Abstract
A new sample preparation procedure combining QuEChERS and dispersive liquid–liquid microextraction (DLLME) was optimized for the determination at trace levels of 13 pesticides from different chemical families (i.e. 2,4-D, acetamiprid, bentazone, cymoxanil, deltamethrin, dicamba, diuron, foramsulfuron, mesotrione, metalaxyl-M, methomyl, pyraclostrobin and tembotrione) in tomato by high-performance liquid chromatography with diode array detection. Target pesticides from tomato samples were isolated by liquid partitioning with acetonitrile and salts and cleaned up by dispersive solid-phase extraction (d-SPE); the analytes were concentrated in trichloromethane by the DLLME procedure. The disperser solvent from DLLME was used at the same time as carrier of analytes form extraction in QuEChERS method. The main factors affecting sample cleanup by d-SPE in QuEChERS and DLLME yield were optimized by means of an experimental design. Under the optimum conditions, good linearity was obtained, the recoveries of pesticides in tomato samples at spiking levels between 0.01 and 1.00 mg/kg ranged from 86 to 116 % (for foramsulfuron and cymoxanil, respectively). Precision was within 15.0 % (RSD) except at the LQ for tembotrione, which was 17.4 %. Limits of quantification achieved (ranging from 0.0058 to 0.15 mg/kg) were below the maximum residue limits established by the European Union.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence of pesticide residues in horticultural products is of concern to human health. European Union and Codex Alimentarius established maximum residue levels (MRLs) to regulate pesticides levels in fruits and vegetables and to ensure consumer safety and regulation of foreign trade (European Commission 2005). Tomato is a typical representative commodity of the category fruiting vegetables (with high water content, Directorate General Health and Consumer Protection, SANCO/10684/2009 2009). Consequently, the search for pesticide residue in this type of vegetable is of major concern.
Development of analytical methods for analyses of pesticides in fruiting vegetables, such as tomato, is difficult due to the fact that compounds of different polarities, solubilities, volatilities and pKa values have to be simultaneously extracted and analysed (Štajnbaher and Zupančič-Kralj 2003; Ravelo-Pérez et al. 2009). Polar pesticides are particularly challenging to analyse (e.g. methomyl, acetamiprid, dicamba, cymoxanil, foramsulfuron, mesotrione, bentazone, 2,4-D). Additionally, the matrix presents a very complex composition and potential interferences can be co-extracted.
QuEChERS sample preparation method stands for “quick, easy, cheap, effective, rugged and safe”. The novelty of this extraction procedure, introduced by Anastassiades et al.(2003) for the multiresidue analysis of pesticides in fruits and vegetables, was the use of magnesium sulphate (MgSO4) for salting out, extractioning/portioning and dispersive solid-phase extraction (d-SPE) for cleanup. This became an AOAC International Official Method 2007.01 and CEN standard method EN 15662, with few modifications (Lehotay et al. 2010). Among other beneficial features, the QuEChERS procedure uses acetonitrile (MeCN), which enables extraction of wide polarity range analytes. Different sorbents and amounts can be used in d-SPE, namely weak ion exchange such as primary secondary amine (PSA), graphitized carbon black (GCB) and C18. Other advantages of QuEChERS methodology are its flexibility, high degree of selectivity and sensitivity (Anastassiades et al. 2003; Lehotay et al. 2005, 2010; Kmellár et al. 2010). However, the major disadvantage is its low enrichment factors (Cunha and Fernandes 2011).
During the last years, miniaturized extraction procedures have been introduced with success, namely dispersive liquid–liquid microextraction (DLLME, Rezaee et al. 2006). The DLLME method is based on the dispersion of an extraction solvent (immiscible in water) combined with a disperser solvent (miscible in both water and extraction solvents) within an aqueous solution. The ternary component solvent system forms a cloudy suspension, which establishes a very high contact area between the aqueous phase and the extraction solvent (Herrera-Herrera et al. 2010; Rezaee et al. 2010; Dadfarnia and Shabani 2010; Caldas et al. 2010).
