Abstract
An ion-exclusion liquid chromatography with mobile phase 0.005 mol L−1 H2SO4 and step flow rate gradient (0.2 mL min−1 in the first 40 min and 0.5 mL min−1 from 41 to 60 min) was used to determine 20 organic acids simultaneously at 17 °C within 51 min. The peak resolutions (Rs) were 0.45∼3.02 and separation factors (α) were all higher than 1. Impurities in fruit vinegar executed with direct injection or C18 cartridge clean-up for analysis would influence the glutaric and oxalic acid measurement; however, SAX cartridge extraction could reduce the interferences (organic acid recoveries were 93.93∼99.98%). Acetic, ascorbic, citric, malic, and malonic acids were the major organic acids in fruit vinegars (apple, apple sparkling, plum, cranberry, and grape).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vinegars are usually used as seasonings and preservatives in food preparation, and also watered done as drink (Tesfaye et al. 2002). Reports indicated that vinegars have antioxidant (Dávalos et al. 2005) and anti-microbial (foodbrone pathogens) (Karapinar and Gönül 1992; Medina et al. 2007) effects. They can also prevent inflammation and hypertension (Murooka and Yamshita 2008), lower serum cholesterol and triacylglycerol (Fushimi et al. 2006), decrease the glycemic index of carbohydrate food for people with and without diabetes (Sugiyama et al. 2003; Johnston et al. 2004), reduce food intake for diet control (Östman et al. 2005) and so on. Due to health benefits, there are many kinds of vinegar products exploited in Taiwan recently. The fruit vinegar is the most popular one.
Organic acids are an important group in fruit vinegars. They may play a protective role against diseases as a result of their antioxidant activities (Silva et al. 2004; Valentão et al. 2005). Ascorbic acid is a widely distributed water-soluble antioxidant in plants. Oxalic acid, the simplest dicarboxylic acid, has remarkable chelating capacity for multivalent cations; furthermore, carboxylic acids, e.g., citric, malic, succinic, and tartaric acid, have a capacity to chelate metals as well (Oliveira et al. 2008). The content of organic acids is also an important quality control index for edible vinegar productions in Taiwan. However, there are rare reports concerning content and component of organic acids in fruit vinegars. High-performance liquid chromatography (HPLC), gas chromatography (GC), and capillary electrophoresis (CE) are the major methods for organic acids determination (Klampfl et al. 1998; Yang and Choong 2001; Suárez-Luque et al. 2002a; Chinnici et al. 2005; Mato et al. 2005). In spite of GC method having excellent separation and sensitivity, it often needs time-consuming derivatization steps and high operation temperature, which may cause artifacts and sample decomposition (Chinnici et al. 2005). CE separation has good resolution, short analysis times, low consumption of reagents and samples, and simple sample preparation; however, poorer reproducibility and precision limit its quantitative application (Mato et al. 2005; Mato et al. 2007). Though HPLC analysis has lower resolution compared with the two methods as described above, it is popularly used due to simplicity, especially ion-exclusion liquid chromatography (Chinnici et al. 2005; Saraduldhat and Paull 2007; Ribeiro et al. 2007).
Organic acids in samples could be purified with C18 or strong anion exchange (SAX) cartridges for analysis or determined directly after filtration (Suárez-Luque et al. 2002a, b; Chinnici et al. 2005).
In the study, we established an ion-exclusion liquid chromatography with step flow rate gradient to determine 20 organic acids in fruit vinegars simultaneously. Three sample preparation methods were also compared: direct injection, C18 cartridge clean-up, and SAX cartridge clean-up. Besides, composition and content of organic acids in five kinds of fruit vinegars (apple, apple sparkling, plum, cranberry, and grape) were also measured.
Materials and Methods
Chemicals and Standards
Organic acid standards: glutaric, oxalic, citric, α-ketoglutaric, tartaric, pyruvic, malic, ascorbic, quinic, malonic, trans-aconitic, lactic, shikimic, succinic, formic, acetic, fumaric, propionic, pyroglutamic and gluconic acid were purchased from Sigma Co. (St. Louis, MO, USA). Their structures are showed in Fig. 1. Methanol (MeOH), hydrochloric acid (HCl) and sulfuric acid (H2SO4) and sodium hydroxide (NaOH) were obtained from Merck Co. (Darmstadt, Germany). Deionized distilled water (dd H2O) was prepared by Ultrapure™ water purification system (Lotun Co., Ltd. Taipei, Taiwan). Bond-Elute SAX (functional group: quaternary ammonium cation) and C18 cartridges (500 mg) were purchased from Varian Co. (Harbor City, CA, USA).
