Abstract
Background
Intensive lifestyle intervention trials in type 2 diabetes contribute evidence on what can be achieved under optimal conditions, but are less informative for translation in applied settings.
Purpose
Living Well with Diabetes is a telephone-delivered weight loss intervention designed for real-world delivery.
Methods
This study is a randomized controlled trial of telephone counseling (n = 151) versus usual care (n = 151); 6-month primary outcomes of weight, physical activity, HbA1c; secondary diet outcomes; analysis was by adjusted generalized linear models.
Results
Relative to usual care, telephone counseling participants had small but significantly better weight loss [−1.12 % of initial body weight; 95 % confidence interval (CI) −1.92, −0.33 %]; physical activity [relative rate (RR) = 1.30; 95 % CI, 1.08, 1.57]; energy intake reduction (−0.63 MJ/day; 95 % CI, −1.01, −0.25); and diet quality (3.72 points; 95 % CI, 1.77, 5.68), with no intervention effect for HbA1c (RR = 0.99; 95 % CI, 0.96, 1.01).
Conclusions
Results are discussed in light of challenges to intervention delivery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid increase in the prevalence of type 2 diabetes, obesity, and associated complications is a major public health problem in most developed and many developing countries [1]. In Australia, data from the 1999–2000 Australian Diabetes and Lifestyle (AusDiab) study estimated that approximately 1 million (7.4 %) Australian adults aged 25 years and over have type 2 diabetes [2], while 60 % are overweight or obese [3], similar to prevalence rates reported in the USA [4] and UK [5].
Weight loss and physical activity are first line approaches in the treatment of type 2 diabetes and its related morbidities [6, 7]. There is substantial evidence that intensive, and most often, clinic-based, lifestyle interventions involving frequent participant contact will produce significant weight loss (5–7 % of body weight) as well as concomitant improvements in glycemic control and dyslipidemia in those with type 2 diabetes [8–10]. The Look AHEAD trial in the USA, which evaluated a multi-year, intensive lifestyle intervention with the aim of reducing the incidence of cardiovascular disease events in type 2 diabetes, is a landmark trial in this regard [11]. However, the intensity of resources involved in intervention delivery and the often highly selected nature of trial participants limit the generalizability of findings from such studies [12]. While these trials have made substantial contributions to the evidence on what is possible to achieve under optimal conditions, they are less informative about what is feasible to achieve in applied settings.
Type 2 diabetes is managed predominately in the primary care setting, with an emphasis on monitoring glycemic control and cardiovascular and neurological complications, with concomitant medication management. While lifestyle advice is part of guideline concordant care [13], intensive lifestyle intervention is not routinely feasible in the general practice setting. Patients are often referred to hospital or community-based weight loss/lifestyle programs, but only a minority of patients with type 2 diabetes attend [14], and such programs are not universally available outside of major metropolitan areas. Thus, there is a need for feasible, effective, broad reach approaches to support the growing numbers of patients with type 2 diabetes to achieve and maintain glycemic control via weight loss and improved physical activity.
Telephone-delivered lifestyle interventions have the potential for widespread and cost-effective population reach and for integration as a primary care referral source. Two systematic reviews have found very strong support for their efficacy in improving physical activity and dietary behaviors, both in healthy adults and those with chronic conditions [15, 16]. A growing number of trials have evaluated telephone-delivered interventions specifically targeting weight loss, with many demonstrating significant intervention effects compared to a control group [17–20]. Only a small number of trials have evaluated telephone-delivered diabetes self-management interventions [21–26]. Most had a primary emphasis on medication management, with less emphasis on weight loss and changes in behaviors that are recommended as part of diabetes management (i.e., physical activity and diet; [13]). The consistency of reporting on weight loss and related health behaviors was also mixed in these trials, as were results for these outcomes. In addition, limited attention was given to sample representativeness.
This paper describes the 6-month outcomes of the Living Well with Diabetes (LWWD) trial which is evaluating a telephone-delivered behavioral weight loss intervention targeting improved glycemic control in adults with type 2 diabetes recruited from primary care practices, compared to usual care. As in the Look AHEAD trial, medical management and related medication adherence issues were the domain of the primary care physician or specialist, allowing LWWD to work in concert with primary care to provide a lifestyle-focussed intervention not possible to be delivered in the context of busy primary care visits. The 6-month endpoint in the LWWD trial corresponds to the end of the intensive phase of intervention involving the highest call frequency, with a 12-month maintenance phase to follow. As described in detail elsewhere [27], intervention protocols were adapted for telephone delivery from clinical practice guidelines for overweight and obesity [6, 9]; our previous trial [28]; and protocols used in the Look AHEAD trial [29, 30]. Thus, the LWWD trial is a pragmatic trial [31], designed to inform translation of intensive lifestyle change and weight loss interventions, such as Look AHEAD, into a feasible broad reach delivery model. It was predicted that compared to usual care, telephone counseling would result in greater changes in the primary outcomes of weight loss, increased physical activity and improved glycemic control; and in reduced energy intake and improved diet quality.