The DLLME method presents important advantages such as simplicity of operation, quickness, low cost, easier manipulation, fewer amounts of organic extraction solvents, high recovery and enrichment factor and easier linkage to analytical methods (Caldas et al. 2010; Sarafraz-Yazdi and Amiri 2010). Its application has been extended to separation, preconcentration and determination of organic and inorganic compounds in liquid samples, but most studies of pesticides evaluated the extraction of few compounds (Fu et al. 2009) or pesticides that belong to the same chemical group, with the same behaviour (Caldas et al. 2010).
Nevertheless the complexity of food samples is a drawback that makes its application difficult in food analysis. DLLME technique in food analysis requires a previous extraction or cleaning step, especially for solid or semisolid samples (Asensio-Ramos et al. 2011). Combination of QuEChERS with DLLME can be of interest due to the simplicity of operation and low costs associated with high recoveries and easier linkage to analytical methods such as GC or LC. The use of powerful mass spectrometric detectors has played a vital role in solving many problems related to food safety (Malik et al. 2010; Sharma et al. 2010). However, these types of detectors are very expensive and not available for all laboratories. Although diode array detection (DAD) offers lower selectivity, sensitivity and specificity, the relatively low cost for equipment setup makes it an attractive detection method (Chou et al. 2009; Al-Rahman et al. 2012). Thus, validations of low cost methods that enable analyses of trace levels of pesticides in complex matrices using high-performance liquid chromatography (HPLC)/DAD are relevant.
The aim of this work is the development of a quick and inexpensive sample preparation combining QuEChERS and DLLME methods for the determination of trace levels of 13 pesticides in tomato using HPLC/DAD. These pesticides (i.e. 2,4-D, acetamiprid, bentazone, cymoxanil, deltamethrin, dicamba, diuron, foramsulfuron, mesotrione, metalaxyl-M, methomyl, pyraclostrobin and tembotrione) belong to different chemical families. Special attention was given to optimization of sample cleanup by d-SPE in QuEChERS procedure and to the nature and amount of extraction solvent, sample pH and salting out effect in DLLME methodology. Experimental design methodology [a full factorial central composite design (CCD)] was used for the optimization of some experimental parameters, as PSA and GCB amounts, extraction solvent volume and sodium chloride (NaCl) percentage, whereas the other parameters were previously optimized by univariate analysis.
Experimental
Chemicals and Reagents
All pesticides had analytical grade (purity >97.8 %) including 2,4-D, acetamiprid, bentazone, cymoxanil, deltamethrin, dicamba, diuron, foramsulfuron, mesotrione, metalaxyl-M, methomyl, pyraclostrobin and tembotrione were purchased from Sigma-Aldrich (Steinheim, Germany). The 4-nitrophenyl acetate (NPA) and and 2-nitrodiphenyl ether (NDPE) with purity >99 % from Sigma-Aldrich were used as internal standard (IS). All the solvents used, including acetonitrile (MeCN), were HPLC grade from Merck (Darmstadt, Germany). Ultrapure water (0.054 μS/cm) was obtained by using a Milli-Q system from Millipore (Milford, MA, USA).
Standard solutions of each pesticide were prepared in acetonitrile and kept with light excluded in a freezer. Working mixtures of appropriate concentrations were prepared weekly and kept at low temperature and protected from light (according SANCO guidelines (Directorate General Health and Consumer Protection, SANCO/10684/2009 2009)).
Buffer solutions with pH 1, 3, 5 and 8 were prepared to study the effect of pH on DLLME pesticide extraction (Perrin and Dempsey 1974). The buffer with pH 1, selected for analysis of real samples, contained 25 mL 0.2 mol dm−3 KCl and 67 mL of 0.2 mol dm−3 HCl, diluted with ultrapure water to 100 mL (Perrin and Dempsey 1974).
