Abstract
Background
Although depression has been linked to insulin resistance, few studies have examined depressive symptom clusters.
Purpose
We examined whether certain depressive symptom clusters are more strongly associated with insulin resistance in a nationally representative sample, and we evaluated potential moderators and mediators.
Methods
Respondents were 4487 adults from NHANES 2005–2010. Depressive symptoms were measured with the Patient Health Questionnaire-9 (PHQ-9), and insulin resistance was indexed by the homeostatic model assessment (HOMA) score.
Results
Positive relationships between PHQ-9 total, somatic, and cognitive-affective scores and HOMA score were detected (ps <0.001). In a simultaneous model, the somatic (p = 0.017), but not the cognitive-affective (p = 0.071), score remained associated with HOMA score. We observed evidence of (a) moderation by race/ethnicity (relationships stronger in non-Hispanic Whites) and (b) mediation by body mass and inflammation.
Conclusions
The depressive symptoms–insulin resistance link may be strongest among non-Hispanic Whites and may be driven slightly more by the somatic symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Considerable evidence suggests that depression and type 2 diabetes have a moderate bidirectional relationship [1–3]. Depression is associated with a 37–60 % increased risk of developing diabetes [4, 5], whereas diabetes is associated with a 15–24 % increased risk of new-onset depression [5, 6]. Several behavioral and biological mechanisms have been proposed to explain this bidirectional relationship. Depression may increase risk of diabetes by promoting maladaptive health behaviors, such as poor diet and sedentary lifestyle [2, 7], which in turn may result in the development of obesity, a risk factor for diabetes [7, 8]. Depression-related biological changes—including autonomic [8], neuroendocrine [9], or inflammatory [10] activation—are other candidate mechanisms of the depression-to-future-diabetes relationship. Conversely, type 2 diabetes may increase risk of depression due to the psychological stress associated with having a chronic illness and the high demands of ongoing self-care [4].
To evaluate whether depression might exert a deleterious influence early in the natural history of diabetes, other studies have examined the relationship between depression and the pre-diabetes condition of insulin resistance. Insulin resistance occurs when there is decreased sensitivity of the insulin receptors, which limits transport of glucose into the cells of muscles, adipose tissue, and organs and increases levels of circulating glucose [11–13]. Although some investigations have reported null results [11], a recent meta-analysis of 18 studies by Kan and colleagues [14] detected a small but significant association (d = 0.19) between measures of depression and insulin resistance. It is worth noting that significant heterogeneity in the effect sizes across the studies was observed.
In closely related research areas, researchers have begun to compare the utility of depressive symptom clusters in predicting health outcomes [15, 16]. Depression can be conceptualized as a multidimensional construct comprised of four symptom clusters—i.e., the affective, cognitive, behavioral, and somatic clusters [17]. Studies in which the depression construct is examined according to symptom clusters could help to elucidate the mechanisms underlying the depression–insulin resistance relationship (by increasing the plausibility of some candidate mechanisms) and could help to inform the design of depression interventions intended to aid in the prevention of diabetes (by identifying the clusters most relevant to target). To date, only two investigations have examined the association between depressive symptom clusters and insulin resistance. In the first study (N = 269, mean age = 61 years), we found that the somatic symptoms of depression, but not the cognitive-affective symptoms, predicted increases in insulin resistance over a 6-year period [18]. This study’s sample, however, was moderate in size and consisted of predominantly older, non-Hispanic White adults of higher socioeconomic status. In the second study (N = 328, mean age = 60 years), both somatic and cognitive symptoms of depression were associated with insulin resistance, but the relationship was stronger for the somatic symptoms [19]. Similar to the aforementioned study, this study’s sample included predominantly older, male, white adults. Therefore, it is not known if these past findings will replicate and extend to other sociodemographic groups.
Accordingly, the primary objective of the present study was to examine whether certain depressive symptom clusters are more strongly associated with insulin resistance in a large, diverse sample of generally healthy community-dwelling adults who are free of diabetes. Our secondary objectives were (a) to test race/ethnicity as a moderator of the depressive symptoms–insulin resistance relationships and (b) to examine potential mediators/confounders of observed relationships. It is important to explore race/ethnicity as a moderator of this relationship for several reasons. First, in their recent meta-analysis, Kan et al. [14] observed substantial heterogeneity, suggesting the presence of unrecognized moderators. Second, the limited existing findings are conflicting. On the one hand, in the 2005–2010 National Health and Nutrition Examination Survey (NHANES) data, we observed an association between somatic depressive symptoms and C-reactive protein among non-Hispanic Whites, but not in the non-Hispanic Black, Mexican American, and Other Hispanic groups [20]. These findings indicate that one mechanism (i.e., increased systemic inflammation) through which depressive symptoms could contribute to insulin resistance may not be present in racial/ethnic minorities, suggesting that depressive symptoms are not as strongly associated with diabetes risk in these groups. On the other hand, studies have found that Hispanics tend to have higher rates of depression-diabetes comorbidity [21, 22], suggesting that depressive symptoms might be more strongly associated with diabetes risk in this group. To achieve our objectives, we examined the 2005–2010 NHANES data, which included a multidimensional measure of depressive symptoms and assessments of fasting plasma glucose and insulin obtained from a large, diverse sample representative of the US population.