Methods
Trial methods are described here in brief as they have been presented in detail previously [27]. Living Well with Diabetes is a two-arm randomized controlled trial. Ethical approval was granted from The University of Queensland Behavioral and Social Sciences Ethical Review Committee. Participants were recruited from nine general practices in the city of Logan (population 270,000), a large ethnically and socioeconomically diverse community in the state of Queensland (Australia), 35 km from Brisbane (the state capital), an urban center of 1.8 million residents.
Patient Recruitment and Randomization
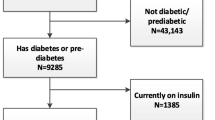
Within practices, 1,407 eligible patients (i.e., diagnosed type 2 diabetes; aged 20–75 years; and having a listed telephone number) were identified using electronic medical records (Fig. 1). Patients not initially excluded by General Practitioner (GP) screening for contraindications to unsupervised physical activity (n = 499) were posted study materials by the GP and if not declining further contact (n = 206), were followed up by study staff to ascertain eligibility and to solicit informed consent. Patients were eligible if they were inactive (self-reported <5 days/week of ≥30 min planned exercise) and/or overweight or obese [body mass index (BMI) ≥25.0 kg/m2], did not currently use weight loss medications, and no previous or planned bariatric surgery. Of the potential participants who were able to be reached via telephone and were established to be eligible (n = 420), 302 (71.9 %) agreed to participate, completed the baseline assessment and were randomized to receive either Telephone Counseling or Usual Care.
Randomization was by the minimization method [32] using the MINIM program (www.sghms.ac.uk/depts/phs/guide/randser.htm). The minimization method aimed to balance treatment groups across the following prognostic factors (without weighting for importance): gender; age (≥55 years); BMI (≥40 kg/m2); HbA1c (≥8 %); self-reported physical activity level (meeting guidelines of ≥150 min and ≥5 days per week) [33]; and self-reported diabetes management (i.e., insulin or combination therapy, traditional oral hypoglycemic medications, new agents, or lifestyle alone). Glucagon-like peptide-1 receptor agonists (e.g., Exenatide) and dipeptidyl peptidase-4 inhibitors (e.g., Sitagliptin) were considered separately as these new agents may cause less weight gain than traditional diabetes medications [34, 35].
Usual Care
Usual Care participants were mailed a brief summary of their assessment results following each assessment, as well as standard, off-the-shelf diabetes self-management education brochures.
Telephone-Delivered Weight Loss Intervention
The weight loss intervention, delivered entirely over the telephone, used a combined approach of increasing physical activity, reducing energy intake, and behavioral therapy [6, 9]. Participants received a detailed workbook at the commencement of the intervention and approximately 14 telephone calls over the first 6 months (four initial weekly calls followed by fortnightly calls), to support initiation of weight loss. The intervention followed a motivational interviewing approach [36] grounded in Social Cognitive Theory constructs of self-efficacy, social support, and outcome expectancies [6, 9], and emphasized building participant skills in behavior change strategies. Accordingly, telephone counsellors worked with participants to identify the benefits of weight loss and lifestyle change, set goals for small, gradual changes to physical activity and dietary intake, self-monitor progress, problem solve, utilize available supports, and focus on achievements with appropriate rewards [6, 37]. Specific intervention targets for weight loss, physical activity, and dietary intake were consistent with management goals for type 2 diabetes [13], with the aim to reduce glycosylated haemoglobin (HbA1c) to less than 7 % [6, 38, 39]. Participants were encouraged to achieve moderate weight loss of 5–10 % of initial body weight, with a loss of 1–2 kg per month [13, 40]. A target of at least 210 min per week (30 min every day) of moderate-intensity, planned activity was recommended, consistent with the level of physical activity necessary to promote weight loss [39], along with resistance exercise (two to three sessions/week) [41]. To allow for specific food preferences and approaches, individualised advice [6, 13, 42] was used to encourage participants to reduce daily energy intake by 2 MJ by following healthy eating principles, including following a low-fat diet (i.e., total fat <30 % of energy and saturated fat <7 % of energy) with sufficient dietary fiber (25 g/day for women and 30 g/day for men) [43]. Participants were provided with a pedometer to monitor daily steps and with a set of digital scales to monitor their body weight. Fidelity of intervention delivery was monitored via feedback to counselors following randomly taped telephone calls and fortnightly clinical supervision meetings. Call attempts, completions, and duration were tracked in the trial database.