Dispersive SPE sorbents used included C18-bonded silica (particle size 55–105 μm) from Waters (Milford, MA, USA), primary secondary amine (PSA; particle size 50 μm) from Supelco (Bellefonte, PA, USA) and GCB (particle size 37–149 μm) from Sigma-Aldrich. Anhydrous MgSO4 and sodium chloride (NaCl) were analytical grade from Riedel-de Haën (Buchs, Switzerland). To ensure efficient removal of phthalates and residual water, MgSO4 was treated for 5 h at 500 °C in a muffle furnace. Extraction solvents trichloromethane, chlorobenzene, trichloroethylene, tetrachloromethane and tetrachloroethylene were high-purity solvents for HPLC analysis obtained from Fluka.
HPLC-DAD Conditions
The chromatographic analysis was carried out in an analytical Jasco HPLC unit (Tokyo, Japan) equipped with PU-1580 HPLC pumps, a Column Heater (Model 7981; Jones Chromatography, Hengoed, UK), with Rheodyne valve 7725i (Supelco, USA) and MD-910 Plus multiwavelength detector. The separation was achieved in a reversed phase column Ultracarb ODS (30) C18 (5 μm, 250 mm 4.6 mm; Phenomenex, Milford, MA, USA). The Borwin PDA Controller Software (JMBS Developments, Le Fontanil, France) was also used. The HPLC was carried out by gradient elution was with a mixture of two solvents and a flow of 1 mL min−1. Solvent A consisted of 10 × 10−3 mol dm−3 formic acid/sodium formate buffer solution at pH 3; solvent B consisted of acetonitrile. The linear gradient programme was 0–5 min, 25 % B in A; 5–22 min, 25–50 % of B in A; 22–30 min, 50–97 % of B in A; 30–38 min, 97 % B in A and 38–50 min, column rinse and re-equilibration. Separations were carried out at 35 °C. Diode array detection was set at maximum absorbance, except for acetamiprid at 250 nm and deltamethrin and pyraclostrobin at 285 nm. Peak identification in samples was carried out by comparing retention times and spectra of unknown peaks with reference standards, as well peak purity.
Combined QuEChERS and DLLME Sample Preparation Procedure
Certified organic tomato samples were bought in a local supermarket from Porto. In the first step, representative portions of unwashed pesticide free fresh tomato sample were chopped using a kitchen hand blender set Silvercrest SSMS 600 B2 Kompernass (Bochum, Germany).
The optimized and validated sample preparation procedure entailed the following steps: (1) weigh 10 ± 0.1 g of thoroughly comminuted tomato sample into a 50-mL centrifuge tube; (2) add the spiking standard and IS solution and vortex for 1 min to let the pesticides better integrate into samples; (3) dispense 10 mL MeCN to the samples, seal the tubes and shake the tubes vigorously by hand for 30 s; (4) transfer to the sample tubes 4 g anhydrous MgSO4 and 1 g NaCl; (5) cap the tubes and shake them vigorously by hand for 1 min (avoiding formation of oversized MgSO4 agglomerates); (6) centrifuge the tubes at 5,000 rpm for 5 min; (7) pick up ca. 8 mL of MeCN extract (upper layer) to 15-mL amber vial; (8) transfer 3 mL of extract to the d-SPE tubes containing 100 mg PSA, 50 mg C18 and 5.26 mg GCB; (9) seal the d-SPE tubes, vortex for 30 s and centrifuge at 3,000 rpm for 3 min; (10) transfer 2 mL of the final extracts into the Sarstedt 15-mL conical tubes; (11) add 400 μL of trichloromethane; (12) add 10 mL of buffer pH = 1 with 400 mg NaCl; (13) cloudy solution that resulted from dispersion of fine droplets of trichloromethane in aqueous solution was formed in the test tube by shaking the tubes vigorously by hand for 1 min; (14) centrifuge conical tubes at 5,000 rpm for 4 min and the upper aqueous phase was removed with a pipette (dispersive particles were sediment at the bottom of the centrifuge tube) and (15) after this process, the organic phase was evaporated in a gentle stream of nitrogen and reconstituted in 50 μL of MeCN and 20 μL was injected into the HPLC system for analysis.
