Abstract
Carotenoids, among which lycopene—the principal pigment found in tomatoes—are lipophilic compounds which play a very important role in human health and nutrition. They are also recognised as strong antioxidants due to their ability to trap singlet oxygen and eliminate the peroxyl radical. The availability of reliable information on lycopene content of foods is essential both for the evaluation of diet and for epidemiological research relating the intake of lycopene. This paper describes a simple and fast HPLC/UV method for lycopene determination in a wide range of food products. All-E-lycopene together with its Z isomers were eluted isocratically using a carotenoid C30 reversed-phase column. The in-house validated HPLC method had a limit of quantification of 60 ng lycopene/g product and high precision and accuracy. The analytical method was successfully applied to several food products such as raw vegetables and fruits and also processed foods. Tomato and tomato-containing products contained the highest amounts of lycopene. While raw foods and minimally processed foods contained above 94% of all-E-lycopene, processed foods (such as soups, pasta sauces, pizza and cheese) contained from 76% to 87% of all-E-lycopene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous epidemiological studies have shown an inverse association between fruit and vegetable consumption and chronic diseases, including different types of cancer and cardiovascular diseases (Yahia 2010). Therefore, interest in the health benefits of fruit and vegetable consumption is increasing, and the interest in understanding the type, concentration and mode of action of the different components in fruits and vegetables that confer health benefits is also increasing. Nowadays, there are epidemiological data indicating that the consumption of tomatoes and tomato products is strongly correlated with several health benefits, mostly due to the presence of carotenoids and, in particular, lycopene. The biological effects of lycopene in humans have been attributed to mechanisms other than vitamin A including antioxidant effects and immune response modulators (Roldan-Gutierrez and Luque de Castro 2007). Therefore, consumption of lycopene has been associated with a number of nutritional and health benefits such as decreased risk of chronic diseases, especially cancer and cardiovascular diseases and the antioxidant properties of lycopene are thought to be primarily responsible for its beneficial effects (Agarwal and Rao 2000; Ellinger et al. 2006). Lycopene is one of the most potent antioxidants, with a singlet oxygen-quenching ability twice as high as that of β-carotene and ten times higher than that of α-tocopherol and, therefore, presumably the most effective in protecting against oxidative damage from free radicals (Di Mascio et al. 1989).
Lycopene is a an acyclic isomer of β-carotene containing linearly arranged 11 conjugated and 2 unconjugated double bonds which cannot form vitamin A (Agarwal and Rao 2000). The unique chemical properties of lycopene derive from its structure makes it extremely hydrophobic and soluble in tissues, milk and organic solvents (Roldan-Gutierrez and Luque de Castro 2007). Lycopene from natural plant sources exists predominantly in an all-E configuration, the most thermodynamically stable form with 11 double carbon bonds (Clinton 1998). Of these 11 double bonds, 7 can be isomerised from the all-E-form to the mono- or poly-Z forms under the influence of excess heat, light or certain chemical reactions typically occurring during food processing. Therefore, it is important to gain more data about the content of lycopene and its Z isomers in foods.
Many methods have been reported for lycopene analysis (HPLC (Ishida and Chapman 2004; Lee and Chen 2002; Xu et al. 2006), spectrophotometric (Davis et al. 2003a, b), high-speed countercurrent chromatography (Baldermann et al. 2008), fibre optic visible reflectance spectroscopy (Choudhary et al. 2009), infrared spectroscopy (De Nardo et al. 2009)). However, they consume too much time (usually >20 min) and solvent in order to achieve separation of the all-E-lycopene with its Z isomers (Böhm 2001; Emenhiser et al. 1996; Huang et al. 2010; Ishida et al. 2001; Lee and Chen 2001). Additionally, lycopene can be used as food colour (European Parliament and Council 1994) and reliable analytical methods are needed in order to verify whether the use of lycopene in foods complies with the adopted legislation. Therefore, an improved simple, fast and validated method was necessary in order to separate and quantify the lycopene and its Z isomers.
In this paper, we describe the development and validation of an analytical methodology based on the HPLC–UV for the determination of lycopene and its isomers in fruits and vegetables but also complex food matrixes such as sauces, soups, pasta and pizza.
Materials and Methods
Materials
Lycopene standard (90% all-E-lycopene) and the β-carotene were purchased from Sigma Aldrich (Bornem, Belgium). Methanol, isopropyl alcohol, tetrahydrofuran (THF) containing 250 ppm butylated hydroxytoluene (BHT), acetone, hexane and ethanol 96% were purchased from Biosolve (Valkenswaard, The Netherlands). Triethylamine (TEA) and the sodium chloride were purchased from BDH Prolabo (VWR International, Leuven, Belgium). Ammonium acetate and potassium hydroxide were purchased from Merck (Overijse, Belgium). Food samples were purchased in local stores and finely ground and homogenised before analysis.