Primary and Secondary Outcomes and Data Collection
Primary outcomes were weight, accelerometer-derived moderate to vigorous intensity physical activity, and HbA1C. Secondary outcomes were energy intake and diet quality. Data were collected at baseline and 6 months via nurse home visits and telephone interviews by registered nurses and research staff blind to participants’ group allocation. Weight was measured in duplicate, without shoes or heavy clothing, using standard calibrated scales (Model TI TBF 350, Tanita Inc., Tokyo, Japan) to the nearest 0.1 kg. Height was measured in duplicate at baseline only using a portable stadiometer (Seca 214 height rod, Seca, Germany). Blood samples were taken by registered nurses early in the morning after an overnight fast (at least 10 h), with participants instructed not to take any glucose-lowering medication prior to the assessment. Current diabetes medications were recorded. HbA1c was measured from whole blood samples by the high-performance liquid chromatography method (Bio-Rad Variant II, Sydney, Australia).
Nurses provided participants with a GT1M accelerometer (Actigraph, LLC, Fort Walton Beach, Florida) to collect physical activity data. This activity monitor, which primarily detects ambulatory movement, was fitted firmly around the waist by elasticised band and positioned on the right midaxillary line. Monitors were set to record in 1-min epochs. Participants were instructed to wear the monitor at all times while awake (except during water-based activities) for a continuous period of 7 days, and to record time worn in a log. Wear time was ascertained by research staff, who compared the monitor data with participants’ wearing logs to determine the precise times movement stopped or began that coincided with participant self-reported wear/removal periods. Using SAS 9.2 (SAS Institute Inc., Cary, NC), moderate to vigorous activity was identified as time spent at ≥1,952 counts/min (cpm; [44]) during worn time on valid days (i.e., ≥10 h of wear, without any excessive counts ≥20,000 cpm). Mean moderate to vigorous activity on valid days was multiplied by seven to yield a weekly estimate of physical activity, with at least one valid day of wear required. Accelerometer compliance was high, with almost all participants (98 %; 97 %; 297/302) and 6-month completers (264/272) providing at least four valid days of data at baseline and 6 months, respectively. Mean (±standard deviation) daily wear time was 13.5 ± 1.6 h at baseline and 13.7 ± 1.7 at 6 months.
Telephone interviews included a previously validated food frequency questionnaire that asked about intake over the previous month (Dietary Questionnaire for Epidemiological Studies, version 2, Cancer Council Victoria, Australia). The questionnaire estimates intakes of most nutrients and energy accurately (within 10 %) and does not systematically under- or over-estimate against weighed records [45]. Coupled with the NUTTAB95 nutrient composition database [46], this questionnaire was used to derive average daily energy intake and nutrient intake. Overall dietary quality was summarised in terms of the revised Diet Quality Index score [47, 48], which ranges from 0 (worst) to 100 (best) quality in terms of 10 dietary characteristics—total fat, saturated fat, dietary cholesterol, fruit, vegetables, grains, calcium, iron, dietary diversity, and dietary moderation—relative to current Australian dietary recommendations in the version used here [49, 50]. Demographic data were also collected during the telephone interview.
Statistical Analyses
Analyses were performed in SPSS version 20.0 (IBM Corp). Significance was set at p < 0.05, two-tailed. The sample size had been chosen to ensure at least 90 % power (with two-tailed significance of 5 %) to detect minimum differences of interest in primary outcomes of 5 % weight loss, 0.6 % HbA1c, and 60 min/week physical activity and provided adequate (≥80 %) power for differences in diet (2 MJ energy intake and 1/2 a standard deviation diet quality). The trial was not powered a priori for moderation analyses.
