Abstract
Background
Chronic pain with comorbid depression is characterized by poor mood regulation and stress-related pain.
Purpose
This study aims to compare depressed and non-depressed pain patients in mood and pain stress reactivity and recovery, and test whether a post-stress positive mood induction moderates pain recovery.
Methods
Women with fibromyalgia and/or osteoarthritis (N = 110) underwent interpersonal stress and were then randomly assigned by pain condition and depression status, assessed via the Center for Epidemiological Studies-Depression scale, to positive versus neutral mood induction.
Results
Depression did not predict stress-related reactivity in despondency, joviality, or clinical pain. However, depression × mood condition predicted recovery in joviality and clinical pain; depressed women recovered only in the positive mood condition, whereas non-depressed women recovered in both mood conditions.
Conclusions
Depression does not alter pain and mood stress reactivity, but does impair recovery. Boosting post-stress jovial mood ameliorates pain recovery deficits in depressed patients, a finding relevant to chronic pain interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Depression is common among individuals with a chronic pain condition. Prevalence estimates suggest that roughly 18 % of pain patients in population-based settings and 27 % of pain patients in primary care clinics meet criteria for depression [1]. The impact of comorbid depression on the health and quality of life of individuals in chronic pain can be substantial; across a range of chronic pain conditions, depressed patients report higher levels of pain and disability [2], display more pain behaviors [3], and respond more poorly to treatments [2] than their non-depressed counterparts.
How might depression contribute to poorer adaptation in chronic pain? One possibility is via its impact on responses to stress. Among chronic pain patients, stress and other negative affective experiences are associated with increases in pain [4–6], an association that is especially strong among those with comorbid depressive symptoms [7]. This vulnerability of patients who are depressed may be due in part to their more frequent use of maladaptive emotion regulation strategies to manage stress compared to their non-depressed counterparts. In particular, depressed individuals have difficulty disengaging their attention from aversive stimuli; rather, they engage in prolonged processing of the information and elaboration about the negative experience [8, 9], which in turn propagates depressive affect. This cycle may interfere with the ability of depressed people to adopt other potentially more useful strategies, and instead fuel ongoing mood disturbance and pain and impede stress recovery [10].
Alternative strategies can enable depressed pain patients to recover quickly in the face of stress. One approach garnering increased support is fostering a shift toward better regulation of emotional life by directing attention to sources of positive emotion [11]. Indeed, attentional redeployment to the positive has been identified as the primary path to restoration of well-being following episodes of stress among individuals in chronic pain [7, 12]. Yet depressed individuals have difficulty tapping into positive emotional resources when they most need them. For example, they are less able to recall positive memories to repair their negative moods and are more likely to use negative rather than positive distractors to shift their attention away from distressing circumstances compared to non-depressed individuals [13, 14]. Thus, depression may both potentiate maladaptive responses to negative experiences and attenuate access to and/or use of positive emotional resources that may aid recovery. Diary studies have shed some light on these processes by observing ecologically valid stressful life events and their aftermath; however, they cannot standardize exposure to negative life experiences and positive emotional resources across individuals [7]. As a result, any differences between depressed and non-depressed pain patients in their stress responses and recovery in the field may hinge on exposure to different stressors and/or positive emotional resources. Missing from the pain literature is the evaluation of stress-related changes in affect and pain to standardized stressors and positive emotion stimuli in the laboratory among individuals with chronic pain.
Study Overview
The current study examined the impact of depression on stress responses and recovery in individuals with chronic pain due to one of two prevalent pain conditions: osteoarthritis (OA) and fibromyalgia (FM). OA is characterized by chronic joint pain and stiffness, whereas FM is marked by widespread pain in soft tissue; OA affects approximately 26 million and FM approximately 5 million adults in USA [15]. Clinically significant depressive symptom levels are common: prevalence estimates range from 22 to 84 % for FM [16, 17], and from 17 to 29 % for OA [18, 19]. Moreover, for both pain conditions, comorbid depression and depressive symptoms are associated with increased pain and disability [20, 21] and less adaptive responses to stress [10, 22, 23].
The aims of the current study were to compare depressed and non-depressed OA and FM patients in mood and pain stress reactivity and recovery, and test whether a post-stress positive mood induction moderates pain recovery. To that end, depressed and non-depressed women with chronic pain due to FM and/or OA underwent an interpersonal stress task and then were randomly assigned by depression status and pain diagnosis to view either an emotionally neutral or a positive video, with repeated assessment of mood and clinical pain. The evaluation of affective reactivity centered on two specific affects: joviality, which reflects high activation positive affect, and despondency, which reflects depressed affect [24]. Joviality and despondency were of particular interest here because they signal key areas of affective disturbance in depression, namely anhedonia and depressed mood.