Sample Preparation
Apple, apple sparkling, plum, condensed cranberry and condensed grape vinegars were provided by Pai Chia Chen Brewery & Foods Co., Ltd., Chiayi County, Taiwan. Sample preparation methods were based on those reported by Chinnici et al. (2005) and Oliveira et al. (2008). One milliliter of condensed vinegars (cranberry and grape) was diluted with 4 mL of dd H2O in advance. For direct injection: 1 mL of each sample was diluted with 9 mL of 0.055 mol L−1 H2SO4 and then filtrated through a 0.22 μm Teflon membrane filter (Millipore Co., Bedford, MA, USA). For C18 cartridge clean-up: 1 mL of each sample was passed through a C18 cartridge (previously conditioned with 3 mL of MeOH and 3 mL of acid water (pH 2 with HCl)) and then diluted with 9 mL of 0.055 mol L−1 H2SO4 (Suárez-Luque et al. 2002a). For SAX cartridge clean-up: 1 mL of each sample was adjusted to pH 9–10 with 1 mol L−1 NaOH, and then loaded into a SAX cartridge conditioned previously with 3 mL of MeOH and 3 mL of dd H2O. The cartridge was washed with 3 mL of dd H2O, eluted with 0.5 mL of 0.5 mol L−1 H2SO4 twice, and then the eluate was diluted with 9 mL of dd H2O (Chinnici et al. 2005).
HPLC Analysis of Organic Acids in Fruit Vinegars
Organic acids were determined by a HPLC system consisted of a PrimeLine™ Gradient Model 500 G HPLC pump system (Analytical Scientific Instruments, Inc., El Sobrante, CA, USA), a S-3210 photodiode-array detector (PDA) (Schambeck SFD GmbH, Bad Honnef, Germany) and an injection valve with a 20 μL loop (Rheodyne Inc., Cotati, CA). The analytical condition was improved from that reported by Ribeiro et al. (2007). The stationary phase was a Rezex ROA organic acid column (300 × 7.8 mm, 8 μm) (Phenomenex, Torrance, CA, USA), which was kept at 17 °C using a Colbox column oven (Hipoint Scientific Co., Kaohsiung, Taiwan). The column resin is polymerized with styrene and divinylbenzene, and 8% of the benzene rings are sulfonated. The mobile phase was an isocratic solvent system (0.01 N H2SO4) with step flow rate gradient: 0.2 mL min−1 in the first 40 min and 0.5 mL min−1 from 41 to 60 min. Detection was at 210 nm. HPLC separation efficiency was evaluated through the separation factor (α) and resolution (Rs). The limits of detection (LODs) and quantification (LOQs) for organic acids were measured by the signal-to-noise ratio (S/N) of 3 and 10, respectively. The reproducibility for each organic acid was determined, and the standard deviation was calculated through six measurements by run-to-run and day-to-day.
Preparation of Standard Curves
Five concentrations of organic acids were injected into HPLC (20 μL), and the linear regression equation for each standard curve was established by plotting the quantity of standard compound injected against the peak area. The regression equation and the correlation coefficients (r 2) were calculated with Chem-Win computer software system (Shuen-Hua, Taipei, Taiwan).
Determination of Recovery
The recoveries were measured by adding a mixture of organic acid standards (each weighing 0.1, 0.25, and 0.5 mg) to 5 mL of fruit vinegars followed by extraction and analysis of organic acids as described above. The recovery of each organic acid was obtained from the analytical result and the original amount of organic acid standard added as the following formula: Recovery (%) = (analytical result − original amount of organic acid in vinegar)/original amount of organic acid standard added.
Statistical Analysis
All determinations were performed in triplicate and the mean values were calculated. The data subjected to analysis of variance and Duncan’s multiple range tests were taken to resolve significance between means, at a level of p < 0.05.