Experimental Design
The nature of extraction solvent and sample pH in DLLME method were optimized by univariate analyses, whereas experimental design methodology (a full factorial central composite design) was used for the optimization of PSA and GCB amounts, extraction solvent volume and NaCl percentage. Optimization of conditions for pesticide extraction from tomato was carried out using response surface methodology. Experiments with four independent variables, PSA amount (X1), GCB amount (X2), extraction solvent volume (X3) and NaCl percentage (X4) were conducted following the experimental design statistical analysis obtained by the full factorial CCD. In this work, the full CCD consisted of (1) a complete two-factorial design (Ravelo-Pérez et al. 2009); (2) n 0, centre point (n 0 >1), and (3) two axial points on the axis of each design variable at a distance of R = 2.000 from the design centre. Hence, a total number of design points of N = 2k + 2k + n 0 was used. The complete design consisted of 30 combinations including six replicates of the centre point with five degrees of freedom for calculation of errors in the experiments. The optimal values of response Y (individual peak area of interest compounds) were obtained by solving the regression equation and by analysing the response surface contour plots.
The minimum and maximum values for PSA amount (X1), GCB amount (X2), extraction solvent volume (X3) and NaCl percentage (X4) are listed in Table 1. The levels of coded independent variables with the respective α rotation are also inserted. The resulting 30 experiments were carried out randomly.
The goodness of fit of the regression model and the significance of parameter estimates were determined through appropriate statistical methods. Design-Expert trial version 8.0.6. was used (Stat-Ease Inc., Minneapolis, MN).
Results and Discussion
Optimized HPLC-DAD Chromatographic Conditions
Thirteen pesticides (2,4-D, acetamiprid, bentazone, cymoxanil, deltamethrin, dicamba, diuron, foramsulfuron, mesotrione, metalaxyl-M, methomyl, pyraclostrobin and tembotrione) were analysed in this study. These compounds have very diverse properties and can be analysed by liquid chromatography (Table 2).
Optimization of HPLC-DAD conditions was first focused on chromatographic separation of the 13 pesticides under study. A mobile phase contained formic acid and sodium formate buffer (10 × 10−3 mol dm−3, pH = 3) as aqueous phase and acetonitrile as organic phase was selected to enable appropriate chromatography behaviour of acidic pesticides. Increasing concentration of organic phase from 25 % up to 97 % enabled separation of different pesticides.
The different steps involved in the extraction of pesticides from the matrix require the use of IS; two compounds were selected: the NPA and NDPE. These compounds are not present in tomato matrix, have appropriate chromatographic elution with well resolved peaks and a UV–Vis spectrum different from that of pesticides and interferences.
The DAD spectra of each pesticide, IS and interferences were analysed and created a library with these spectra. The quantification of compounds was made at the maximal absorbance, except for acetamiprid (250 nm was preferred) and deltamethrin and pyraclostrobin (285 nm was the most appropriate for these pesticides). Figure 1 presents optimum separation of the selected group of pesticides added to a tomato sample analysed by HPLC-DAD at their maximum UV absorbance wavelengths and a tomato sample without addition of pesticides. As shown in Fig. 1, QuEChERS and DLLME extractions from certified organic tomato samples revealed the absence of the selected pesticides and some interfering compounds were also present; however, they do not influence the detection and quantification of the selected pesticides.
