter paper, adjusted to 100 mL with the same solutions, and then filtered on 0.2-μm Millipore filters before the analysis.
The purpose of using different environments for propolis extraction was to increase the solubility of certain compounds (especially flavonoids) in aqueous solution. It is known that the concentration ranges of flavonoids in extracts are limited due to their restricted solubility, but there are some parameters that can improve the solubility, such as temperature (Srinivas et al. 2009, 2010), nature of the solvents (Ferreira and Pinho 2012) and the pH (Luo et al. 2012). Depending on pH, the hydroxyl groups of polyphenols are more or less ionized and this could influence the solubility of compounds in aqueous solutions.
The plants (Mentha aquatica and Origanum from Plafar Company) were dried for 1 week at room temperature (RT) and finely grinded with a Grindomix GM200 grinder. The extraction was made at RT, during 7 days, with a mixture of ethanol/water (70 % (v/v)) in 1:10 ratio (w/v). After that, the extracts were centrifuged for 15 min at 5000 rpm and supernatants were collected, adjusted to 10 mL and filtered (0.2 μm Millipore; Bedford, MA, USA). Samples were diluted (where necessary) in BGE.
Results and Discussions
Total Polyphenols and Flavonoids
The results obtained from total polyphenol and flavonoid analysis in samples are presented in Table 1. All the samples contained important concentrations of polyphenols (between 2027.0 ± 5.0 and 7748.3 ± 17.2) and flavonoids (between 270.2 ± 3.1 and 2192.3 ± 3.5), respectively. It could be noticed that the concentrations for these compounds in the propolis extracts in accordance with pH increase were increasing.
Method Development
Several CE methods were considered for polyphenolic compound separation (McGhie and Markham 1994; Markham and McGhie 1996; Petr et al. 2008; Fukuji et al. 2010; Ballus et al. 2012, 2014; Franquet-Griell et al. 2012). In CZE, the analytes are separated inside a narrow bore capillary containing only a buffer solution across which a voltage is applied creating an electric field. In general, the migration of charged compounds in CZE is based on two major components, namely the electro-osmotic flow (EOF) and the electrophoretic mobility of the analytes. These parameters are influenced by buffer (background electrolyte) type, concentration and pH, capillary type and dimensions, temperature, voltage and injection mode, etc.
Several BGEs were examined for polyphenolic compound separation (e.g. phosphate, borate) individually or combined with different surfactants (SDS). Our CE method belongs to CZE category with direct UV detection. The anionic surfactant, SDS, improves the separation but was found under critical concentration level for the standard micellar chromatography. The procedure based on tetraborate buffer at alkaline pH was assessed for the best separation of 20 compounds. Tetraborate concentration, SDS concentration and pH value were slightly varied in order to improve the separation in the shortest possible time.
Effect of Concentration of BGE and Anionic Surfactant
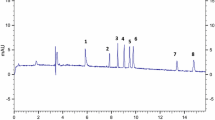
Figure 1 presents the results for simultaneous separation of 20 polyphenolic compounds in three different migration buffers (40–50 mM) at the same value of pH and SDS.
Effect of sodium borate and SDS concentrations for the separation of 20 polyphenolic compounds: 1 resveratrol, 2 pinostrobin, 3 acacetin, 4 chrysin, 5 rutin, 6 naringenin, 7 isoquercitrin, 8 umbelliferone, 9 cinnamic acid, 10 chlorogenic acid, 11 galangin, 12 sinapic acid, 13 syringic acid, 14 ferulic acid, 15 kaempferol, 16 luteolin, 17 coumaric acid, 18 quercetin, 19 rosmarinic acid and 20 caffeic acid; a 45 mM borate and 0.9 mM SDS, pH 9.35 (working conditions); b 40 mM borate and 0.9 mM SDS, pH 9.35; c 50 mM borate and 0.9 mM SDS; pH 9.35; d 45 mM borate and 0.45 mM SDS, pH 9.35; e 45 mM borate and 1.35 mM SDS, pH 9.35
With the increasing of the buffer concentration (EOF reduced), the resolution increases but the migration time also increases (Whatley 2001). However, it can be seen that at 50 mM concentration, the migration time increased and the peaks resolution decreased compared to concentration of BGE considered the most efficient.
The effect of SDS concentrations was studied within the domain 0.45–1.35 mM. The buffer concentration of sodium tetraborate was maintained at 45 mM and pH 9.35. As shown in Fig. 1(d), at 0.45 mM SDS concentration, the migration time increased and pinostrobin could not be separated, while at a higher concentration of SDS (Fig. 1(e)), it was observed that peaks resolution decreases along with increasing migration times. Therefore, the most suitable concentration of SDS was considered to be 0.9 mM (Fig. 1(a)).