Methods
Study Design and Sample
This study used cross-sectional data from the 2005–2010 waves of NHANES, a survey conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention to assess the health and nutritional status of the US population. Detailed information regarding the study design (a stratified, multistage, probability sample) is provided elsewhere (www.cdc.gov/nchs/nhanes.htm). Briefly, data were collected from a nationally representative sample of civilian, non-institutionalized adults and children. Approximately 5000 people were recruited each survey year, and non-Hispanic Blacks and Hispanics were among the groups oversampled. Those selected to participate were initially interviewed in their homes by trained personnel, who administered sociodemographic and health-related questionnaires using computer-assisted technology. One to two weeks after the household interview, respondents traveled to a Mobile Examination Center (MEC), where they completed other interviews and underwent laboratory and examination assessments. Our archival study was approved by the institutional review board at Indiana University–Purdue University Indianapolis.
From the total sample for the 2005–2010 survey years (N = 31,034), we selected all respondents aged 18 years and older (n = 18,318). We included those with complete glucose and insulin data, which was only collected from a subsample respondents who attended the morning MEC session and adhered to the fasting requirements (n = 6921), and we included respondents who responded to eight or more of the nine depressive symptom items (n = 6407). We then excluded 1524 adults who reported a history of one or more of the following medical conditions due to their likely impact on depressive symptoms and/or insulin resistance: diabetes (n = 669) [1, 23], cardiovascular disease (coronary heart disease, angina, myocardial infarction, stroke, or congestive heart failure; n = 460) [24, 25], liver conditions (n = 144) [26], kidney conditions (n = 66) [27], and current pregnancy (n = 185) [28]. We then excluded individuals with clinically elevated glucose levels (≥126 mg/dL) to rule out cases of unrecognized diabetes (n = 200) [29]. We further excluded the 196 respondents in the Other Race group (see “Other Factors” section). The Other Race group was similar to the other racial/ethnic groups on key variables (mean age = 39 years, 54 % female, 5 % less than 9th grade, mean PHQ-9 total score = 2.5, mean HOMA score = 2.7). The characteristics of the resulting 4487 individuals, which comprised our final sample, are shown in Table 1.
Measures and Procedures
Depressive symptoms
The Patient Health Questionnaire-9 (PHQ-9) [30] was administered during the MEC interview to assess depressive symptom severity over the past 2 weeks. On a 0–3 scale, respondents indicated the frequency with which they experienced the following symptoms of major depressive disorder: (1) anhedonia, (2) depressed mood, (3) sleep disturbance, (4) fatigue, (5) appetite changes, (6) low self-esteem, (7) concentration problems, (8) psychomotor retardation/agitation, and (9) suicidal ideation. Total scores range from 0 to 27, and scores ≥10 represent clinically significant depressive symptoms [31]. The PHQ-9 has high internal consistency and good sensitivity and specificity for identifying cases of major depressive disorder in community samples [30–34].
For 71 respondents missing one PHQ-9 item, we imputed the missing value using the mean of the other eight items for that respondent. Next, we computed the PHQ-9 total score by summing the nine items. Finally, we calculated PHQ-9 somatic and cognitive-affective subscale scores. The somatic subscale score was computed by summing the items 3, 4, 5, and 8, and the cognitive-affective subscale score was computed by summing items 1, 2, 6, 7, and 9. Previous factor analyses supported a one-factor structure comprised of all nine items [35, 36]; however, recent confirmatory factor analyses have established that a two-factor structure provides a better fit [37, 38]. Of note, other confirmatory factor analyses support including item 8 (psychomotor retardation/agitation) in the somatic subscale rather than in the cognitive-affective subscale [39]. To facilitate comparisons between this study and past studies (including our investigation) [20], we computed our subscale scores based on the two-factor structure that includes item 8 in the somatic subscale, which is more often used in studies examining relationships between depressive symptom clusters and health outcomes.
Insulin resistance
Blood samples were obtained from respondents who attended the morning MEC session and who reported abstaining from food, beverages other than water, and certain over-the-counter medications for at least 9 h before their visit. Samples were collected by trained NHANES personnel through venipuncture using standard phlebotomy techniques. Samples were processed and then stored at −20°C until time of assay at the University of Minnesota Medical Center, Fairview. Fasting plasma glucose concentration was determined by the enzyme hexokinase-mediated reaction method using the Roche/Hitachi 911 Analyzer (Roche Diagnostics, Indianapolis, IN) between 2005 and 2006 and by the glucose oxidase method using the Beckman Synchron LX20 (Beckman Coulter Inc., Brae, California) in 2007 and the Beckman Coulter UniCel® DxC800 Synchron (Beckman Coulter Inc., Brae, California) between 2008 and 2010. Fasting insulin concentration was determined by a two-site immunoenzymometric assay using the Beckman Coulter Biomek 2000 Workstation (Beckman Coulter Inc., Brae, California) between 2005 and 2008 and the Roche Elecsys 2010 (Roche Diagnostics, Indianapolis, IN) between 2009 and 2010.