Results and Discussion
HPLC Separation of Organic Acids
Ion-exclusion liquid chromatography is a practical method for organic acid analysis (Soyer et al. 2003; Chinnici et al. 2005; Oliveira et al. 2008). The characteristic feature of the chromatography technique for organic acid separation is that dissociated acidic compounds (negatively charged ions) are separated on cation exchanged resins with anionic functional groups (usually sulfonic acid groups). Ion exclusion is the primary mechanism of ion retention, which depends on the ratio of the concentrations of ionized to neutral forms of the analyzed compounds (Glód 1997). Besides pKa value (Fig. 1), length of aliphatic chain, structure, molecular size, electrostatic interaction, and operating temperature would influence the sample elution (Glód 1997).
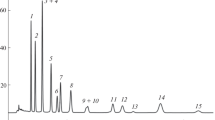
However, the conditions of ion-exclusion liquid chromatography presenting in reports were generally used to separate fewer organic acids and some compounds did not show good resolutions (even overlap). Chinnici et al. (2005) used 0.00167 mol L−1 phosphoric acid (0.4 mL min−1, at 25 °C) to analyze nine organic acids (oxalic, citric, malic, quinic, galacturonic, ascorbic, succinic, and fumaric) within 20 min. Oliveira et al. (2008) and Ribeiro et al. (2007) separated 6 organic acids (oxalic, citric, malic, quinic, shikimic, and fumaric acid) within 55 min through 0.005 mol L−1 H2SO4 (0.2 mL min−1, at 30 °C). Soyer et al. (2003) determined 5 organic acids (citric, tartaric, malic, succinic, and shikimic acid) within 13 min by 0.005 mol L−1 H2SO4 (0.6 mL min−1, at room temperature). In our work, 0.005 mol L−1 H2SO4 was adopted as mobile phase performed with step flow rate gradient (0.2 mL min−1 in the first 40 min and 0.5 mL min−1 from 41 to 60 min) to analyze 20 organic acids simultaneously at 17 °C. All organic acids could be determined within 51 min (Fig. 2 and Table 1). The condition for the organic acid separation exhibited good reproducibility, RSD < 1.93% for retention times and RSD < 4.52% for integrated areas (Table 2). The separation factors (α) for all peaks were higher than 1 (Table 1). Rs for peaks 3 (citric acid) and 4 (α-ketoglutaric acid), 7 (malic acid) and 8 (ascorbic acid), 8 and 9 (quinic acid), 9 and 10 (malonic acid), and 13 (shikimic acid) and 14 (succinic acid) were in the range of 0.45–0.71, whereas others were higher than 0.8 (Table 1). The LODs and LOQs for these organic acids at 210 nm were 0.1 (aconitic acid) ∼5.8 (propionic acid) mg L−1 and 0.4 (aconitic acid) ∼19.5 (propionic acid) mg L−1, respectively (Table 1). Chinnici et al. (2005) found that LODs (S/N = 3) for nine organic acids at 210 nm were 0.5 (fumaric acid) ∼7.3 mg L−1 (quinic acid). Their results were higher than ours (0.3 mg L−1 for fumaric acid and 4.1 mg L−1 for quinic acid). The established condition was used to determine the organic acids in fruit vinegars.
HPLC chromatograms of organic acids added to plum vinegars through various preparation procedures: a direct injection, b C18 cartridge clean-up, and c SAX cartridge clean-up. HPLC conditions: column, Rezex ROA (300 × 7.8 mm, 8 μm); column temperature, 17 °C; mobile phase, 0.005 mol L−1 H2SO4 = 0.2 mL min−1 in the first 40 min and 0.5 mL min−1 from 41 to 60 min; detection, UV 210 nm
Comparison of Sample Preparation
Suitable preparation method is important for sample analysis, which could obtain the accurate analytical results. For organic acid analysis, direct determination after sample filtration is a simple preparation method; however, more impurities might interfere with the analytical results. Sample passing through C18 cartridge for purification could remove some compounds as the anthocyanins. Though sample purified with SAX cartridge needs more steps, it could remove the neutral and positively charged species (Suárez-Luque et al. 2002a, b; Chinnici et al. 2005; Mato et al. 2005). In the investigation, we compared the three preparation methods for determination of organic acids in fruit vinegar samples. Figure 2 shows that glutaric (peak 1) and oxalic acid (peak 2) were interfered seriously by impurities in the samples injected directly or cleaned up with C18 cartridge for analysis; however, samples purified through SAX cartridges could effectively eliminate the interference. Though direct injection and C18 cartridge clean-up could obtain higher recoveries for most organic acids in plum vinegar than SAX cartridge extraction (Table 3) (other samples also showed the similar results (data not show)), SAX cartridge was adopted to prepare samples before analysis in our work. Because plum vinegar contained more organic acids, we showed the representative data here. Mato et al. (2005) found that recoveries of organic acids added to grape juice and wine samples were 99.0∼104.3% and 92.7∼105.8%, respectively; these samples were done by water dilution and direct injection into HPLC for analysis. Suárez-Luque et al. (2002b) observed that recoveries of organic acids added to honey were 62.9∼99.4% after C18 cartridge clean-up. Analytical recoveries of organic acids added to brewed coffee and apple juice were 24∼112% (Rodrigues et al. 2007) and 94.2∼102.3% (Chinnici et al. 2005), respectively, through SAX cartridge extraction. Our results showed that recoveries of organic acids added to fruit vinegar samples were 95.04∼101.17% for direct injection (except glutaric and oxalic acid), 94.62~100.23 for C18 cartridge clean-up (except glutaric and oxalic acid), and 93.93∼99.98% for SAX cartridge clean-up, respectively (Table 3).