Three hypotheses were tested regarding the impact of depression status on stress reactivity and recovery: (1) stress-induced increases in clinical pain and mood disturbance will be more pronounced among depressed versus non-depressed pain patients; (2) clinical pain and mood recovery following stress will be impaired among depressed versus non-depressed patients; and (3) impairment in clinical pain during recovery in depressed patients will be ameliorated by post-stress induction of high activation positive affect (i.e., joviality) compared to the neutral mood condition, whereas clinical pain recovery in non-depressed patients will not differ based on mood condition assignment.
Method
Participants
Participants for this study were recruited in the Phoenix, Arizona metropolitan area through use of flyers and advertisements placed in local newspapers, physician offices, and community centers, and mailings to members of the Arthritis Foundation inviting them to participate in a longitudinal study of adaptation to chronic pain. Individuals who responded to these outreach efforts by calling the research team for more information and were willing to be screened were assessed for eligibility. To be eligible for the study, individuals had to meet the following criteria: (1) be female aged 18 or over, (2) have a physician-confirmed diagnosis of OA and/or FM, (3) report no autoimmune disorders, (4) report a pain rating above 20 on a 0 to 100 scale, and (5) report that they were not involved in litigation regarding their condition. In addition, individuals with OA were asked to report the highest level of pain they had experienced in the past month on a 0 to 100 scale; they needed to report that they had experienced clinical pain at a level greater than 40 within that time frame. This inclusionary criterion for OA participants ensured that they had recently experienced clinical pain levels comparable to those typical of FM participants recruited from the community [25]. The current study draws on health measures from the initial questionnaire and laboratory data gleaned from 110 women with pain due to OA (n = 38) and/or FM (n = 72) who underwent the laboratory stress session (described below).
Procedure
All procedures for the study were approved by the Institutional Review Board at Arizona State University. Participants for this study were screened by phone to determine initial eligibility and subsequently provided informed consent and written verification from their physicians regarding their FM and/or OA diagnosis. They then completed and returned an initial questionnaire packet that included assessment of demographics, depressive symptoms, and physical and mental health. Subsequently, one half of the participants were randomly selected to undergo a laboratory assessment of responses to a stress recall task and post-stress mood induction.
The laboratory session began between 1 pm and 3 pm. Women were seated in a comfortable chair, fitted with a blood pressure cuff, and asked to relax for 15 min. Next, they were asked to describe a recurring conflict that they had recently experienced with an important person in their lives that was “stressful in some way and that provoked strong feelings at the time.” During the 20-min stressor, an interviewer used the following prompts to elaborate participants' recall of the event: (1) who was involved in the conflict and when did it occur; (2) describe the conflict and the events that led up to it; (3) what emotions came up for you during the event; (4) what kinds of thoughts were you having during the event; (5) what was the worst part of the situation and why; and (6) how did you cope with the event [5]. The stress task followed a format used in prior work to mimic daily interpersonal stress in a laboratory environment [5, 10, 26]. Participants' responses to the prompts were coded to yield scores for conflict recency (less than 1 month, 1–6 months, greater than 6 months, greater than 1 year) and the identity of the network member involved (family, coworker, spouse, other).
Immediately following the stress interview, participants were randomly assigned by pain diagnosis (OA vs. FM) and depression status (described below) to view a 2.5-min video clip selected to induce either high activation positive mood or neutral mood (i.e., mood condition). The positive film clip was a scene from a family comedy, and the neutral clip was a scene from a documentary on ocean tide patterns across the globe. The clips were originally piloted in eight participants (all female, aged 30 to 60, depression status unknown) to assure that (1) both clips induced increases in the attentiveness subscale and (2) the positive clip but not the neutral clip induced increases in the joviality subscale of the Positive and Negative Affect Scale-Expanded Form (PANAS-X, described below). Pain, mood, and attentiveness ratings were obtained immediately following completion of each laboratory period.
Measures
Physical and Mental Health
Depressive symptoms were measured via the 20-item Center for Epidemiologic Studies-Depression Scale (CES-D) [27], a widely used measure that has good reliability and predictive validity in chronic pain patients [28]. Items were summed to create a score with a possible range of 0–60, with higher scores reflecting higher levels of depressive symptoms. The CES-D showed good internal consistency (Cronbach's α = 0.83). A cutpoint of 27 or higher was used to distinguish depressed versus non-depressed participants, consistent with the optimal cutoff value identified for individuals in chronic pain by Geisser and colleagues [29]. A cutoff score of 27 shows both good sensitivity and specificity in discriminating between depressed and non-depressed pain patients [29].