Fasting glucose and insulin values were used to compute the Homeostatic Model of Assessment (HOMA) score. HOMA was computed as [fasting plasma insulin (mU/L) × fasting plasma glucose (mmol/L)]/22.5 [40]. We selected the HOMA2-IR modeling approach, which has been found to improve upon the HOMA-IR approach though the use of advanced computer programming to account for the complexity of the association between insulin and glucose [41]. Higher HOMA scores represent increased insulin resistance or decreased insulin sensitivity [42]. Although the euglycemic clamp method and some insulin sensitivity indices have been suggested as superior measures [14], HOMA is an established index of insulin resistance that correlates highly with the gold standard euglycemic clamp (rs = 0.85–0.88) [40, 43]. Because HOMA is a less invasive approach than the traditional clamp method, it is an appropriate measure of basal insulin resistance in epidemiologic studies [42, 44].
Other factors
Statistical models included the following variables as covariates: age (years), sex (0 = male, 1 = female), three dummy variables for race/ethnicity, and education level (see Table 1). The primary NHANES race/ethnicity variable has five levels: non-Hispanic White, non-Hispanic Black, Mexican American, Other Hispanic, and Other Race including Multi-Racial. NHANES recommends against combining data for the Mexican American and Other Hispanic groups, as the sampling structure changed over the course of the NHANES waves included in this study (http://www.cdc.gov/nchs/data/nhanes/analyticnote_2007-2010.pdf). We excluded the 196 respondents in the Other Race group because it was much smaller and more heterogeneous than the other racial/ethnic groups, which made interpretation difficult. Thus, our race/ethnicity variable had four categories, which we used to compute three dummy variables comparing the non-Hispanic Black group (RE1), Mexican American group (RE2), and Other Hispanic group (RE3) to the non-Hispanic White reference group. Respondents reported their highest level of education during the household interview. For those aged 20+ years, the categories were less than 9th grade, 9–12th grade with no diploma, high school diploma or GED, some college or associates degree, and college graduate or above. For those aged 18–19 years, the categories were grade level ranging from 1st to 12th grade with no diploma, high school graduate, GED or equivalent, or more than high school. We reclassified the education level of respondents aged 18–19 years using the categories for respondents 20+ years.
We examined four additional variables as potential mediators/confounders of detected depressive symptom–HOMA associations—namely, body mass index (BMI; kg/m2), C-reactive protein (CRP; mg/L), current smoking (0 = no, 1 = yes), and alcohol use (drinks per day). We selected these factors because (a) they were assessed in 2005–2010 waves of NHANES and (b) they have each been found to be associated with both depression and insulin resistance [45–52]. BMI was calculated from measurements of height and weight taken at the MEC. CRP levels were determined from serum samples obtained during the MEC visit, which were frozen at −20 °C until the time of assay at the University of Washington. Serum CRP was quantified by latex-enhanced nephelometry using a Dade Behring Nephelometer II Analyzer System (Dade Behring Diagnostics Inc., Somerville, NJ). Additional details are available elsewhere [20]. For adults aged 20+ years, smoking and alcohol use data were collected during the MEC interview. NHANES does not report data for alcohol use for adults aged 18–19 years, so respondents of those ages were excluded from the mediation analyses.
To assess current smoking, respondents who completed the NHANES Smoking-Cigarette Use Questionnaire were first asked, “Have you smoked at least 100 cigarettes in your entire life?” Only respondents who responded “yes” were additionally asked, “Do you now smoke cigarettes every day, some days, or not at all?” Respondents who reported smoking every day or some days were coded as current smokers, and respondents who reported smoking not at all or who reported smoking fewer than 100 cigarettes in their lifetime were coded as not current smokers. To assess current alcohol use, respondents who completed the NHANES Alcohol Use Questionnaire were first asked, “In any one year, have you had at least 12 drinks of any type of alcoholic beverage?” Only respondents who responded “yes” were asked follow-up questions regarding the number of days drinking per week, month, or year and the average number of drinks consumed on an average drinking day. Respondents who reported drinking less than 12 drinks in any given year were assigned a value of 0 for drinks per day. For all other respondents, we first computed the number of days drinking alcohol during the past year. This value was then multiplied by the average number of drinks consumed per drinking day during the past year, yielding the total number of drinks consumed during the past year. Finally, we divided this value by 365 to compute drinks per day during the past year, which we log transformed to normalize the distribution.
Data Analysis
One-way analyses of variance (ANOVAs) were performed to test for differences in the depressive symptom measures and HOMA score across the racial/ethnic groups. We evaluated the internal consistency of the three depressive symptom measures (Cronbach’s α) and their interrelationships (Pearson correlations).
To examine whether certain depressive symptom clusters are more strongly associated with insulin resistance, we conducted a series of linear regression analyses involving the entire sample with HOMA score as the criterion variable. First, the PHQ-9 total, somatic subscale, and cognitive-affective subscale scores were entered as predictor variables into separate models that included demographic factors (age, sex, three dummy variables for race/ethnicity, and education level) and the NHANES sampling design variables. Second, in simultaneous models, the somatic and cognitive-affective subscale scores were entered into the same model as predictors along with the demographic factors and sampling design variables.