Lycopene Extraction Without Saponification
All steps of the sample preparations were performed in subdued light. Food samples (1 ± 0.05 g) were mixed for 1 min with 20 mL of extraction buffer (hexane/acetone/ethanol 2:1:1) using an Ultra-Turrax (15,000 rot/min) on ice. The obtained mixture was filtered and transferred into a separatory funnel. An aliquot of 50 mL of saturated NaCl solution was added and mixed well for 1 min. Once the phases were well-separated, the aqueous phase was decanted and the hexane phase was recovered and filtered over anhydrous NaSO4 (5 g) which was rinsed two times with 2.5 mL of extraction buffer. The filtrate was further dried under nitrogen and the obtained residue was redissolved in THF with BHP and 0.05% TEA to appropriate concentrations before being injected.
Lycopene Extraction with Saponification
Food samples containing more than 1% of lipids were saponified before analysis according to the method described by Lee and Castle (2001) with slight modifications. Briefly, 1 g (±0.05) of sample was mixed for 1 min with 20 mL of extraction buffer (hexane/acetone/ethanol 2:1:1) using an Ultra-Turrax (15,000 rot/min) on ice. The obtained mixture was filtered and transferred into a separatory funnel containing 50 mL of saturated NaCl solution and mixed well. The aqueous phase was removed and 10 mL of 10% methanolic KOH solution was added to the organic phase. The sample was stored in the dark for 2 h with subsequent shaking each 20 min. After incubation, the sample was washed three times with 20 mL of ammonium acetate solution (50 mM) in order to neutralise the samples. The obtained organic phase was further dried and redissolved in THF as mentioned previously.
HPLC/UV Analysis of the Lycopene
Lycopene was analysed using reversed-phase high-performance liquid chromatography using isocratic elution and UV detection at 472 nm (Waters, Zellik, Belgium). A carotenoid C30 reversed-phase column (250 × 4.6 id, 3 μm) from YMC corporation (Waters, Zellik, Belgium) was used with MeOH/isopropyl alcohol/THF (30:30:35) containing 250 ppm BHT and 0.05% TEA as mobile phase. The flow rate was 1 mL/min, column temperature was 35 °C and the injection volume 20 μL.
Preparation of the Standard Solutions
The lycopene standard (1 mg vials) was quantitatively transferred to a 25-mL volumetric flask with THF containing 250 ppm BHT and 0.05% TEA. The obtained solution was stored at −80 °C, and before use, the concentration of the lycopene was spectrophotometrically determined after dilution in hexane up to solutions giving an absorbance value of below 0.7 units. The concentration of the stock lycopene solution was calculated using the A1% of 3,450 in hexane at 472 nm (Craft 2001). Furthermore, calibration curves were prepared by diluting stock lycopene with methanol. All-E-lycopene was identified by comparing the retention time with the reference standard and the quantification was done by external standard calibration based on peak area. The β-carotene (1.25 μg/mL) was added to the lycopene standard and used as a control for the analytical process. Total lycopene was quantified by summing the peak area of all-E-lycopene and the Z isomers and based on the standard curve of all-E-lycopene (Lee and Chen 2001).
Analytical Method Validation
Linearity was determined between 0 and 8.27 μg/mL using eight levels of calibration in triplicate. The Mandel’s fitting test was used to evaluate the linearity of the straight line regression model (Van Loco et al. 2002).
Limit of detection (LOD) and limit of quantification (LOQ) were determined according to the method described by the International Conference on Harmonization (Dias et al. 2008; International Conference on Harmonization 2005; Lee and Chen 2001). For this, standards of 36.5 to 547.5 ng lycopene/mL were prepared and injected three times each. The mean of the slopes (S) and standard deviation of the intercepts (σ) were calculated from the three calibration curves obtained. The LOD and the LOQ were calculated according to the formulas below: (International Conference on Harmonization 2005)
For the assessment of the matrix effect, calibration curves (0 to 4.96 μg lycopene/mL) were prepared in the methanol or in the extract of dried apricots (prepared without saponification) and cheese spread (prepared with prior saponification) and analysed in triplicates. The matrix effect was evaluated based on the slopes of the obtained calibration curves in the methanol or dried apricot/cheese spread extract.
The precision, as a measure of the repeatability and the within-lab reproducibility of the whole analytical method (including sample preparation and analysis) was assessed by analysing a sample with and without saponification on at least two different days in triplicate and expressed as the relative standard deviation.