a Chromatographic separation of the selected group of pesticides added (concentration 1.0 mg kg−1 for dicamba and metalaxyl-M; 0.5 mg kg−1 for 2,4-D and deltamethrin, 0.1 mg kg−1 for the other nine pesticides) to a sample of organic tomato analysed by HPLC-DAD at their maximum UV absorbance wavelengths. b Chromatographic separation of a sample of organic tomato sample without addition of pesticides
QuEChERS and DLLME Parameters Selection
Unbuffered QuEChERS method (modified from literature for sample preparation of vegetables (Anastassiades et al. 2003)) was used as first step extraction procedure of pesticide compounds from matrix. The procedure involves initial single-phase extraction of 10 g sample with 10 mL acetonitrile, followed by liquid–liquid partitioning formed by addition of 4 g anhydrous MgSO4 plus 1 g NaCl and the extract was recovered (±8 mL). Major advantage of QuEChERS methodology is its high degree of selectivity and sensitivity (Anastassiades et al. 2003; Lehotay et al. 2005, 2010). However, the low enrichment factors observed are its main disadvantage (Cunha and Fernandes 2011). Thus, the DLLME procedure was tested using several extraction solvents: trichloromethane, chlorobenzene, tetrachloromethane, trichloroethylene and tetrachloroethylene. Different buffers (pH 1, 3, 5 and 8) were tried as aqueous phase.
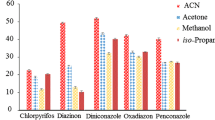
Preliminary experiments were carried out with the aim of selecting the best extracting solvent and the pH of aqueous phase. For selection of the best extraction solvent, the experiments were performed using 5 mL of ultrapure water with 1 mL of MeCN extract of QuEChERS (after d-SPE with 100 mg of C18 and 175 mg of PSA). A cloudy solution resulted from dispersion of fine droplets of a volume of 100 μL of extraction solvent (trichloromethane, chlorobenzene, trichloroethylene, tetrachloromethane, tetrachloroethylene) when injected into the 5 mL water placed in the test tube. Figure 2 shows the effect of different extraction solvents on total chromatographic peak area of the different pesticides. As can be seen from Fig. 2, trichloromethane gave the highest peak area for all pesticides under study, except for cymoxanil, dicamba, pyraclostrobin and deltamethrin. Therefore trichloromethane was selected as the extraction solvent.
Effect of different extraction solvents on the total chromatographic peak area of pesticides. Extraction conditions: 5 mL of ultrapure water with 1 mL of MeCN QuEChERS extract obtained with a tomato sample spiked with a concentration of 3.0 mg kg−1 for dicamba and metalaxyl-M; 1.5 mg kg−1 for 2,4-D and deltamethrin, 0.150 mg kg−1 for the other nine pesticides and 100 μL of each extraction solvent
The pH influences the ionization form of certain compounds and, consequently, their water solubility and extractability. The effect of the pH upon pesticides extractability with DLLME was investigated at pH 1, 3, 5 and 8 (Perrin and Dempsey 1974), using 5 mL of buffer solution, with 1 mL of MeCN QuEChERS extract and 100 μL of trichloromethane as extraction solvent (Fig. 3). The pH values close to 1 enhance the analytical signal of dicamba, foramsulfuron, mesotrione, bentazone and 2,4-D, whereas the signal of other pesticides was not significantly affected by pH variation (p > 0.05). At pH 1 acidic pesticides present a neutral form and a higher tendency to partition into the organic solvent if compared with ionized form.
Effect of pH from aqueous phase on the total chromatographic peak area of pesticides. Extraction conditions: 5 mL of buffer with 1 mL of MeCN QuEChERS extract obtained with a tomato sample spiked with a concentration of 3.0 mg kg−1 for dicamba and metalaxyl-M; 1.5 mg kg−1 for 2,4-D and deltamethrin, 0.150 mg kg−1 for the other nine pesticides and 100 μL of trichloromethane as extraction solvent
After selection of extraction solvent and aqueous phase pH, the next step was to study the range of variation for these parameters and the volume of QuEChERS extract to guarantee that cloudy suspension was formed as well as a sedimented phase was clearly obtained in the bottom of the tube. For this purpose, 10 mL of buffer solution and 2 mL of QuEChERS are required, whereas the volume of extraction solvent was further optimized.