To test whether race/ethnicity moderated the depressive symptoms-HOMA relationship, we computed nine cross-product interaction terms by multiplying the PHQ-9 total, somatic, and cognitive-affective scores (first converted to z scores) by each of the race/ethnicity dummy variables (RE1, RE2, and RE3). The three PHQ-9 total interaction terms were entered into a linear regression model with HOMA score as the criterion variable. This model also included age, sex, three dummy variables for race/ethnicity, education level, PHQ-9 total, and the NHANES sampling design variables. We constructed parallel models for the somatic and cognitive-affective subscales. Given the presence of significant interactions, we reran the linear regression models after stratifying by race/ethnicity. We performed additional exploratory moderation analyses examining depressive symptoms × sex (all ps > 0.40) and depressive symptoms × education level (all ps > 0.60).
We examined four potential mediators/confounders (i.e., BMI, CRP, smoking, and alcohol use) of the depressive symptoms–HOMA relationship observed in the non-Hispanic White group. Because 322 of the non-Hispanic White respondents had missing data for at least one potential mediator, the final sample for these analyses was 1885. First, we reran the separate model for the PHQ-9 total for this new sample. Then, we added BMI, CRP, smoking, and alcohol use one at a time to this model. To quantify the effect of each potential mediator/confounder, percent change in the effect size was computed as [(B mediator/confounder − B separate)/B separate] × 100, where B mediator/confounder is the unstandardized coefficient for the PHQ-9 total from the model with the selected mediator/confounder and B separate is the unstandardized coefficient for the PHQ-9 total from the model without the selected mediator/confounder. Sobel tests were also performed to test for statistical mediation. Finally, because both BMI and CRP reduced the effect size of the depressive symptoms–HOMA relationship and had significant Sobel tests, we entered both in the same model and repeated the mediation analyses.
Analyses were performed using SAS statistical software (version 9.3). Estimates from the linear regression models were weighted using the appropriate NHANES sampling design variables—i.e., strata, primary sampling unit, and fasting subsample sample weights—which account for survey design factors including oversampling, non-response, and post-stratification (see www.cdc.gov/nchs/tutorials/nhanes/SurveyDesign/Weighting/intro.htm for further details). We used sample weights from the fasting subsample because HOMA scores were calculated from insulin and glucose values. For each respondent, the sample weight indicates the proportion of people in the population s/he represents. Analyses utilizing NHANES sampling design variables provide estimates representative of the US civilian non-institutionalized population.
Results
Depressive Symptoms and HOMA Score
Although the mean PHQ-9 total score for the entire sample fell in the subclinical range (see Table 1), 301 (7 %) respondents had a score ≥10, reflecting clinically significant depressive symptoms [31]. Compared to respondents with a PHQ-9 score <10, those with a PHQ-9 score ≥10 were more likely to be female (p < 0.001), be current smokers (p < 0.001), have less than 9th grade education (p < 0.001), and have a higher mean CRP level (p = 0.001) and mean HOMA score (p = 0.033). As is shown in Table 1, no racial/ethnic differences in the total, somatic, or cognitive-affective subscale scores were observed. Both the PHQ-9 total and cognitive-affective subscale had at least adequate internal consistency (Cronbach’s α ≥ 0.70) in all racial/ethnic groups; however, the somatic subscale tended to fall just short of this cut point (see Table 2). The somatic and the cognitive-affective subscales were moderately correlated in each racial/ethnic group (see Table 2).
The mean HOMA score for the entire sample was 3.0. A pattern of group differences was observed, as the non-Hispanic White group had a lower mean than the three other race/ethnicity groups (see Table 1). Although there is no established HOMA cut point for determining insulin resistance, past studies have used HOMA cut points ranging from 1.8 to 4.0 [40, 53–55].
Associations of Depressive Symptoms with HOMA Score in the Entire Sample
Separate regression models adjusted for demographic factors and the NHANES sampling design demonstrated that there were positive relationships between the PHQ-9 total, somatic subscale, and cognitive-affective subscale and HOMA score (all ps < 0.001) in the entire sample (see Table 3). In the simultaneous model that included both PHQ-9 subscales, the somatic subscale remained positively associated with HOMA score (p = 0.017), whereas the cognitive-affective subscale fell short of significance (p = 0.071). Although one PHQ-9 subscale was significantly related to HOMA and the other was not, the magnitudes of these relationships do not appear to be meaningfully different (see βs in Table 3).
Associations of Depressive Symptoms with HOMA Score Moderated by Race/Ethnicity
To examine race/ethnicity as a moderator of the depressive symptoms–HOMA relationship, we first tested interaction terms involving the PHQ-9 total and the three dummy variables for race/ethnicity. The PHQ-9 total × RE2 (non-Hispanic White vs. Mexican American) interaction was significant (β = −0.15, p < 0.001); however, the PHQ-9 total × RE1 (non-Hispanic White vs. non-Hispanic Black) interaction (β = −0.04, p = 0.45) and the PHQ-9 total × RE3 (non-Hispanic White vs. Other Hispanic) interaction (β = −0.07, p = 0.27) were not. These results demonstrate that the PHQ-9 total was more strongly associated with HOMA score among non-Hispanic Whites (p < 0.001) than among Mexican Americans (p = 0.22; see Table 3).