Effect of pH of BGE
Buffer pH is a very important parameter for its effect on zeta potential (ζ), the EOF as well as the overall charge of all the analytes, which affect the migration time and the separation of the analytes. The experiments were performed, varying the pH from 9.25 to 9.45 while preserving the other conditions. As presented in Fig. 2, the best result for the separation of 20 polyphenolic compounds at different pH values was obtained at pH 9.35 (Fig. 2(c)), respectively, the elution of all compounds including pinostrobin (see peak 2 in Fig. 2(c)) and the shortest runtime.
Other Operating Conditions
The 72-cm-length capillary was used for separation, and the highest 30 kV voltage was applied for reducing the runtime to ~27 min.
The addition of organic solvent in the BGE was also tested, and a small amount of organic solvent (methanol, acetonitrile) was added, leading to a decrease in resolution. Hydrodynamic injection time (5–15 s) was also studied in order to increase sensitivity. An injection time of 12 s (35 mbar) was selected for a good resolution.
Validation of the Electrophoretic Procedure
The main parameters used in the validation of the methodology are the following: the selectivity, linearity, precision, accuracy (recovery), limit of detection and limit of quantification. The results are presented in Tables 2, 3 and 4. Limit of detection (LoD) and limit of quantification (LoQ) used to assess sensitivity were estimated using a signal-to-noise ratio of 3 and 10, respectively. Detection limits for the samples resulted between 0.02 μg mL−1 for quercetin and acacetin and 1.75 μg mL−1 for pinostrobin. Linearity ranges used for compound quantification were satisfactory, presenting correlation coefficients (r 2) between 0.997 and 0.999 for all 20 compounds (Table 2).
The repeatability of the method was studied by repeated injections of the polyphenol mixtures (standards) five times in the same day (intra-day precision), whereas the reproducibility assimilated to inter-day precision was assessed by triplicate injections in three different days (Table 3). The results are reported in terms of relative standard deviation (RSD). The RSD values for repeatability did not exceed 4.86 % for intra-day assays and 5.07 % for inter-day assays. Quantification limits were maintained between 0.07 μg mL−1 for quercetin and 5.77 μg mL−1 for pinostrobin.
In order to verify the applicability of the proposed method for various types of polyphenolic extracts, the recovery tests were performed for an ethanolic sample of Origanum vulgare (diluted 20 times) and an aqueous sample of propolis (diluted 50 times) spiked with known concentrations of standard solutions (Table 4). The results of the recovery assays were between 87.4 and 114, 2 % for O. vulgare sample and between 85.0 and 111.0 % for propolis sample. Regarding all validation parameters, the method complies with validation requirements and it is suitable for the analysis of selected samples.
Sample Analysis
Different plant and propolis extracts (see “Sample Preparation”) were analyzed for polyphenolic compounds using the CE method validated above. The results obtained were compared with other literature data (Table 5). Generally, our method showed a higher accuracy than most CE methods presented in Table 5. Also, the time of analysis is short for the separation of 20 compounds and the peaks are completely separated (Fig. 1(a)).
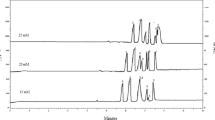
The content of polyphenolic compounds found in analyzed samples is shown in Table 6, and electropherograms of two samples are presented in Fig. 3. Due to the presence of other components in propolis and plant extracts, the method of standard additions was used for the identification of polyphenols in samples, comparing their migration time with the migration times obtained for standard polyphenols.
Electropherograms of a Origanum sample and b propolis sample (pH 9); 2 pinostrobin, 3 acacetin, 4 chrysin, 5 rutin, 6 naringenin, 7 isoquercitrin, 9 cinnamic acid, 10 chlorogenic acid, 11 galangin, 12 sinapic acid, 13 syringic acid, 14 ferulic acid, 15 kaempferol, 16 luteolin, 17 coumaric acid, 18 quercetin, 19 rosmarinic acid and 20 caffeic acid
The composition of propolis depends on the vegetation of the area from where it is collected (Marcucci 1995). Propolis from temperate zones (Europe, Asia, North America, etc.) contains usually phenolic compounds, including some flavonoids, aromatic acids and their esters originated mainly from the poplar buds (Populus spp.) exudates, which appear to be the principal source of propolis (Tomas-Barberan et al. 1993; Bankova et al. 2000). Looking at the results obtained in our study on aqueous Romanian propolis extracts (Table 6), the major components were flavonoids (chrysin, pinostrobin, quercetin, naringenin, galangin) and phenolic acids (caffeic, coumaric, ferulic, cinnamic). These results are in accordance with data of other authors: Kujumgiev et al. (1999), Uzel et al. (2005), Kalogeropoulos et al. (2009), Mărghita et al. (2013) who found flavonoids and phenolic acid esters as main constituents in Bulgarian, Anatolian, Greek and Romanian propolis samples, respectively.