Combined QuEChERS and DLLME sample preparation procedure requires slight modification of the QuEChERS protocol usually used for fruits and vegetables in order to increase the detection limits (DLs). The cleanup has to be made taking in account the further DLLME extraction. Thus, 3 mL of QuEChERS extract was used in d-SPE (instead of 1 mL of MeCN extract usually used (Anastassiades et al. 2003; Lehotay et al. 2005, 2010; Cunha and Fernandes 2011)) in order to recover 2 mL of cleaned extract to be used in DLLME method.
Co-extracted Matrix Components: Chromatographic Interferences
Chlorogenic acid and rutin are polar compounds and elute before 20 min under the chromatographic conditions used. Carotenoids (mainly lycopene) elute in the chromatogram after 20 min and exhibit a peculiar visible spectrum characterized by the presence of three maxima between 400 and 500 nm. These compounds present in tomato matrix are chromatographic interferences in the proposed extraction procedure.
Dispersive SPE permits the use of different combinations of sorbents in appropriate amounts to act as a “chemical filter” and remove matrix interferences without retaining the analytes. The use of C18 sorbent retains trace amounts of lipid matrix components and is commonly used to remove apolar interferences, in combination with PSA (Lehotay et al. 2005). HPLC/DAD analyses of tomato extracts using cleanup with PSA indicated that this sorbent removed and/or decreased chromatographic peaks of some less polar interfering compounds (Lehotay et al. 2010). This type of sorbent has high capacity of removing fatty acids, flavonoids, organic acids, sugars and pigments (Lehotay et al. 2010). GCB has a strong affinity towards planar molecules and removes pigments, such as chlorophylls and carotenoids (Anastassiades et al. 2003). The combination of PSA and GCB gave improved cleanup of matrix components from tomato, but their amounts must be carefully selected to prevent that analytes are also removed. The use of a mixture of three d-SPE dispersive sorbents (C18, PSA and GCB) was needed to minimize the presence of co-extracted matrix components (results not shown).
Experimental Design
Taking in account the results obtained in preliminary studies described above, a full factorial central composite design was built with 30 experimental points (24 full factorial design, with eight axial points with α = 2 and six replicates for centre point) for appropriate optimization of most important factors affecting QuEChERS cleanup (i.e. amount of PSA and GCB) and DLLME extraction yield (i.e. trichloromethane amount, NaCl percentage). The individual peak areas of all analytes were introduced separately as the response in the statistical program. Each model structure was evaluated by means of analysis of variance namely by ensuring statistical significance of regression coefficients, regression and evaluating the lack of fit (Khodadoust and Hadjmohammadi 2011). The results from individual analysis of each compound were studied. In general, higher amount of extraction solvent leads to a higher analytical signal in almost all analytes. The NaCl percentage also gives an increase of signal, but in this case only was for the first compounds eluted, namely methomyl, acetamiprid, dicamba, cymoxanil and 2,4-D. The amount of PSA and GCB reduces the peak area of interfering compounds but also reduces the chromatographic peak area of the analytes that are significant variables in the statistical program.
The simultaneous extraction of pesticides with very different properties from a complex matrix such as tomato requires a compromise between each individual extraction optimum condition. For that purpose the optimization tool of the statistical program was used that explores the response surface and gave a point prediction as a result of defined goals. To achieve this goal, the analytical signal of one pesticide that has the lowest MRL (foramsulfuron), the two internal standards (NAP and NDPE) and one usual interfering peak that appear in tomato samples were selected. The goals for each response were set: to maximize the NAP, NDPE and foramsulfuron and minimize the interfering compound to construct desirability indices. The program combines the individual desirabilities into a single number and then searches for the greatest overall desirability. A value of one represents the ideal case. A zero indicates that one or more responses fall outside desirable limits. Graphical analysis of desirability is presented in Fig. 4. The following optimum experimental conditions were obtained: 100 mg PSA, 5.26 mg GCB, 4 % NaCl and 400 μL of trichloromethane. These optimum conditions were tested (six runs), and the predicted response value of each studied compound was in agreement with experimental results (Table 3).