We observed a similar pattern of results for the PHQ-9 subscales. The somatic × RE2 interaction (β = −0.13, p = 0.005) was significant, but the somatic × RE1 interaction (β = −0.05, p = 0.23) and the somatic × RE3 interaction (β = −0.08, p = 0.18) were not. The somatic subscale was more strongly related to HOMA score among non-Hispanic Whites (p < 0.001) than among Mexican Americans (p = 0.58; see Table 3). The cognitive-affective × RE2 interaction (β = −0.14, p < 0.001), but not the cognitive-affective × RE1 interaction (β = −0.003, p = 0.94) and the cognitive-affective × RE3 interaction (β = −0.04, p = 0.53), was significant. The cognitive-affective subscale was more strongly related to HOMA score among non-Hispanic Whites (p < 0.001) than among Mexican Americans (p = 0.074; see Table 3). Of note, the relatively smaller sample size of the Other Hispanic group may result in reduced statistical power to detect differences in the magnitude of the depressive symptoms–HOMA relationships between the non-Hispanic White and Other Hispanic groups. A simultaneous model was run for the non-Hispanic White group only because that was the only group in which we observed depressive symptoms–HOMA associations (see Table 3). Results paralleled those in the entire sample. While the somatic subscale remained positively associated with HOMA score (p = 0.006), the cognitive-affective subscale again fell short of significance (p = 0.10).
Potential Mediators/Confounders of Depressive Symptoms–Insulin Resistance Relationship among Non-Hispanic Whites
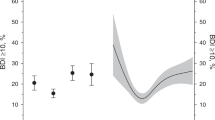
We examined four potential mediators/confounders of PHQ-9 total–HOMA score relationship detected in the non-Hispanic White group. As can be seen in Fig. 1, only adjustment for BMI (52.4 % reduction) and CRP (41.0 % reduction) reduced the effect size by more than 5 %. Adjusting for alcohol use had no impact, whereas adjusting for smoking slightly increased the effect size. Sobel tests support these descriptive results, with BMI (p < 0.001) and CRP (p < 0.001) each partially explaining the association between the PHQ-9 total and HOMA score. When both of these potential mediators were entered into the same model, they collectively explained 62.5 % of the PHQ-9 total–HOMA score relationship.
Linear regression analyses examining potential mediators/confounders of the relationship between total depressive symptoms and HOMA score among non-Hispanic Whites (n = 1885). The y-axis represents the percent change in the effect size of the depressive symptoms–HOMA score relationship after inclusion of the selected potential mediator(s)/confounder(s) in the separate model for the PHQ-9 total. The β for the PHQ-9 total in the separate model with no potential mediator/confounder was 0.09 in the mediation sample. HOMA Homeostatic Model of Assessment, BMI body mass index, CRP C-reactive protein, PHQ-9 Patient Health Questionnaire-9. *p < 0.05; **p < 0.01
Discussion
We found that greater depressive symptom severity was cross-sectionally associated with greater insulin resistance in a large, diverse, and nationally representative sample of generally healthy adults free of diabetes. In addition, our results suggest that both the somatic and cognitive-affective clusters contribute to the overall depressive symptoms–insulin resistance relationship, with a slightly greater contribution from the somatic cluster (i.e., sleep disturbance, fatigue, appetite changes, and psychomotor retardation/agitation). There was also evidence of moderation by race/ethnicity. Total depressive symptoms, somatic symptoms, and cognitive-affective symptoms were more strongly associated with HOMA score in non-Hispanic Whites than in Mexican Americans. Analyses stratified by race/ethnicity revealed that the pattern of results in the non-Hispanic White group mirrored that of the entire sample; however, no associations between depressive symptoms and HOMA score were observed in the non-Hispanic Black, Mexican American, or Other Hispanic groups. Analyses examining potential mediators/confounders indicated that BMI and CRP explained over half of the depressive symptoms–insulin resistance relationship in non-Hispanic Whites. As a set, our findings suggest that (a) the link between depressive symptoms and insulin resistance may be strongest among non-Hispanic Whites and (b) this relationship in non-Hispanic Whites may be driven slightly more by the somatic depressive symptoms and may be partially explained by increased body mass and systemic inflammation. It should be noted, however, that the observed effect sizes were small, and the difference in effect size between the somatic and cognitive-affective subscales was minimal. The use of a depressive symptom self-report measure instead of a diagnostic interview [14] and restriction of range in the PHQ-9 and HOMA scores may have contributed to these smaller effect sizes.
To our knowledge, this study is the first to examine race/ethnicity as a moderator of the depressive symptoms–insulin resistance relationship. Our results are inconsistent with past findings of higher rates of depression-diabetes comorbidity among Hispanics [21, 22]; however, depression may have been secondary to diabetes diagnosis, management, and/or complications in those studies. Our findings also suggest moderation by race/ethnicity may partly explain the substantial heterogeneity in the effect sizes observed in Kan et al.’s meta-analysis [14]. The present findings are consistent with the two previous studies that examined the association between depressive symptom clusters and insulin resistance [18, 19]. In an older, predominantly non-Hispanic White sample, we found that the somatic-vegetative symptoms, but not the cognitive-affective symptoms, predicted increases in insulin resistance over 6 years [18].