Physical and mental health were assessed with the RAND 36-Item Short Form Health Survey (SF-36) developed as part of the Medical Outcomes Study [30]. The measure is widely used to assess self-reported physical and mental quality of life in adults and performs well as a measure of health status [31]. For the current study, the SF-36 physical component and mental component summary scores were used as measures of functional health. Raw scores were transformed to a 0–100 point scale, with higher scores reflecting better functioning. Internal reliabilities were good for both components (Cronbach's α = 0.78 and 0.81 for physical and mental health components, respectively).
Pain, Affect, and Attention
Clinical pain was assessed via an item asking participants to rate their current pain on a 101-point scale, with ratings ranging from 0 = no pain to 100 = pain as bad as it can be [32]. Current jovial and despondent affect were assessed via items drawn from the PANAS-X [24]. Participants rated adjectives describing jovial affect (i.e., excited, happy, enthusiastic, cheerful) and three items describing despondent affect (i.e., blue, sad, ashamed) on a 5-point scale ranging from 1 = very slightly or not at all to 5 = extremely. Mean joviality and despondency scores were calculated for the baseline, stress, and mood induction periods. Internal consistencies for joviality were 0.86, 0.72, and 0.87, and for despondency were 0.76, 0.76, and 0.83 for baseline, stress, and mood induction periods, respectively. Mean attentiveness was assessed for the rest, stress, and mood induction periods by calculating the mean of two additional items (i.e., attentiveness, interest) gleaned from the PANAS-X. Items were moderately to highly correlated within each period (r values = 0.56–0.77, p values < 0.0001).
Analytic Strategy
The first step in the data analysis was to establish that mood condition groups were equivalent at baseline by conducting t test and chi-square comparisons of demographic, general physical and mental health, depression, and pain diagnoses. Comparability of resting levels of pain and mood measures for mood condition and depression groups were tested with a series of 2 (mood condition) × 2 (depression) analyses of variance (ANOVAs), with pain and mood scores as dependent variables. The main hypotheses that depression status, mood condition, and their interaction would predict pain and mood changes in response to the stress interview and mood induction were evaluated via a series of 2 (depression) × 2 (mood condition: neutral versus positive) × 3 (period: rest, stress, mood induction) repeated measures ANOVAs. The magnitude of the effects was evaluated through the use of partial eta squared effect sizes (η p 2), with values of 0.01, 0.06, and 0.16 reflecting small, medium, and large effect sizes, respectively [33].
Finally, the extent to which changes in mood could account for the relationship between a depression status × mood condition interaction and pain were evaluated through a series of regression analyses followed by product-of-coefficients analyses, as detailed by MacKinnon [34]. To that end, the regression analyses examined whether (1) the depressive symptom level × mood condition interaction predicted the proposed mood mediator, joviality (i.e., coefficient a), and (2) the mediator predicted the pain outcome with the independent variable, depressive symptom × mood condition, in the model (i.e., coefficient b). To evaluate whether the extent of mediation was significant, the product of the coefficients (a × b) was then calculated, and PRODCLIN [35] was employed to estimate asymmetric 95 % confidence limits for the mediated effects.
Significance was set at p < 0.05. To evaluate study hypotheses, significant interaction effects involving period and mood, depression, or both were followed by planned comparisons.
Results
Participant Characteristics and Baseline Comparisons
Table 1 shows the sample demographic and health characteristics, and the resting clinical pain and mood scores based on mood condition. Women comprising the sample reported an average age of 57 years (SD = 8.12; range = 37–72) and half indicated that they were married or living with a romantic partner. The sample was primarily Caucasian (91 %) and employed (56 %), and reported attending at least some college (84 %) and earning an annual household income greater than $30,000 (59 %). The average CES-D score was 20.6 (SD = 11.9). Twenty-nine percent of the 110 participants scored at or above a CES-D cutoff of 27 (13 % of women with OA and 37 % of women with FM), indicating a clinical level of depression. Roughly 50 % of the sample reported using antidepressant medication. Mood condition groups were comparable in terms of age, household income, educational attainment, ethnicity, and employment status (all p values > 0.35). A single difference in demographics emerged between mood condition groups: the neutral mood condition was characterized by a greater proportion of partnered individuals compared to the positive mood condition (χ 2 (1) = 9.39, p < 0.002). With regard to health status, mood condition groups were comparable on measures of physical and mental health, pain diagnosis and time since diagnosis, and antidepressant use (p values > 0.12). Moreover, scores on the SF-36 physical and mental health outcomes in the current sample were similar to those of other samples of patients with chronic pain in [36].