Results indicated that the content of polyphenols in propolis extracts varies, the highest concentrations of flavonoids being found in propolis extract at pH 9, while the highest concentrations of caffeic, coumaric and ferulic acids were found in propolis extract at pH 7.6 (Table 6). The 0.1 M carbonate buffer, pH = 9, was the most efficient environment for polyphenol extraction from propolis. Chrysin, considered as the reference flavonoid in poplar propolis, which was reported in high amounts in Romanian propolis samples (1.6 mg/g propolis, Mărghita et al. 2013), was found in our samples in higher amounts, ~3.7 mg/g in neutral environment and 7.2 mg/g in alkaline environment (pH = 9), respectively. Generally, in propolis extracts, all the representative peaks were identified (Fig. 3(b)).
Concerning the sample of Origanum, rosmarinic acid was representatively found, in accordance with other studies from Romania (Cioanca et al. 2013; Danila et al. 2011) and with other studies on O. vulgare. Our results regarding polyphenol composition of O. vulgare ethanolic extract are similar to those reported in Lithuania (Radušienė et al. 2008), India (Hithamani and Ramalakshmi 2013) and Greece (Grevsen et al. 2009). As could be observed in Fig. 3(a), some unidentified signals appear in Origanum sample which could be attributed to hispidulin, apigenin, diosmetin, herbacetin, eriodictyol, taxifolin or carnosic acid as other studies previously reported (Cavero et al. 2006; Skoula et al. 2008).
The sample of M. aquatica presented the same compounds previously reported in the literature (Mišan et al. 2011), namely rosmarinic acid, quercetin, naringenin, caffeic acid, chlorogenic acid and other compounds such as rutin and ferulic acid found by HPLC-DAD-MS in our previous study (Teodor et al. 2014, submitted manuscript). In addition, cinnamic acid, isoquercitrin and kaempferol were identified using CE method in the M. aquatica sample.
All the analytical data obtained by capillary electrophoresis method were situated in the concentration range obtained by spectrophotometric methods.
Conclusions
Capillary electrophoresis is a good alternative technique to HPLC for the investigation of various compounds due to its good resolution, versatility, simplicity, short analysis time and low consumption of chemicals and samples. This simple, reliable and fast CE method developed and partially validated for simultaneous detection of 20 polyphenolic compounds run in less than 27 min.
The method was experimentally improved with a BGE consisting of sodium tetraborate and SDS and is well suited for separation of polyphenolic compounds in propolis and plant extracts.
Regarding all the validation parameters, the method complies with validation requirements and it is suitable for the analysis of selected samples. The results obtained from the analysis of samples are in correlation with other literature data and bring new information about less studied samples such as Romanian propolis and M. aquatica.