Organic Acid Content in Fruit Vinegars
The linear ranges of each organic acid used to establish standard curves were showed in Table 4. Their correlation coefficients (r 2) of linear regression equations were higher than 0.9986. If organic acid concentrations in fruit vinegars were higher, the samples should be diluted to enter into the linear ranges during quantitative process. Table 5 shows that the sequence for organic acid contents in fruit vinegars was in the order of cranberry (56.5 g L−1) > grape (41.0 g L−1) > plum (13.9 g L−1) > apple (9.7 g L−1) > apple sparkling (5.7 g L−1). Acetic, ascorbic, citric, malic, and malonic acids were the major organic acids in these vinegar samples, and the acetic acid level was the highest (Table 5). Other organic acids such as glutaric, oxalic, α-ketoglutaric, tartaric, pyruvic, quinic, lactic, shikimic, succinic, formic, fumaric, pyroglutamic, and gluconic could also be determined in these samples. Shahidi et al. (2008) indicated that acetic, citric, malic, lactic, succinic, tartaric, and fumaric acid could be found in fruit vinegars (including apple, grape, and so on) as our results; however, content of each organic acid was not showed in their report. Giumanini et al. (2001) found that apple vinegar contained lactic, succinic, malic, glutaric, tartaric, α-ketoglutaric and citric acid. Nevertheless, lactic acid could not be detected in our apple vinegar. Organic acids in fruit vinegars might source from the original materials and be generated during the fermentation process (Shahidi et al. 2008).
In the investigation, a simple ion-exclusion liquid chromatography using step flow rate gradient was developed, which could simultaneously determine 20 organic acids in one time injection. As compared with direct injection and C18 cartridge purification, SAX cartridge purification could reduce the interference of impurities for determination of organic acids in fruit vinegars. The study could provide more information for content and composition of organic acids in different fruit vinegars.
References
Chinnici F, Spinabelli U, Riponi C, Amati A (2005) Optimization of the determination of organic acids and sugars in fruit juices by ion-exclusion liquid chromatography. J Food Compos Anal 18:121–130
Dávalos A, Bartolomé B, Gómez-Cordovés C (2005) Antioxidant properties of commercial grape juices and vinegars. Food Chem 93:325–330
Fushimi T, Suruga K, Oshima Y, Fukiharu M, Tsukamoto Y, Goda T (2006) Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br J Nutr 95:916–924
Giumanini AG, Verardo G, Martina DD, Toniutti N (2001) Improved method for the analysis of organic acids and new derivatization of alcohols in complex natural aqueous matrixes: application to wine and apple vinegar. J Agric Food Chem 49:2875–2882
Glód BG (1997) Ion exclusion chromatography: parameters influencing retention. Neurochem Res 22:1237–1248
Johnston CS, Kim CM, Bullar AJ (2004) Vinegar improves insulin sensitivity to a high-carbohydrate meal in subjects with insulin resistance or type 2 diabetes. Diabetes Care 27:281–282
Karapinar M, Gönül ŞA (1992) Effects of sodium bicarbonate, vinegar, acetic and citric acids on groeth and survival of Yerinia entrocolitica. J Food Microbiol 16:343–347
Klampfl CW, Katzmayr MU, Buchberger W (1998) Separation of inorganic and organic anions by capillary zone electrophoresis with simultaneous indirect UV and conductivity detection. Electrophoresis 19:2459–2464
Mato I, Suárez-Luque S, Huidobro JF (2005) A review of the analytical methods to determine organic acids in grape juices and wines. Food Res Int 38:1175–1188