To evaluate whether groups differed on initial ratings of mood or clinical pain, 2 (depression) × 2 (mood condition: neutral versus positive) ANOVAs were conducted, with resting levels of despondency, joviality, clinical pain, and attentiveness serving as dependent measures. Results yielded only two marginally significant effects: depressed individuals had marginally lower joviality and attentiveness scores at rest compared to non-depressed individuals [F values (1, 106) > 3.44, p values < 0.06]. Levels of the mood and pain variables based on depression and mood condition at rest, stress, and mood induction period of the laboratory session are presented in Table 2.
Overall, these findings suggest that random assignment to mood condition yielded groups that were largely comparable in terms of demographic background, health status, and resting levels of clinical pain and mood.
Stress and Mood Manipulation Effects: Depression as a Moderator
In the sample as a whole, the conflict selected for discussion occurred recently; 67 % reported that it occurred less than 1 month, 18 % 1 to 6 months prior, 9 % less than 1 year prior, and 6 % greater than 1 year prior. The conflict involved a family member (54 %), coworker (13 %), spouse/partner (12 %), or other individual (21 %). Depressed and non-depressed participants were similar in terms of the recency of the conflict event (χ 2 (3) = 6.83, ns) and the identity of the other individual involved (χ 2 (3) = 1.17, ns).
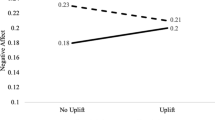
Figure 1 depicts levels of despondency across the session based on depression group and mood condition, and shows that despondency changed over time, period effect F (2, 212) = 81.17, p < 0.0001, η p 2 = 0.43. Planned comparisons revealed that despondency levels increased from rest to stress and then declined during the mood induction period to resting levels for all groups (p values < 0.05). No other main effects or interaction terms achieved significance. Thus, the stress manipulation had its intended effect on levels of despondency, but depression did not enhance stress-related increases in despondency nor impede recovery during the post-stress mood induction. Moreover, inducing a positive mood did not alter the extent to which despondency levels declined following stress.
Figure 2 depicts levels of joviality across the session based on depression group and mood condition. Results showed that joviality changed over time [period effect F (2, 212) = 23.21, p < 0.0001, η p 2 = 0.18], but the magnitude of the change depended on mood condition [period × mood condition interaction F (2, 212) = 10.41, p < 0.0001, η p 2 = 0.09], and the combination of depression and mood condition [period × depression × mood condition interaction, F (2, 212) = 3.26, p < 0.04, η p 2 = 0.03]. Planned comparisons indicated that joviality significantly decreased from baseline to stress to a similar extent in depressed and non-depressed participants (M change = −0.28 and −0.60 for depressed and non-depressed groups, respectively; ns). Although joviality increased from stress to mood induction, the magnitude of the increase varied based on both depression status and mood condition. Among depressed participants, those assigned to the neutral mood condition showed no increase in joviality whereas those assigned to the positive mood condition showed a substantial increase (M change = −0.18 and 1.00 for neutral and positive mood conditions, respectively; p < 0.05). In contrast, among non-depressed participants, those assigned to the neutral and positive mood conditions showed comparable, significant increases in joviality from stress to mood induction (M change = 0.61 and 0.99 for neutral and positive mood conditions, respectively; ns). At post-mood induction, depressed individuals had lower levels of joviality than non-depressed individuals in the neutral mood condition (p < 0.05); in contrast, depressed and non-depressed individuals had similar levels of joviality in the positive mood condition (ns). In sum, depression did not enhance stress-related decreases in joviality, but did dampen joviality recovery following stress for those in the neutral mood condition. However, the impairment in recovery of joviality among the depressed versus non-depressed groups in the neutral mood condition was offset in the positive mood condition.