References
Afanasev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI (1989) Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol 38:1763–1769
Anastasiadi M, Pratsinis H, Kletsas D, Skaltsounis A-L, Haroutounian SA (2010) Bioactive non-coloured polyphenols content of grapes, wines and vinification by-products: evaluation of the antioxidant activities of their extracts. Food Res Intern 43:805–813
Bankova VS, De Castro SL, Marcucci MC (2000) Propolis: recent advances in chemistry and plant origin. Apidologie 31:3–15
Ballus CA, Meinhart AD, Grando de Oliveira R, Godoy HT (2012) Optimization of capillary zone electrophoresis separation and on-line preconcentration of 16 phenolic compounds from wines produced in South America. Food Res Intern 45:136–144
Ballus CA, Meinhart AD, de Souza Campos FA, Bruns RE, Godoy HT (2014) Doehlert design-desirability function multi-criteria optimal separation of 17 phenolic compounds from extra-virgin olive oil by capillary zone electrophoresis. Food Chem 146:558–568
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Cavero S, Garcia-Risco MR, Marin FR, Jaime L, Santoyo S, Senorans FJ, Reglero G, Ibanez E (2006) Supercritical fluid extraction of antioxidant compounds from oregano. Chemical and functional characterization via LC–MS and in vitro assays. J Supercrit Fluids 38:62–69
Choudhari MK, Haghniaz R, Rajwade JM, Paknikar KM (2013) Anticancer activity of Indian stingless bee propolis: an in vitro study. Evid Based Complement Alternat Med 2013; ID: 842985
Cioanca O, Mircea C, Iancu C, Gille E, Hăncianu M (2013) Intraspecific variability of Origanum crop varieties depending on growing conditions: secondary metabolites accumulation. Sgem2013 Conference Proceedings, doi: 10.5593/Sgem2013/Bf6/S25.015
Danila AO, Gatea F, Radu GL (2011) Polyphenol composition and antioxidant activity of selected medicinal herbs. Chem Nat Compds 47:22–26
Darvesh AS, Bishayee A (2013) Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutr Cancer 65:329–344
Ferreira O, Pinho SP (2012) Solubility of flavonoids in pure solvents. Ind Eng Chem Res 51:6586–6590
Franquet-Griell H, Checa A, Núñez O, Saurina J, Hernández-Cassou S, Puignou L (2012) Determination of polyphenols in Spanish wines by capillary zone electrophoresis. Application to wine characterization by using chemometrics. J Agric Food Chem 60:8340–8349
Fukuji TS, Tonin FG, Tavares MFM (2010) Optimization of a method for determination of phenolic acids in exotic fruits by capillary electrophoresis. J Pharm Biomed 51:430–438
Ghisalberti EL (1979) Propolis: a review. Bee World 60:59–84
Giada MLR (2012) Phenolic acids as additives in the food industry. In: Munné-Bosch S (ed) Phenolic acids: composition, applications and health benefits. Nova Science, New York, pp 125–145
Grevsen K, Fretté XC, Christensen LP (2009) Content and composition of volatile terpenes, flavonoids and phenolic acids in Greek oregano (Origanum vulgare L. ssp. hirtum) at different development stages during cultivation in cool temperate climate. Eur J Hortic Sci 74:193–203
Hithamani G, Ramalakshmi K (2013) Microwave assisted extraction of phenolics from Origanum vulgare. Int J Agric Soil Sci 1:7–12
Ho L, Ferruzzi MG, Janle EM, Wang J, Gong B, Chen T-Y, Lobo J, Cooper B, Wu QL, Talcott ST, Percival SS, Simon JE, Pasinetti GM (2013) Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J 27:769–782
Hurtado-Fernández E, Gomez-Romero M, Carrasco-Pancorbo A, Fernandez-Gutierrez A (2010) Application and potential of capillary electroseparation methods to determine antioxidant phenolic compounds from plant food material. J Pharm Biomed Anal 53:1130–1160
Ignat I, Volf I, Popa VI (2011) A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem 126:1821–1835
Kalogeropoulos N, Konteles SJ, Troullidou E, Mourtzinos I, Karathanos VT (2009) Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem 116:452–461
Kiss AK, Derwińska M, Granica S (2011) Quantitative analysis of biologically active polyphenols in evening primrose (Oenothera paradoxa) seeds aqueous extracts. Pol J Food Nutr Sci 61:109–113
Knekt P, Jarvinen R, Seppanen R, Hellovaara M, Teppo L, Pukkala E et al (1997) Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am J Epidemiol 146:223–230
Kujumgiev A, Tsvetkova I, Serkedjieva Yu, Bankova V, Christov R, Popov S (1999) Antibacterial, antifungal and antiviral activity of propolis from different geographic origin. J Ethnopharmacol 64:235–240
Luo Z, Murray BS, Ross AL, Povey MJ, Morgan MR, Day AJ (2012) Effects of pH on the ability of flavonoids to act as Pickering emulsion stabilizers. Colloids Surf B: Biointerfaces 92:84–90
Marcucci MC (1995) Propolis: chemical composition, biological properties and therapeutic activity. Apidologie 26:83–99