Significance of changes within groups was assessed by paired t tests (normal data) or signed ranks test (physical activity). Analyses were by generalized linear models with normal distribution and identity link for data that followed an approximately normal distribution (weight loss, log-transformed HbA1c, energy intake, and diet quality) or with a gamma distribution and log link for physical activity, which approximately followed a gamma distribution. Means for each group and differences between groups are reported with corresponding 95 % confidence intervals (CI) from these models. Means for HbA1c are presented as back-transformed means; for HbA1c and physical activity, differences between groups are presented in exponentiated form, as rate ratios (RR, i.e., ratio of mean for Telephone Counseling/Usual Care). There was no evidence of collinearity (variance inflation factors all <2.5) or outliers (Cook’s distance <1). Plots of residuals versus predicted values suggested no problems due to non-normality or heteroscedasticity. Models adjusted for baseline values, potential confounders partly controlled through minimization (i.e., baseline age, gender, HbA1c, BMI, accelerometer-assessed physical activity, nurse-assessed diabetes medications), progression onto diabetes medication or onto insulin from baseline to 6 months, and other a priori identified potential confounders that had some association with at least one outcome at p < 0.1 [i.e., baseline employment (retired yes/no), smoking status (never-/ex-/current), cardiovascular-related condition (cardiovascular disease, stroke, hypertension, high cholesterol), musculoskeletal condition (arthritis, osteoporosis), and lung disease]. Duration of diabetes, income, education, use of weight loss aids, and depression/anxiety had no association at p < 0.1 with any outcome; intervention effects were unchanged (to within 20 %) by removal of these variables from models. Moderation of weight loss by participant characteristics and baseline behaviors was examined by adding interaction terms to the models; only results of p < 0.1 are reported.
Missing data (12.6 % Telephone Counseling, 7.3 % Usual Care) were handled using the baseline-value-carried-forward (BVCF) method, to bias results towards the null in view of the possible systematic loss of participants who were not benefitting from the program. Completers analysis (n = 136 Telephone Counseling, n = 141 Usual Care) examined the extent to which results were affected by assuming no change among dropouts (i.e., BVCF). A per-protocol analysis (in completers) examined results for those who completed the majority of the telephone counseling program (i.e., ≥11 calls).
Results
Baseline characteristics of the study participants are detailed in Table 1. The sample had a mean (±standard deviation, SD) age of 58.0 (±8.6) years and a median duration of diabetes of 5 years (25th, 75th percentile, 2; 10 years). Nearly all participants were either overweight (26.2 %) or obese (68.2 %), over two thirds were not engaging in guideline levels of physical activity (69.5 %), most were Caucasian (87.4 %), and 56.3 % were men. Compared with the general diabetes population as reported from the large AusDiab study, study participants were similar in terms of gender, use of insulin, median duration of diabetes, and HbA1c, but were more likely to use traditional oral hypoglycemic medication [Electronic Supplementary Material (ESM) Table 1], and, consistent with the study inclusion criteria, were slightly younger and less variable in age, more commonly obese, and had a lower prevalence of cardiovascular disease. Participation rate was high (72 % of those reached and eligible), and participants mostly did not differ from non-participants (ESM Table 2) except for statistically significant differences in self-report-derived BMI, smoking status, educational level, and diabetes duration. Loss to follow-up was minimal and non-differential, with 87.4 % of the telephone counseling group and 92.7 % of the usual care group completing all 6-month assessments (Fig. 1). Most characteristics did not differ between those with complete (n = 272) and those with missing data at 6 months (n = 30) (ESM Table 3); the only statistically significant differences were in use of insulin (p = 0.023) and smoking status (p = 0.036).
Table 2 shows the mean values at baseline and follow-up for the outcome variables and the results of the regression analyses that examined intervention effects, adjusted for baseline values and potential confounders. There were statistically significant differences between groups at follow-up, favoring the telephone counseling group, in weight loss, physical activity, energy intake, and diet quality, but not in HbA1c. The intervention effects showed, relative to usual care, that the intervention group achieved: more weight loss (−1.12 % of initial body weight; 95 % CI, −1.92, −0.33, which was equivalent to −1.14 kg); 30 % higher mean physical activity (95 % CI, 8 %, 57 %), equating to an absolute difference of 30.8 min/week; and lower energy intakes (−0.63 MJ; 95 % CI, −1.01, −0.25) coupled with better dietary quality (3.72 points; 95 % CI, 1.77, 5.68). Expressed as Cohen’s d, the adjusted between-group differences at follow-up were “small” for weight loss (d = −0.322; 95 % CI, −0.548, −0.094), physical activity (d = 0.322; 95 % CI, 0.095, 0.549), and diet (energy intake d = −0.382; 95 % CI, −0.610, −0.154; diet quality d = 0.439; 95 % CI, 0.211, 0.667). For all these outcomes, the telephone counseling group improved significantly from baseline, while the usual care group showed no substantial or significant changes. Despite the behavioral and anthropometric improvements, there was no substantial or statistically significant difference between groups in HbA1c at follow-up (RR = 0.99; 95 % CI, 0.96, 1.01, equivalent to an absolute difference in means of 0.10 in favor of the telephone counseling group), with no significant change from baseline being observed in either telephone counseling or usual care groups.