Figure 3 depicts levels of clinical pain across the session based on depression group and mood condition, and largely mirror patterns for joviality. Results showed that pain ratings changed over time [period effect F (2, 212) = 9.78, p < 0.0001, η p 2 = 0.08], but the magnitude of the change varied by mood condition [period × mood condition interaction F (2, 212) = 3.15, p < 0.05, η p 2 = 0.03], and the combined effects of depression and mood condition [period × depression × mood condition interaction F (2, 212) = 4.80, p < 0.009, η p 2 = 0.04]. Planned comparisons indicated that pain significantly increased from baseline to stress to a similar extent in depressed and non-depressed participants (M change = 7.16 and 1.17 for depressed and non-depressed groups, respectively; ns). Although pain decreased from stress to mood induction, the magnitude of the decrease varied based on both mood condition and depression. Among depressed participants, those assigned to the neutral mood condition showed no decrease in pain, whereas those assigned to the positive mood condition showed a substantial decrease (M change = −0.67 and −17.71 for neutral and positive mood conditions, respectively; p < 0.05). In contrast, among non-depressed participants, those assigned to the neutral and positive mood conditions showed comparable, significant decreases in pain from stress to mood induction (M change = −9.09 and −7.63 for neutral and positive mood conditions, respectively; ns). At post-mood induction, depressed individuals had higher levels of pain than non-depressed individuals in the neutral mood condition (p < 0.05); in contrast, depressed and non-depressed individuals had similar levels of pain in the positive mood condition (ns). Taken together, the findings suggest that depression did not enhance stress-related increases in pain, but did impede pain recovery following stress for those in the neutral mood condition. However, the impairment in pain recovery among the depressed versus non-depressed groups in the neutral mood condition was offset in the positive mood induction.
To evaluate whether the depression status × mood condition effects on joviality and pain could be due to differential engagement with either the stress task or the mood induction, a repeated measures ANOVA evaluated change in attentiveness ratings over the session. Results indicated that attentiveness increased over the course of the session [period F (2, 212) = 19.30, p < 0.0001, η p 2 = 0.15], but the magnitude of the change did not vary over time based on depression status, mood condition, or their interaction (all p values > 0.29) (see Table 2). These findings suggest that the participants in all groups were equally attentive to the stress task and the mood inductions.
Because initial comparisons of mood condition groups indicated that participants in the neutral versus positive mood condition were more likely to be married or partnered, analyses that evaluated stress reactivity and recovery in pain and mood reports were repeated, including partner status as a covariate. The findings indicated that partner status was unrelated to stress reactivity and recovery on any outcome measure, and including partner status as a covariate did not alter the main findings, reported above. Thus, differences in clinical pain and joviality responses between mood condition and depression groups are not accounted for by partner status differences.
Joviality as a Mediator of Post-Stress Pain Recovery
Finally, two regression models evaluated whether the depression × mood condition effect on change in clinical pain from stress to mood induction was mediated by change in jovial mood. (The depression × mood condition interaction was unrelated to variation in despondency, indicating that the a path in the mediational chain was nonsignificant. Thus, despondency was not tested as a potential mediator.) [34] These models are depicted in Table 3. Table 3, model A, presents analyses to derive coefficient a, the link between the independent variable (i.e., depression × mood condition interaction) and the purported mediator (i.e., change in jovial mood). Table 3, model B, presents analyses to derive coefficient b, the link between the mediator and the outcome (i.e., clinical pain recovery following stress), controlling for the independent variable. Result of mediation tests indicated that the depression × mood condition interaction → change in pain recovery relation was significantly mediated by change in joviality (indirect effect = −0.25926, 95 % CI = −0 0.5936, −0.0258). Of note, although it was diminished in magnitude, the direct effect of depression × mood condition on pain recovery remained significant, indicating only partial mediation. About 22 % of the relation was mediated by change in joviality. Together, these findings suggest that the beneficial effect of positive mood induction on clinical pain recovery for depressed patients was mediated in part by increases in joviality.
Discussion
Investigating the impact of current depression on pain regulation in chronic pain patients has important implications for understanding their longer term adaptation. In the present study, we examined whether depression among women in chronic pain predicts disturbance in two specific processes that are implicated in the regulation of pain—emotional reactivity to and recovery from stress. We further examined whether a specific emotion regulation strategy—positive mood induction—is effective in reducing post-stress clinical pain in depressed women. The results of this study indicated that stress-related decrements in jovial mood and exacerbations in despondent mood and clinical pain were comparable in depressed and non-depressed pain patients, contrary to expectation. Depressed patients did show deficits compared to non-depressed patients in their capacity to rebound following stress in terms of their joviality and clinical pain levels in the neutral mood condition, but these deficits were completely eliminated if women were exposed to a positive mood induction, as expected. Moreover, the post-stress benefits of the positive mood induction for clinical pain among depressed patients were mediated in part by improvements in joviality. These results suggest that both depressed and non-depressed individuals show immediate stress-related changes in affect and clinical pain of comparable magnitude to personally relevant stress; however, non-depressed individuals show a natural capacity to repair their mood and clinical pain following stress whereas depressed individuals recover only when provided with access to a potent external source of positive mood.