Mărghita DA, Dezmirean DS, Bobi O (2013) Important developments in Romanian propolis research. Evid Based Complement Alternat Med 2013; ID: 159392
Markham KR, McGhie TK (1996) Separation of flavones by capillary electrophoresis: the influence of pKa on electrophoretic mobility. Phytochem Anal 7:300–304
McGhie TK, Markham KR (1994) Separation of flavonols by capillary electrophoresis—the effect of structure on electrophoretic mobility. Phytochem Anal 5:121–126
Mišan AČ, Mimica-Dukić NM, Mandić AI, Sakač MB, Milovanović ILj, Sedej IJ (2011) Development of a rapid resolution HPLC method for the separation and determination of 17 phenolic compounds in crude plant extracts. Cent Eur J Chem 9:133–142
Naczk M, Shahidi F (2004) Extraction and analysis of phenolics in food. J Chromatogr A 1054:95–111
Nazari QA, Kume T, Takada-Takatori Y, Izumi Y, Akaike A (2013) Protective effect of luteolin on an oxidative-stress model induced by microinjection of sodium nitroprusside in mice. J Pharmacol Sci 122:109–117
Petr J, Vitkova K, Ranc V, Znaleziona J, Maier V, Knob R, Sevcik J (2008) Determination of some phenolic acids in Majorana hortensis by capillary electrophoresis with online electrokinetic preconcentration. J Agric Food Chem 56:3940–3944
Popova M, Bankova V, Butovska D, Petkov V, Nikolova-Damyanova B, Sabatini AG, Marcazzan GL, Bogdanov S (2004) Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem Anal 15:235–240
Radušienė J, Ivanauskas L, Janulis V, Jakštas V (2008) Composition and variability of phenolic compounds in Origanum vulgare from Lithuania. Biology 54:45–49
Skoula M, Grayer RJ, Kite GC, Veitch NC (2008) Exudate flavones and flavanones in Origanum species and their interspecific variation. Biochem Syst Ecol 36:646–654
Soleas GJ, Grassc L, Josephy PD, Goldberg DM, Diamandis EP (2002) A comparison of the anticarcinogenic properties of four red wine polyphenols. Clin Biochem 35:119–124
Srinivas K, King JW, Monrad JK, Howard LR, Hansen CM (2009) Optimization of subcritical fluid extraction of bioactive compounds using Hansen solubility parameters. J Food Sci 74:E342–E354
Srinivas K, King JW, Howard LR, Monrad JK (2010) Solubility of gallic acid, catechin, and protocatechuic acid in subcritical water from (298.75 to 415.85) K. J Chem Eng Data 55:3101–3108
Taviano MF, Marino A, Trovato A, Bellinghieri V, Melchini A, Dugo P, Cacciola P, Donato P, Mondello L, Güvenç A, De Pasquale R, Miceli N (2013) Juniperus oxycedrus L. subsp. oxycedrus and Juniperus oxycedrus L. subsp. macrocarpa (Sibth. & Sm.) Ball. “berries” from Turkey: comparative evaluation of phenolic profile, antioxidant, cytotoxic and antimicrobial activities. Food Chem Toxicol 58:22–29
Tomas-Barberan FA, Garcia-Viguera C, Vit-Oliver P, Ferreres F, Tomas-Lorente F (1993) Phytochemical evidence for botanical origin of tropical propolis from Venezuela. Phytochemistry 34:191–196
Uzel A, Sorkun K, Oncag O, Cogulu D, Gencay O, Salih B (2005) Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol Res 160:189–195
Wang J, Ho L, Zhao Z, Seror I, Humala N, Dickstein DL, Thiyagarajan M, Percival SS, Talcott ST, Pasinetti GM (2006) Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J 20:2313–2320
Whatley H (2001) Basic principles and modes of capillary electrophoresis. In: Petersen JR, Mohammad AA (eds) Clinical and forensic applications of capillary electrophoresis pathology and laboratory medicine. Humana, Totowa, pp 21–58
Woisky RG, Salatino A (1998) Analysis of propolis: some parameters and procedures for chemical quality control. J Apic Res 37:99–105
Yu Y, Deng Y, Lu B-M, Liu Y-X, Li J, Bao J-K (2014) Green tea catechins: a fresh flavor to anticancer therapy. Apoptosis 19:1–18
Zhao J, Hu D-J, Lao K, Yang Z-M, Li S-P (2014) Advance of CE and CEC in phytochemical analysis (2012–2013). Electrophoresis 35:205–224
Acknowledgments
The research was supported by Romanian Agency for Scientific Research, Nucleu-Biodiv grant PN09-360101/2009.
Compliance with Ethics Requirements
ᅟ
Conflict of Interest
Florentina Gatea has no conflict of interest. Eugenia Dumitra Teodor has no conflict of interest. Alina Oana Matei has no conflict of interest. Georgiana Ileana Badea has no conflict of interest. Gabriel Lucian Radu has no conflict of interest. All the funds are provided by Romanian Agency for Scientific Research. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gatea, F., Teodor, E.D., Matei, A.O. et al. Capillary Electrophoresis Method for 20 Polyphenols Separation in Propolis and Plant Extracts. Food Anal. Methods 8, 1197–1206 (2015). https://doi.org/10.1007/s12161-014-0006-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-0006-5