Although few studies have empirically examined candidate mediators of the link between depressive symptoms and insulin resistance [11], our findings generally agree with the available evidence. In the prospective study described above [18], we found that 6-year change in BMI was a partial mediator and explained 23 % of the association between somatic depressive symptoms and insulin resistance. Evidence from our study suggests that BMI and CRP may be partial mediators, together accounting for 63 % of the depressive symptoms–insulin resistance relationship among non-Hispanic Whites. These observations provide support for the notion that depressive symptom-related increases in adiposity and systemic inflammation may be pathways through which depressive symptoms contribute to insulin resistance and diabetes onset [2, 8, 10]. However, because the present data are cross-sectional and BMI and CRP have been found to predict future depression [56, 57], we cannot rule out the possibility that these factors are operating as confounders instead of mediators here.
We considered possible methodological and conceptual explanations for why the link between depressive symptoms and insulin resistance was observed only among non-Hispanic Whites. First, depressive symptom measurement variance across the racial/ethnic groups might be responsible. However, evidence suggests that the PHQ-9 performs equally well for each of the racial/ethnic groups in our study [36]. Second, the racial/ethnic groups might differ in their depressive symptom levels and variability, which could increase the likelihood of detecting an association in certain groups. We detected only one significant mean difference between the groups, and the standard deviations for the PHQ-9 total and subscales were remarkably similar across groups (see Table 1). Third, race/ethnicity may be proxy for socioeconomic status. The depressive symptom × education level interactions were not significant, rendering this possibility unlikely. Fourth, key pathways may be stronger or present only among non-Hispanic Whites. For instance, in a separate study examining the NHANES 2005–2010 data, we found that depressive symptom severity was associated with circulating CRP levels in non-Hispanic Whites but not in the other racial/ethnic groups [20]. Assuming that elevated systemic inflammation is a crucial step in the promotion of insulin resistance in depressed adults [8, 10], the depressive symptoms–insulin resistance relationship might not be observed in groups with a weak or nonexistent depressive symptoms–inflammation relationship. Currently, there is a need for additional studies examining race/ethnicity as a moderator of the associations of depressive symptoms with insulin resistance and diabetes onset and for studies evaluating potential mechanisms underlying this moderator effect.
There are several potential explanations for why the link between depressive symptoms and insulin resistance may be driven slightly more by the somatic depressive symptoms. First, the somatic symptoms may have stronger associations with the mechanisms thought to underlie the depressive symptoms-to-insulin resistance relationship than the cognitive-affective symptoms. To illustrate, recent studies have observed that somatic depressive symptoms are more strongly related to abdominal obesity [58] and circulating inflammatory markers [59–62]. Second, because the NHANES data are cross-sectional, it is possible that insulin resistance may produce somatic symptoms that overlap with those of depression. Specifically, insulin resistance produces elevated levels of circulating glucose and insulin [63, 64], which can result increased appetite and fatigue [64, 65]. Among people with diabetes, symptom overlap between depression and diabetes can lead to over-identification of depression when using the PHQ-9 [66, 67]. In the present study, mild symptoms of increased appetite and fatigue in some respondents with insulin resistance could have resulted in an overestimation of the somatic symptoms–insulin resistance association. However, unlike diabetes, insulin resistance is often asymptomatic [29]. Third, the somatic depressive symptoms could be caused by another underlying condition, such as insomnia or sleep apnea, which in turn may promote insulin resistance [54, 55]. Fourth, elevated somatic depressive symptoms may be a marker of greater depressive symptom severity or a history of clinical depression [16], considering that residual symptoms after depression treatment tend to be somatic [68, 69].
Our study has important limitations that are worth mentioning. First, due to the cross-sectional nature of the NHANES study design, we were unable to determine the directionality of the detected depressive symptoms–insulin resistance associations. As is discussed above, both directions are plausible. Furthermore, because the data are cross-sectional, we could also not determine whether BMI and CRP were operating as mediators or confounders, and both roles are supported by past evidence. Second, to minimize likelihood of diabetes and other medical conditions operating as confounders, we excluded adults based on their self-report of receiving such diagnoses from a physician or another health professional. Consequently, our final sample likely included some respondents with undiagnosed diabetes or other conditions. To partially address this concern, we excluded respondents with clinically elevated levels of fasting glucose (≥126 mg/dL) to minimize the likelihood of including respondents with unrecognized diabetes. Third, the available data could not be used to verify that respondents adhered to the fasting instructions before the blood draw, and the methods for assessing fasting glucose and insulin changed over the 2005–2010 NHANES years. Both of these factors could have introduced noise into the insulin and glucose assessments, thereby making it more difficult to detect associations with HOMA score. Fourth, because history of depression and past exposure to depression treatments were not assessed in NHANES, we could not examine the effect of controlling for these factors on the associations between current depressive symptoms and insulin resistance. Fifth, the range of our PHQ-9 and HOMA scores was likely restricted, which may have contributed to the smaller effect sizes we observed. PHQ-9 scores of most respondents were indicative of low depressive symptom levels. In addition, respondents with a diabetes diagnosis or a fasting glucose level ≥126 mg/dL were excluded, which likely limited variability in the HOMA scores. Our study also has important strengths, including a large, diverse sample representative of the US population, the use of a validated, multidimensional depressive symptoms measure, and the testing of multiple candidate mediators.