Depression did not worsen stress-related pain and mood reports in the current study, an unexpected finding. However, an earlier investigation also reported that depressive symptoms were unrelated to changes in positive and negative mood following a stress interview in a sample of OA patients [10]. One plausible explanation for the lack of a difference between groups in stress reactivity is the constraints imposed by the structure of the interpersonal stress tasks used in both studies. In each case, participants were interviewed in detail by a research team member. In the current study, this format called for discussion of the same type of stressor, conflict, and required a similar level of engagement and processing of the conflict for depressed and non-depressed individuals. In fact, participant reports regarding the recency of the conflict, the identity of the individual involved, and the primary emotion the conflict elicited were similar for depressed and non-depressed individuals. Moreover, ratings of attentiveness increased to a similar extent from baseline to the stressor among depressed and non-depressed women. The evidence linking depression with enhanced stress-related changes in pain and mood to this point has been derived primarily from diary studies that assess a wide variety of stressors in everyday life [37, 38]. It may be that in naturalistic settings, depressed individuals are more likely to show pronounced negative cognitive biases in attention to and processing of stressful events [39]. Hence, differences in stress reactivity based on depression status may be maximized in more real-world contexts, where individuals are active agents in determining the stressors they experience and the quality of their responses to those stressors.
Stress routinely provokes increases in pain among chronic pain patients [4, 40], but one antidote that appears to help restore homeostasis following stress is positive affect. Resilient individuals appear to “bounce back” physiologically from stressful experiences quickly through use of positive emotions [41, 42], a pattern evidenced by the non-depressed individuals in the current study. On the other hand, depressed individuals examined here were not as resilient; they were unable to rebound quickly from the dampened joviality and increased pain evoked by stress on their own. Yet when depressed patients were exposed to a film intervention to boost positive mood, they showed even more robust pain recovery than did the non-depressed patients, recovery that was partly due to their increased joviality. Thus, bolstering positive emotions in depressed individuals with chronic pain appears to give them the help they need to “undo” the effects of stress [11].
A growing body of evidence gleaned from diary reports of pain patients consistently points to the link between daily boosts in state positive affect and same-day decreases in clinical pain [43–45]. Because diary findings in those studies were correlational, the directionality of the positive affect–pain relationship has remained an open question. Experimental manipulations of negative emotions have documented their role in amplifying clinical pain (e.g., 46). However, to our knowledge, the current study is the first to document the causal role of positive emotions in dampening clinical pain in pain patients.
Although use of a positive film clip produced substantial, immediate increases in positive mood in depressed participants, it is possible and perhaps even likely that the elevations would be difficult for depressed participants to sustain over time. In a recent investigation employing fMRI, depressed and non-depressed individuals were exposed to emotion slides for a 36-min period [47]. Both depressed and non-depressed individuals showed comparable increases in positive emotion and neural activation in response to pleasant slides for the first half of the slide exposure. However, only non-depressed individuals could sustain positive affective responding over the second half of the session. Thus, a focus on promoting the development of habits that lead to frequent, brief, and sustainable opportunities for positive engagement may be a fruitful avenue for addressing positive emotion deficits among the depressed, including those in chronic pain.
The study had several strengths, the most important of which is its experimental design. The positive mood induction was contrasted with a neutral mood condition designed to control for the effects of attending to a video stimulus. Random assignment to mood condition by both depression status and pain condition (OA versus FM) yielded groups that were comparable in demographic, health, and mood and pain measures, increasing confidence that the findings are not due to confounding factors. A second strength is the use of a standardized stress induction procedure designed to capture personally relevant stress common in everyday life. Third, the study had a large sample size, yielding adequate power to detect small-to-moderate depression status × mood condition interactions.
Several limitations are noteworthy because they have implications for the interpretation of the findings. First, we categorized participants as depressed versus not depressed based on their scores on a depression inventory instead of a diagnostic interview. Although we used a high cutoff score to increase the likelihood that we captured clinical levels of depression in a pain sample, nevertheless, the gold standard diagnostic method is a validated, structured interview. A second limitation is the reliance on self-report measures to assess emotion and pain outcomes. A growing literature draws on new imaging technologies to elaborate the neural mechanisms underlying depression, emotion, and pain regulation, and use of such methods in the future may provide an integrated view of these interlocking processes. Third, despite random assignment to mood condition, groups were not comparable on relationship status. Statistically controlling for this group difference by including relationship status as a covariate did not change any of the findings; however, only replication of the study with equivalent groups can establish with certainty that the mood induction itself accounted for the benefits in clinical pain and joviality. Fourth, we induced changes in neutral and jovial mood states via use of film clips. Although films are reliable elicitors of emotion, they represent only one type of possible stimulus—one that is external, visual, and auditory. Replicating these findings using alternative means of eliciting emotion and eliciting emotions across a range that includes low arousal levels (e.g., serenity) are important. In particular, it will be useful to contrast the stress recovery benefits of an external source of positive affect, like a film clip, with one generated by individuals themselves. Some data suggest that depressed individuals may find it difficult to use their own positive distractors to turn attention away from distressing information even though they understand the utility of such an approach [14]. Fifth, only one third of the women in the sample were diagnosed with OA, and of those, only 13 % were categorized as depressed. As a result, the study did not have sufficient power to examine whether the depression × mood condition effects varied by pain condition. Finally, because our sample included only women with chronic pain due to FM and/or OA, these findings cannot generalize to men, or to individuals with other types of chronic pain conditions.