The accuracy of the method was determined by recovery test using the addition of known amounts of lycopene to apricot when samples without saponification were analysed and to spreadable cheese (containing 24.5% lipids) when samples with saponification were analysed.
Results and Discussion
Like other carotenoids, lycopene may occur in various geometrical isomers. In raw foods the all-E-isomer is the most predominant with lower amounts of other Z isomers (Schierle et al. 1997). Food products are subjected to a large variety of processing conditions such as cooking in aqueous media, microwave cooking, extrusion, blanching, boiling, steaming or dry heat (oven roasting, oil roasting, infrared heating). During such processing steps, environmental factors such as air, light and temperature may be very important for the isomerization and autoxidation of lycopene in foods which can further have an impact on the lycopene bioavailability as well (Chen et al. 2009).
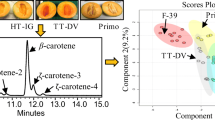
Nowadays, the C30 columns are frequently used for the separation of the carotenoid isomers in food and biological samples. However, the acyclic carotenoid lycopene is much more strongly retained on the C30 stationary phases compared to other bicyclic carotenoids such as α- and β-carotene (Emenhiser et al. 1996). In this study, an isocratic HPLC method was developed which allowed separation of all-E-lycopene and several of its Z isomers on a C30 column in less than 15 min (Fig. 1). The β-carotene eluted with a retention time of about 4.2 min and the lycopene of about 11 min. The standard purchased from Sigma contained about 90% of all-E-lycopene. Therefore, upon injection of higher concentrations of lycopene, four additional peaks could be observed in the retention time range between 5.5 and 8 min. These were named CX1 up to CX4 since it was difficult to assign what type of Z isomer they were without proper standards available. Nevertheless, according to literature data, these peaks seem to represent the 13-Z-, 15-Z-, 5-Z-; 9-Z- and the 9-Z-lycopene isomers respectively (Fröhlich et al. 2007).
Prior to analysis, the lycopene was extracted from foods either with or without a saponification step involved. The extraction procedure without saponification could be only employed for samples containing low amounts of lipids (<1%). However, sauces and ready to eat foods contain higher amounts of lipids. Therefore, these samples had to be saponified prior to the analysis in order to remove the lipids and other interfering substances. However, the impact of the saponification on the lycopene content had to be tested first. As can be seen from Fig. 2, no significant difference could be seen in the amount of lycopene detected by either using the extraction method with or without saponification.
The newly developed method was further validated by means of determination of the linearity, matrix effect, LOD and LOQ, precision and accuracy. A typical calibration curve obtained for lycopene is illustrated in Fig. 3. The method linearity was tested by analysing several standard solutions and by determining the Mandel’s test values. The Mandel’s test value obtained was 2.50 which was below the tabulated F value of the 99% confidence level (F 99 = 13.75) indicating that a linear model was preferred.
Matrix effect was evaluated to determine whether there is a concentration-dependent systematic error due the presence of the matrix. This was performed by comparing the slope of the standard calibration with slopes of the addition curves by means of a statistical t test. The calculated t is compared with the tabulated t at the 95% confidence level. The standard calibration was performed in methanol and addition curves performed in dried apricot and cheese spread extracts. The t test values obtained for dried apricot and cheese spread were 0.47 and 1.14. These values were below the tabulated test value (representing 2.01) indicating that no interference on quantification based on the calibration curves is likely to take place.
Furthermore, the LOD and the LOQ values of the method were determined. According to this method, an LOD value of 10 ng/mL and an LOQ value of 30 ng/mL were obtained. This would correspond to an LOD of 20 ng lycopene/g of product and LOQ of 60 ng lycopene/g product considering that the extract of 1 g product was mainly redissolved in 2 mL of THF.
Repeatability standard deviation and within-laboratory reproducibility standard deviation were calculated according to ISO 5725-2 (1994) together with the coefficients of variation. This was done by performing spiking on the dried apricot or cheese spread by performing several replicate analyses. The coefficient of variation for repeatability was of 4.96% and 5.67% and for the within-laboratory reproducibility of 11.45% and 12.84% for the samples analysed without and with saponification, respectively. Moreover, these calculated coefficients did not exceed the predicted values as determined by the Horwitz equation for the coefficient of variation for repeatability and within-laboratory reproducibility (14.7% and 22.0%, respectively) (Horwitz and Albert 2006). Because no certified reference material was found available for lycopene, the recovery of the lycopene was determined by spiking dried apricot and cheese spread. Dried apricot was spiked at four different levels and the recovery varied from 76.28% to 114.14% (Table 1). Additionally, the recovery from dried apricot and cheese spread was determined daily by spiking with a fixed volume of stock lycopene solution (50 μL). The recovery represented on average 77% which was within the limits of acceptance set in the commission decision 2002/657 (European Commission Decision 2002/657/EC 2002).