Conclusion
From a large sample of generally healthy adults free of diabetes, we report evidence that the depressive symptoms–insulin resistance relationship may be strongest among non-Hispanic Whites, may be driven slightly more by the somatic symptoms, and may be partially explained by increased body mass and systemic inflammation. Our results raise the possibility that non-Hispanic Whites with elevated depressive symptoms, especially the somatic symptoms, may be at increased risk of type 2 diabetes. In addition, our findings combined with similar results could provide the rationale for examining the effect of depression interventions, perhaps specifically targeting the somatic symptoms, on diabetes risk markers in depressed adults.
Abbreviations
- HOMA:
-
Homeostatic model of assessment
- BMI:
-
Body mass index
- MEC:
-
Mobile Examination Center
- NHANES:
-
National Health and Nutritional Examination Survey
- CRP:
-
C-reactive protein
- PHQ-9:
-
Patient Health Questionnaire-9
References
Renn BN, Feliciano L, Segal DL. The bidirectional relationship of depression and diabetes: a systematic review. Clin Psychol Rev. 2011; 31: 1239-1246.
Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012; 142: S8-S21.
Rustad JK, Musselman DL, Nemeroff CB. The relationship of depression and diabetes: pathophysiological and treatment implications. Psychoneuroendocrinology. 2011; 36: 1276-1286.
Knol MJ, Twisk JW, Beekman AT, et al. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006; 49: 837-845.
Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008; 31: 2383-2390.
Nouwen A, Winkley K, Twisk J, et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010; 53: 2480-2486.
Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008; 299: 2751-2759.
Ismail K. Unraveling the pathogenesis of the depression-diabetes link. In: Katon W, Maj M, Norman S, eds. Depression and diabetes. Chichester, UK: Wiley & Sons; 2010.
Golden SH. A review of the evidence for a neuroendocrine link between stress, depression and diabetes mellitus. Curr Diabetes Rev. 2007; 3: 252-259.
Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002; 53: 873-876.
Silva N, Atlantis E, Ismail K. A review of the association between depression and insulin resistance: pitfalls of secondary analyses or a promising new approach to prevention of type 2 diabetes? Curr Psychiatry Rep. 2012; 14: 8-14.
Shepherd PR, Kahn BB. Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999; 341: 248-257.
Ryan JP, Sheu LK, Critchley HD, Gianaros PJ. A neural circuitry linking insulin resistance to depressed mood. Psychosom Med. 2012; 74: 476-482.
Kan C, Silva N, Golden SH, et al. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. 2013; 36: 480-489.
Stewart JC, Zielke DJ, Hawkins MA, et al. Depressive symptom clusters and 5-year incidence of coronary artery calcification: the coronary artery risk development in young adults study. Circulation. 2012; 126: 410-417.
Carney RM, Freedland KE. Are somatic symptoms of depression better predictors of cardiac events than cognitive symptoms in coronary heart disease? Psychosom Med. 2012; 74: 33-38.
Davidson KW, Rieckmann N, Rapp MA. Definitions and distinctions among depressive syndromes and symptoms: implications for a better understanding of the depression–cardiovascular disease association. Psychosom Med. 2005; 67(Suppl 1): S6-9.
Khambaty T, Stewart JC, Muldoon MF, Kamarck TW. Depressive symptom clusters as predictors of 6-year increases in insulin resistance: data from the Pittsburgh Healthy Heart Project. Psychosom Med. 2014; 76: 363-369.
Austin AW, Gordon JL, Lavoie KL, et al. Differential association of insulin resistance with cognitive and somatic symptoms of depression. Diabet Med. 2014; 31: 994-1000.
Case SM, Stewart JC. Race/ethnicity moderates the relationship between depressive symptom severity and C-reactive protein: 2005–2010 NHANES data. Brain Behav Immun. 2014; 2014(41): 101-108.
Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med. 2008; 25: 1096-1101.
Katon W, Fan MY, Unutzer J, et al. Depression and diabetes: a potentially lethal combination. J Gen Intern Med. 2008; 23: 1571-1575.
Bunt JC, Krakoff J, Ortega E, Knowler WC, Bogardus C. Acute insulin response is an independent predictor of type 2 diabetes mellitus in individuals with both normal fasting and 2-h plasma glucose concentrations. Diabetes Metab Res Rev. 2007; 23: 304-310.
Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000; 106: 453-458.
Van der Kooy K, van Hout H, Marwijk H, et al. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007; 22: 613-626.
Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006; 91: 4753-4761.
Liao MT, Sung CC, Hung KC, et al. Insulin resistance in patients with chronic kidney disease. J Biomed Biotechnol. 2012; 2012: 691369.
Barbour LA, McCurdy CE, Hernandez TL, et al. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007; 30(Suppl 2): S112-119.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010; 33(Suppl 1): S62-69.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001; 16: 606-613.
Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatric Annals. 2002; 32: 509-515.