In summary, our findings provide evidence that depressed and non-depressed pain patients show equivalent mood and clinical pain responses to personally relevant stress, but depressed patients are able to recover following stress only when they receive a substantial external boost in positive emotion. Although depressed pain patients show deficits in positive emotion regulation, particularly in the context of stress recovery, these individuals can experience rewarding emotions when presented with a ready, potent source of positive stimuli. To our knowledge, this is the first study to test the causal effects of a positive mood induction on pain recovery in chronic pain patients based on depression status, and findings suggest that increasing attention to the promotion of positive engagement as a key aspect of treatment for chronic pain is warranted.
References
Bair M, Robinson R, Katon W, Kroenke K. Depression and pain comorbidity: A literature review. Arch Intern Med. 2003; 163: 2433-2445.
Kerns RD, Haythornthwaite JA. Depression among chronic pain patients: Cognitive–behavioral analysis and effect on rehabilitation outcome. J Consult Clin Psychol. 1988; 56: 870-876.
Keefe FJ, Wilkins RH, Cook WA Jr, Crisson JE, Muhlbaier LH. Depression, pain, and pain behavior. J Consult Clin Psychol. 1986; 54: 665-669.
Schwartz L, Slater MA, Birchler GR. Interpersonal stress and pain behaviors in patients with chronic pain. J Consult Clin Psychol. 1994; 62: 861-864.
Davis MC, Zautra AJ, Reich JW. Vulnerability to stress among women in chronic pain from fibromyalgia and osteoarthritis. Ann Behav Med. 2001; 23: 215-226.
van Middendorp H, Lumley MA, Moerbeek M, et al. Effects of anger and anger regulation styles on pain in daily life of women with fibromyalgia: A diary study. Eur J Pain. 2010; 14: 176-182.
Zautra AJ, Smith BW. Depression and reactivity to stress in older women with rheumatoid arthritis and osteoarthritis. Psychosom Med. 2001; 63: 687-696.
Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Pers Psychol Sci. 2008; 3: 400-424.
Joormann J. Cognitive inhibition and emotion regulation in depression. Curr Dir Psychol Sci. 2010; 19: 161-166.
Devellis RF, Carl KL, Devellis BM, Blalock SJ, Patterson CC. Correlates of changes in mood following a mood induction in osteoarthritis patients. Arthritis Rheum. 1998; 11: 234-242.
Fredrickson BL, Branigan C. Positive emotions broaden the scope of attention and thought–action repertoires. Cogn Emot. 2005; 19: 313-332.
Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol. 2005; 73: 212-220.
Joormann J, Siemer M. Memory accessibility, mood regulation, and dysphoria: Difficulties in repairing sad mood with happy memories? J Abnorm Psychol. 2004; 113: 179-188.
Wenzlaff RM, Wegner DM, Roper DW. Depression and mental control: The resurgence of unwanted negative thoughts. J Pers Soc Psychol. 1988; 55: 882-892.
Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008; 58: 26-35.
Epstein SA, Kay G, Clauw D, et al. Psychiatric disorders in patients with fibromyalgia: A multicenter investigation. Psychosomatics. 1999; 40: 57-63.
Aguglia A, Salvi V, Maina G, Rossetto I, Aguglia E. Fibromyalgia syndrome and depressive symptoms: Comorbidity and clinical correlates. J Affect Disorders. 2011; 128: 262-266.
Hawker GA, Gignac MA, Badley E, et al. A longitudinal study to explain the pain–depression link in older adults with osteoarthritis. Arthrit Care Res. 2011; 63: 1382-1390.
Gleicher Y, Croxford R, Hochman J, Hawker G. A prospective study of mental health care for comorbid depressed mood in older adults with painful osteoarthritis. BMC Psychiatry. 2011; 11: 147.
Sale JE, Gignac M, Hawker G. The relationship between disease symptoms, life events, coping and treatment, and depression among older adults with osteoarthritis. J Rheumatol. 2008; 35: 335-342.