Once the analytical method was validated, it was further used for monitoring the lycopene content in different food commodities. Fresh fruits and vegetables (tomatoes, papaya, water melon, grapefruit and fruit salad) were analysed together with processed foods (soups, sauces, pasta and pizza). Table 2 shows the mean, maximum and minimum values of total and all-E-lycopene contents in the analysed samples. The most remarkable observation was the variability in the lycopene content between different samples. In the case of tomatoes, total lycopene content ranged from 33.3 to 103.7 μg/g. Similarly, variability was observed for watermelon and papaya. Such variability in the lycopene content is not unusual and has been previously reported as well (Gayosso-Garcia et al. 2010; Olives Barba et al. 2006; Perkins-Veazie et al. 2006). Moreover, in these fresh fruits and vegetables, the ratio of the all-E-lycopene constituted from about 94% to 99%.
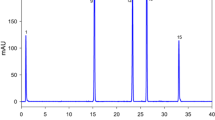
Considering that tomatoes are the most important source of lycopene, it was evident that the tomato products such as tomato juice, tomato concentrates and double concentrates, canned tomatoes, sun-dried tomatoes and ketchup were rich in lycopene as well. Similar as for the fresh tomatoes, the ratio of the all-E-lycopene constituted about 94% in these samples. On the contrary, for the severely processed foods such as soups, pizzas, pasta with tomato sauces and cheeses, the all-E-lycopene ratio represented from 76% to 87%. This was moreover evident from the chromatograms of such samples (Fig. 4) where an additional peak eluting after the all-E-lycopene could be observed which could potentially represent the 5Z-lycopene as previously shown (Ishida and Chapman 2004; Fröhlich et al. 2007). However, besides these products, tomato powder or tomato extract can be sometimes added to snacks and chips as coating or candies. In these products, the lycopene content is rather low (±1 μg/g product) mostly represented by the all-E-lycopene.
Conclusions
In conclusion, this paper describes a fast a reliable method for lycopene determination in a broad spectrum of food samples. A wide variation in the lycopene content in the analysed foods was seen with fresh and slightly processed foods containing primarily all-E-lycopene while highly processed foods contain 76–87% all-E-lycopene. This method can be further used for the fast screening of food products rich in lycopene and the data presented can be used by nutritionist and epidemiologists to help evaluate the lycopene intake by the population.
References
Agarwal S, Rao AV (2000) Tomato lycopene and its role in human health and chronic diseases. CMAJ 163:739–744
Baldermann S, Ropeter K, Kohler N, Fleischmann P (2008) Isolation of all-trans lycopene by high-speed counter-current chromatography using a temperature-controlled solvent system. J Chromatogr A 1192:191–193
Böhm V (2001) Use of column temperature to optimize carotenoidisomer separation by C30 high performance liquidchromatography. J Sep Sci 24:955–959
Chen J, Shi J, Xue SJ, Ma Y (2009) Comparison of lycopene stability in water- and oil-based food model systems under thermal- and light-irradiation treatments. LWT- Food Sci Technol 42:740–747
Choudhary R, Bowser TJ, Weckler P, Maness NO, McGlynn W (2009) Rapid estimation of lycopene concentration in watermelon andtomato puree by fiber optic visible reflectance spectroscopy. Postharvest Biol Tec 52:103–109
Clinton SK (1998) Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev 56:35–51
European Parliament and Council (1994) European Parliament and council directive 94/36/EC of 30 June 1994 on colours for use in foodstuffs. Official Journal of the European Union
Craft NE (2001) Chromatographic techniques for carotenoid separation. In: current protocols in food analytical chemistry. John Wiley & Sons
Davis AR, Fish WW, Perkins-Veazie P (2003a) A rapid hexane-free method for analyzing lycopene content in watermelon. J Food Sci 68:328–332
Davis AR, Fish WW, Perkins-Veazie P (2003b) A rapid spectrophotometric method for analysing lycopene in tomato and tomato products. Postharvest Biol Tec 28:425–430
De Nardo T, Shiroma-Kian C, Halim Y, Francis D, Rodriguez-Saona LE (2009) Rapid and simultaneous determination of lycopene and b-carotene contents in tomato juice by infrared spectroscopy. J Agric Food Chem 57:1105–1112
Di Mascio P, Kaiser S, Sies H (1989) Lycopene as the most efficient biological carotenoid singlet oxygen quencer. Arch Biochem Biophys 274:532–538
Dias MG, Camoes MFGF, Oliveira L (2008) Uncertainty estimation and in-house method validation of HPLC analysis of carotenoids for food composition data production. Food Chem 109:815–824
Ellinger S, Ellinger J, Stehle P (2006) Tomatoes, tomato products and lycopene in the prevention and treatment of prostate cancer: do we have the evidence from intervention studies? Curr Opin Clin Nutr Metab Care 9:722–727
Emenhiser C, Simunovic N, Sandler LC, Schwartz S (1996) Separation of geometrical carotenoid isomers in biological extracts using a polymeric C30 column in reversed-phase liquid chromatography. J Agric Food Chem 44:3887–3893
European Commission Decision 2002/657/EC (2002) Concerning the performance of analytical methods and the interpretation of results.