Analytical Performance
Verification of analytical performance of method was carried out by matrix matched calibration, recovery and accuracy studies. The linearity was investigated over the ranges indicated in Table 4 for each pesticide. For this purpose the certified organic tomato samples were spiked with the different amounts of pesticide standards and the same amount of the two internal standards before acetonitrile addition.
Calibration curves were calculated from the quotient between analytes area to internal standard versus the concentration of each compound. Analysis of the surface response curves of the experimental design indicate that NPA, 2,4-D, acetamiprid, bentazone, cymoxanil, dicamba, diuron, foramsulfuron, mesotrione, metalaxyl-M and methomyl were more influenced by NaCl percentage, volume of trichloromethane and PSA amount, thus NPA has been used as internal standard for these pesticides. Whereas for NDPE, deltamethrin, pyraclostrobin and tembotrione, the GCB amount was the major factor that conditioned the analytical signal. Thus, NDPE was used as internal standard for these three pesticides.
Calibration curves have been obtained using a weighted least squares linear regression model, and the concentration range between levels is presented in Table 4, using seven matrix calibration standards of each analyte. The calibration parameters, related standard deviations and uncertainties were calculated according to Miller and Miller (2005) and Mansilha et al. (2010). The weighting factors applied were calculated using the inverse of the variances (s i −2), and the sums of relative errors (in percent) were used as quality indicators of adjustment in the assessment of weighted linear regression. Correlation coefficients (r 2) higher than 0.9980 were obtained. Each test was performed in at least five independent experiments. The quantification limits (LQs) and LDs were calculated based on the standard deviations of the weighted intercept and calibration curve parameters.
It should be noted that the purposed analytical method, combining QuEChRES/DLLME and HPLC/DAD, allowed the quantitation of pesticides at trace levels. For all pesticides under study, the LQs were lower than the MRLs established in the EU regulation (Table 4).
A statistical comparison was performed between the calibration equations obtained from standards dissolved in acetonitrile and in spiked sample extracts (matrix matched calibration) in order to evaluate the matrix effect. Comparison of the slopes of the calibration curves was statistically evaluated by F and p values (Zar 2010). For all pesticides statistical differences were observed (p values for the comparison of the slopes were <0.001), and as a result, according to EU Guidelines (SANCO; Directorate General Health and Consumer Protection, SANCO/10684/2009 2009), quantification should be developed using the calibration curves obtained with the tomato sample.
Recovery studies at two concentration levels were performed to evaluate the accuracy and repeatability of the whole method (n = 3 for intraday assays and n = 9 for interday assays; Table 5). The lower spiking level was near the MRLs of each pesticide for tomato. Mean recoveries ranged between 86 and 116 % (for foramsulfuron and cymoxanil, respectively). With respect to intraday RSD, it was lower than 3.5 % for retention time and lower than 14.2 % for area. Interday RSD was lower than 5.4 % for retention time and lower than 17.4 % for area. The results obtained highlight that the optimized method achieves acceptable recoveries according to EU Guidelines (SANCO; Directorate General Health and Consumer Protection, SANCO/10684/2009 2009). It is required that precision be within 15 % (RSD) except at the LQ, which can assume a value ±20 %.
Conclusions
The combination of QuEChERS with DLLME is a simple, quick and inexpensive way to obtain low detection limits for 13 pesticides from different chemical classes analysed in tomato samples. After isolation of the target pesticides from samples with liquid partitioning with acetonitrile and salts and cleanup by d-SPE, the analytes were concentrated in trichloromethane by the DLLME procedure. The disperser solvent from DLLME was used at the same time as carrier of analytes from extraction in QuEChERS method. The novel sample preparation procedure combined with HPLC-DAD method provided appropriate accuracy and repeatability. The quantification limits achieved were below the maximum residue levels established in EU regulation.