Mato I, Suárez-Luque S, Huidobro JF (2007) Simple determination of main organic acids in grape juice and wine by using capillary zone electrophoresis with direct UV detection. Food Chem 102:104–112
Medina E, Romero C, Brenes M, deCastro A (2007) Antimicrobial activity of olive oil, vinegar, and various beverages against foodbrone pathogens. J Food Prot 70:1194–1199
Murooka Y, Yamshita M (2008) Traditional healthful fermented products of Japan. J Industr Microbiol Biotech 35:791–798
Oliveira AP, Pereira JA, Andrade PB, Valentão P, Seabra RM, Silva BM (2008) Organic acids composition of Cydonia oblonga Miller leaf. Food Chem 111:393–399
Östman E, Granfeldt Y, Persson L, Bjorck I (2005) Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr 59:983–988
Ribeiro B, Rangel J, Valentão P et al (2007) Organic acids in two Portuguese chestnut (Castanea sativa Miller) varieties. Food Chem 100:504–508
Rodrigues CI, Marta L, Maia R, Miranda M, Ribeirinho M, Máguas C (2007) Application of solid-phase extraction to brewed coffee caffeine and organic acid determination by UV/HPLC. J Food Compos Anal 20:440–448
Saraduldhat P, Paull RE (2007) Pineapple organic acid metabolism and accumulation during fruit development. Pineapple organic acid metabolism and accumulation during fruit development. Sci Hortic 112:297–303
Shahidi F, McDonald J, Chandarasekara A, Zhong Y (2008) Phytochemicals of foods, beverages and fruit vinegars: chemistry and health effects. Asia Pac J Clin Nutr 17:380–382
Silva BM, Andrade PB, Valentão P, Ferreres F, Seabra RM, Ferreira MA (2004) Quince (Cydonia oblonga Miller) fruit (pulp, peel, and seed) and jam: Antioxidant activity. J Agric Food Chem 52:4705–4712
Soyer Y, Koca N, Karadeniz F (2003) Organic acid profile of Turkish white grapes and grape juices. J Food Compos Anal 16:629–636
Suárez-Luque S, Mato I, Huidobro JF, Simal-Lozano J, Sancho M (2002a) Rapid determination of minority organic acids in honey by high-performance liquid chromatography. J Chromatogr A 955:207–214
Suárez-Luque S, Mato I, Huidobro JF, Simal-Lozano J (2002b) Solid-phase extraction procedure to remove organic acids from honey. J Chromatogr B 770:77–82
Sugiyama M, Tang AC, Wakaki Y, Koyama W (2003) Glycemic index of single and mixed meal foods among common Japanese foods with white rice as a reference food. Eur J Clin Nutr 57:743–752
Tesfaye W, Morales ML, Garía-Parrilla MC, Troncoso AM (2002) Wine vinegar: technology, authenticity and quality evaluation. Trends Food Sci Technol 13:12–21
Valentão P, Andrade PB, Rangel J et al (2005) Effect of the conservation procedure on the contents of phenolic compounds and organic acids in chanterelle (Cantharellus cibarius) mushroom. J Agric Food Chem 53:4925–4931
Yang MH, Choong YM (2001) A rapid gas chromatographic method for direct determination of short-chain (C2–C12) volatile organic acids in foods. Food Chem 75:101–108
Acknowledgments
All fruit vinegar samples were kindly provided by Pai Chia Chen Brewery & Foods Co., Ltd., Chiayi County, Taiwan, to whom we are deeply appreciative. This work was supported by the Chun Shan Medical University, Taichung, Taiwan (project no. CSMU 97-OM-A-062).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, JT., Liu, SC., Shen, YC. et al. Comparison of Various Preparation Methods for Determination of Organic Acids in Fruit Vinegars with a Simple Ion-Exclusion Liquid Chromatography. Food Anal. Methods 4, 531–539 (2011). https://doi.org/10.1007/s12161-011-9204-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-011-9204-6