Kurtze N, Gundersen KT, Svebak S. Quality of life, functional disability and lifestyle among subgroups of fibromyalgia patients: The significance of anxiety and depression. Brit J Med Psychol. 1999; 72: 471-484.
Homann D, Facco Stefanello JM, Goes SM, Breda C, Paiva Edos S, Leite N. Stress perception and depressive symptoms: Functionality and impact on the quality of life of women with fibromyalgia. Revista Brasileira De Reumatologia. 2012; 52: 319-330.
Dailey P, Bishop G, Russell I, Fletcher E. Psychological stress and the fibrositis/fibromyalgia syndrome. J Rheumatol. 1990; 17: 1380-1385.
Watson D, Clark LA: Manual for the Positive and Negative Affect Schedule—Expanded Form. Ames: University of Iowa, 1994.
Giesecke T, Williams DA, Harris RE, et al. Subgrouping of fibromyalgia patients on the basis of pressure–pain thresholds and psychological factors. Arthritis Rheum. 2003; 48: 2916-2922.
Dimsdale J, Stern M, Dillion E. The stress interview as a tool for examining physiological reactivity. Psychosom Med. 1998; 50: 64-71.
Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. App Psych Meas. 1977; 1: 385-401.
Turk DC, Okifuji A. Detecting depression in chronic pain patients: Adequacy of self-reports. Beh Res Ther. 1994; 32: 9-16.
Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: A comparative analysis. Clin J Pain. 1997; 13: 163-170.
Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992; 30: 473-483.
McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993; 31: 247-263.
Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: A comparison of six methods. Pain. 1986; 27: 117-126.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988.
MacKinnon DP. Introduction to statistical mediation analysis. New York: Lawrence Erlbaum; 2008.
MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Beh Res Meth. 2007; 39: 384-389.
Garratt AM, Ruta DA, Abdalla MI, Buckingham JK, Russell IT. The SF 36 Health Survey Questionnaire: An outcome measure suitable for routine use within the NHS? Brit Med J. 1993; 306: 1440-1444.
Zautra AJ, Smith BW. Depression and reactivity to stress in older women with rheumatoid arthritis and osteoarthritis. Psychosomatic Medicine. 2001; 63: 687-696.
Tennen H, Affleck G, Zautra A. Depression history and coping with chronic pain: A daily process analysis. Health Psychol. 2006; 25: 370-379.
Joormann J, Siemer M. Affective processing and emotion regulation in dysphoria and depression: Cognitive biases and deficits in cognitive control. Soc Pers Psychol Comp. 2011; 5: 13-28.
Affleck G, Tennen H, Urrows S, Higgins P. Person and contextual features of daily stress reactivity: Individual differences in relations of undesirable daily events with mood disturbance and chronic pain intensity. J Pers Soc Psychol. 1994; 66: 329-340.
Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. J Pers Soc Psychol. 2004; 86: 320-333.
Fredrickson BL, Mancuso RA, Branigan C, Tugade MM. The undoing effect of positive emotions. Motiv Emotion. 2000; 24: 237-258.
Finan PH, Quartana PJ, Smith MT. Positive and negative affect dimensions in chronic knee osteoarthritis: Effects on clinical and laboratory pain. Psychosom Med. 2013; 75: 463-470.
Litt MD, Shafer D, Napolitano C. Momentary mood and coping processes in TMD pain. Health Psychol. 2004; 23: 354-362.
Litt MD, Shafer DM, Ibanez CR, Kreutzer DL, Tawfik-Yonkers Z. Momentary pain and coping in temporomandibular disorder pain: Exploring mechanisms of cognitive behavioral treatment for chronic pain. Pain. 2009; 145: 160-168.
van Middendorp H, Lumley MA, Jacobs JW, Bijlsma JW, Geenen R. The effects of anger and sadness on clinical pain reports and experimentally‐induced pain thresholds in women with and without fibromyalgia. Arthrit Care Res. 2010; 62: 1370-1376.
Heller AS, Johnstone T, Shackman AJ, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. P Natl Acad Sci USA. 2009; 106: 22445-22450.
Acknowledgments
The authors wish to acknowledge grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR046034: Alex J. Zautra, P.I.).
Conflict of Interest
The authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Davis, M.C., Thummala, K. & Zautra, A.J. Stress-Related Clinical Pain and Mood in Women with Chronic Pain: Moderating Effects of Depression and Positive Mood Induction. ann. behav. med. 48, 61–70 (2014). https://doi.org/10.1007/s12160-013-9583-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12160-013-9583-6