References
Al-Rahman SHA, Almaz MM, Ahmed NS (2012) Food Anal Methods 3(5):564
Anastassiades M, Lehotay SJ, Štajnbaher D, Schenck FJ (2003) J AOAC Int 2(86):412
Asensio-Ramos M, Ravelo-Pérez LM, González-Curbelo MÁ, Hernández-Borges J (2011) J Chromatogr A 1218(42):7415
Caldas SS, Costa FP, Primel EG (2010) Anal Chim Acta 665(1):55
Chou TY, Lin SL, Fuh MR (2009) Talanta 2(80):493
Directorate General Health and Consumer Protection, SANCO/10684/2009 (2009). http://www.crlpesticides.eu/library/docs/allcrl/AqcGuidance_Sanco_2009_10684.pdf. Accessed 10 May 2012
Cunha SC, Fernandes JO (2011) J Chromatogr A 1218(43):7748
Dadfarnia S, Shabani AMH (2010) Anal Chim Acta 658(2):107
European Commission (2005) Commission Regulation (EC) 396/2005. Off J Eur Comm L70:1
Fu L, Liu X, Hu J, Zhao X, Wang H, Wang X (2009) Anal Chim Acta 632(2):289
Herrera-Herrera AV, Asensio-Ramos M, Hernández-Borges J, Rodríguez-Delgado MA (2010) Trend Anal Chem 297:728
Khodadoust S, Hadjmohammadi M (2011) Anal Chim Acta 699(1):113
Kmellár B, Abrankó L, Fodor P, Lehotay SJ (2010) Food Addit Contam Part A Chem Anal Control Expo Risk Assess 10(27):1415
Lehotay SJ, Maštovska K, Yun SJ (2005) J AOAC Int 2(88):630
Lehotay SJ, Son KA, Kwon H et al (2010) J Chromatogr A 16(1217):2548
Malik AK, Blasco C, Picó Y (2010) J Chromatogr A 1217(25):4018
Mansilha C, Melo A, Rebelo H et al (2010) J Chromatogr A 43(1217):6681
Miller JN, Miller JC (2005) Statistics and chemometrics for analytical chemistry, 5th edn. Pearson Education Limited, England
Perrin DD, Dempsey B (1974) Buffers for pH and metal ion control. Chapman & Hall, London
Ravelo-Pérez LM, Hernández-Borges J, Asensio-Ramos M, Rodríguez-Delgado MA (2009) J Chromatogr A 1216(43):7336
Rezaee M, Assadi Y, Milani Hosseini MR, Aghaee E, Ahmadi F, Berijani S (2006) J Chromatogr A 1116(1–2):1
Rezaee M, Yamini Y, Faraji M (2010) J Chromatogr A 1217(16):2342
Sarafraz-Yazdi A, Amiri A (2010) Trend Anal Chem 29(1):1
Sharma D, Nagpal A, Pakade YB, Katnoria JK (2010) Talanta 82(4):1077
Štajnbaher D, Zupančič-Kralj L (2003) J Chromatogr A 1015(1–2):185
Zar JH (2010) Biostatistical analysis, 5th edn. Pearson Education, New Jersey
Acknowledgments
One of the authors (A. Melo) wishes to thank Fundação Ciência Tecnologia the grant SFRH/BD/41764/2007. This work has been supported by Fundação para a Ciência e a Tecnologia through Project PTDC/AGR-ALI/101583/2008 and PEst-C/EQB/LA0006/2011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melo, A., Mansilha, C., Pinho, O. et al. Analysis of Pesticides in Tomato Combining QuEChERS and Dispersive Liquid–Liquid Microextraction Followed by High-Performance Liquid Chromatography. Food Anal. Methods 6, 559–568 (2013). https://doi.org/10.1007/s12161-012-9469-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-012-9469-4