Fröhlich K, Conrad J, Schimidt A, Breithoupt DE, Böhm V (2007) Isolation and structural elucidation of different geometrical isomers of lycopene. Int J Vitam Nutr Res 77(6):369–375
Gayosso-Garcia SLE, Yahia EM, Gonzalez-Aguilar GA (2010) Identification and quantification of phenols, carotenoids, and vitamin C from papaya (Carica papaya L., cv. Maradol) fruit determined by HPLC–DAD–MS/MS–ESI. Food Res Int 44(5):1284–1291
International Conference on Harmonization (2005) Validation of analytical procedures: text and methodology Q2(R1).
Horwitz W, Albert R (2006) The Horwitz ratio (HorRat): a useful index of method performance with respect to precision. J AOAC Int 89(4):1095–1109
Huang XY, Liu YW, Di DL, Liu JX, Li C (2010) An improved LC-DAD method for simultaneous determination of lutein, β-carotene and lycopene in tomato and its products. Chromatographia 71:331–334
Ishida BK, Chapman MH (2004) A comparison of carotenoid content and total antioxidant activity in catsup from several commercial sources in the United States. J Agric Food Chem 52:8017–8020
Ishida BK, Ma J, Chan B (2001) A simple, rapid method for HPLC analysis of lycopene isomers. Phytochem Anal 12:194–198
ISO 5725-2 (1994) Accuracy (trueness and precision) of measurement methods andresults—basic method for the determination of repeatability and reproducibilityof a standard measurement method
Lee SH, Castle WS (2001) Seasonal changes of carotenoid pigments and color in Hamlin, Earlygold and Budd Blood Orange Juices. J Agric Food Chem 49:877–882
Lee MT, Chen B (2001) Separation of lycopene and its cis isomers by liquid chromatography. Chromatographia 54:613–617
Lee MT, Chen BH (2002) Stability of lycopene during heating and illumination in a model system. Food Chem 78:425–432
Olives Barba AI, Címara Hurtado M, Sanchez Mata MC, Fernandez Ruiz V, Lopez Saenz De Tejada M (2006) Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and b-carotene in vegetables. Food Chem 95:328–336
Perkins-Veazie P, Collins JK, Davis AR, Roberts W (2006) Carotenoid content of 50 watermelon cultivars. J Agric Food Chem 54:2593–2597
Roldan-Gutierrez JM, Luque de Castro MD (2007) Lycopene: the need for better methods for characterization and determination. TrAC—Trends in Analytical Chemistry 26:163–170
Schierle J, Bretzel W, Buhler I, Faccin N, Hess D, Steiner K et al (1997) Content and isomeric ratio of lycopene in food and human blood plasma. Food Chem 59:459–465
Van Loco J, Elskens M, Croux C, Beernaert H (2002) Linearity of calibration curves:use and misuse of the correlation coefficient. Accred Qual Assur 7:281–285
Xu F, Yuan QP, Dong HR (2006) Determination of lycopene and b-carotene by high-performance liquid chromatography using sudan I as internal standard. J Chromatogr B Analyt Technol Biomed Life Sci 838:44–49
Yahia EM (2010) The contribution of fruit and vegetable consumption to human health. In: de la Rosa LA, Alvarez-Parrilla, E, Gonzalez-Agu GA (eds), Fruit and vegetable phytochemicals
Acknowledgements
Financial support by the Federal Public Service of Health, Food Chain Safety and Environment is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cucu, T., Huvaere, K., Van Den Bergh, MA. et al. A Simple and Fast HPLC Method to Determine Lycopene in Foods. Food Anal. Methods 5, 1221–1228 (2012). https://doi.org/10.1007/s12161-011-9354-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-011-9354-6