Wittkampf KA, Naeije L, Schene AH, Huyser J, van Weert HC. Diagnostic accuracy of the mood module of the Patient Health Questionnaire: a systematic review. Gen Hosp Psychiatry. 2007; 29: 388-395.
Patten SB, Schopflocher D. Longitudinal epidemiology of major depression as assessed by the Brief Patient Health Questionnaire (PHQ-9). Compr Psychiatry. 2009; 50: 26-33.
Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ. 2012; 184: E191-196.
Cameron IM, Cardy A, Crawford JR, et al. Measuring depression severity in general practice: discriminatory performance of the PHQ-9, HADS-D, and BDI-II. Br J Gen Pract. 2011; 61: e419-426.
Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006; 21: 547-552.
Chilcot J, Rayner L, Lee W, et al. The factor structure of the PHQ-9 in palliative care. J Psychosom Res. 2013; 75: 60-64.
Krause JS, Bombardier C, Carter RE. Assessment of depressive symptoms during inpatient rehabilitation for spinal cord injury: is there an underlying somatic factor when using the PHQ? Rehabil Psychol. 2008; 53: 513-520.
de Jonge P, Mangano D, Whooley MA. Differential association of cognitive and somatic depressive symptoms with heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Psychosom Med. 2007; 69: 735-739.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28: 412-419.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004; 27: 1487-1495.
Everson-Rose SA, Meyer PM, Powell LH, et al. Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care. 2004; 27: 2856-2862.
Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000; 23: 57-63.
Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002; 19: 527-534.
Baliunas DO, Taylor BJ, Irving H, et al. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009; 32: 2123-2132.
Eliasson B. Cigarette smoking and diabetes. Prog Cardiovasc Dis. 2003; 45: 405-413.
Freedland KE, Carney RM, Skala JA. Depression and smoking in coronary heart disease. Psychosom Med. 2005; 67(Suppl 1): S42-46.
Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009; 71: 171-186.
Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006; 444: 840-846.
Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010; 67: 220-229.
Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006; 116: 1793-1801.
Sullivan LE, Fiellin DA, O’Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med. 2005; 118: 330-341.
Yin J, Li M, Xu L, et al. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol Metab Syndr. 2013; 5: 71.
Reinehr T, Andler W. Cortisol and its relation to insulin resistance before and after weight loss in obese children. Horm Res. 2004; 62: 107-112.
Esteghamati A, Ashraf H, Khalilzadeh O, et al. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr Metab (Lond). 2010; 7: 26.
Blaine B. Does depression cause obesity? A meta-analysis of longitudinal studies of depression and weight control. J Health Psychol. 2008; 13: 1190-1197.
Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006; 27: 24-31.
Wiltink J, Michal M, Wild PS, et al. Associations between depression and different measures of obesity (BMI, WC, WHtR, WHR). BMC Psychiatry. 2013; 13: 223.
Duivis HE, Vogelzangs N, Kupper N, de Jonge P, Penninx BW. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands Study of Depression and Anxiety (NESDA). Psychoneuroendocrinology. 2013; 38: 1573-1585.
Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav Immun. 2009; 23: 936-944.
Michal M, Wiltink J, Kirschner Y, et al. Differential associations of depressive symptom dimensions with cardio-vascular disease in the community: results from the Gutenberg health study. PLoS One. 2013; 8, e72014.
Deverts DJ, Cohen S, DiLillo VG, et al. Depressive symptoms, race, and circulating C-reactive protein: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosom Med. 2010; 72: 734-741.
Gallagher EJ, LeRoith D, Karnieli E. The metabolic syndrome—from insulin resistance to obesity and diabetes. Endocrinol Metab Clin North Am. 2008; 37: 559-579. vii.
Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J. 2005; 149: 33-45.
Vgontzas AN, Bixler EO, Chrousos GP. Obesity-related sleepiness and fatigue: the role of the stress system and cytokines. Ann N Y Acad Sci. 2006; 1083: 329-344.
Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014; 31: 764-772.
Twist K, Stahl D, Amiel SA, et al. Comparison of depressive symptoms in type 2 diabetes using a two-stage survey design. Psychosom Med. 2013; 75: 791-797.
Hybels CF, Steffens DC, McQuoid DR, Rama Krishnan KR. Residual symptoms in older patients treated for major depression. Int J Geriatr Psychiatry. 2005; 20: 1196-1202.
Conradi HJ, Ormel J, de Jonge P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol Med. 2011; 41: 1165-1174.
Author information
Authors and Affiliations
Corresponding author
Additional information
Potential conflicts of interest
All authors declare that there are no conflicts of interest.
Author’s statement of conflict of interest
Elizabeth A. Vrany, Jessica M. Bertnson, Tasneem Khambaty, and Jesse C. Stewart declare that they have no conflict of interest. All procedures were conducted in accordance with the standards of institutional review board of Indiana University–Purdue University Indianapolis and with the Helsinki Declaration of 1975, as revised in 2000.
About this article
Cite this article
Vrany, E.A., Berntson, J.M., Khambaty, T. et al. Depressive Symptoms Clusters and Insulin Resistance: Race/Ethnicity as a Moderator in 2005–2010 NHANES Data. ann. behav. med. 50, 1–11 (2016). https://doi.org/10.1007/s12160-015-9725-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12160-015-9725-0