Viewed in terms of program targets, few telephone counseling (12.6 %) and even fewer usual care (4.6 %) participants met the program target for weight loss of ≥5 % of initial body weight (Fig. 2a), with most experiencing either minor weight loss (1 to <5 % loss) or no change (±1 %). Weight gain (≥1 % of bodyweight) was much less common in the telephone counseling (17.2 %) than the usual care participants (34.4 %). While the program had no significant impact on HbA1c, the proportion of participants meeting the HbA1c target (≤7; Fig. 2b) showed a slight tendency towards a favorable increase in the telephone counseling group (+2.7 %) and towards a decrease in the usual care group (−5.0 %). According to accelerometer measures, 27.2 % of telephone counseling and 19.2 % of usual care participants met program targets for physical activity (≥210 min/week) at 6 months, with these figures having been at 17.9 and 17.2 %, respectively, at baseline (Fig. 2c). Only a minority (19.9 % of telephone counseling and 17.2 % of usual care) met the targeted 2 MJ reduction in energy intake (Fig. 2d).
Sensitivity analyses showed significant intervention effects for physical activity were still present (RR = 2.09; 95 % CI, 1.39, 3.15, absolute difference = 17.4 min) when applying one of the highest cutpoints for moderate physical activity (≥2,743 cpm) [51], but not when applying one of the lowest moderate cutpoints, designed to capture lifestyle activities [51] (RR = 1.03; 95 % CI, 0.92, 1.12, absolute difference = 25.3 min), which also led to unrealistic mean estimates of physical activity (approximately 13 h/week).
Sensitivity analyses in completers showed that BVCF had either reduced the differences between groups or had not affected results for: weight loss (−1.27; 95 % CI, −2.14, −0.40 % of initial weight); HbA1c (RR = 0.99; 95 % CI, 0.96, 1.02, absolute difference = 0.09); physical activity (RR = 1.29; 95 % CI, 1.06, 1.57, absolute difference = 31.0 min/week); energy intake (−0.71; 95 % CI, −1.12, −0.30 MJ); and diet quality (4.28; 95 % CI, 2.16, 6.39 points). BVCF can underestimate the variability in the outcomes but did so only slightly in this study. The standard errors for intervention effects using BVCF were all 92 % the size of those in completers and widening the 95 % confidence intervals for the BVCF results accordingly did not alter study conclusions regarding statistical significance.
Those in the telephone counseling group received from none to 17 calls over the first 6 months (median = 10; 25th, 75th percentile, 6,12), with 46 % receiving the majority of calls (≥11) and 91 participants (60.3 %) receiving the majority of the four initial weekly calls. Mean (SD) call duration was 28.2 (11.2) min. Call receipt was not significantly associated with most participant characteristics, except for employment, with retirees receiving the most calls (ESM Table 4).
Per protocol analyses showed that differences in outcomes for the telephone counseling participants who completed the majority of calls (n = 68), relative to the usual care (n = 141), were much stronger than in the main analysis or completers analysis: weight loss (−2.17; 95 % CI, −3.24, 1.10 % of initial weight); HbA1c (RR = 0.96; 95 % CI, 0.93, 1.00, p = 0.056, absolute difference = −0.26); physical activity (RR = 1.44; 95 % CI, 1.13, 1.83, absolute difference = 47.1 min/week); energy intake (−0.71; 95 % CI, −1.22, −0.21 MJ); and diet quality (5.72; 95 % CI, 3.27, 8.2 points). There was a statistically significant (p = 0.027) reduction in HbA1c within the telephone counseling participants adhering to protocol, with means (95 % CI) shifting from 7.3 (7.0, 7.6) at baseline to 7.1 (6.9, 7.3) at follow-up.
We did not detect significant (p < 0.05) moderation of weight loss by age, gender, race/ethnicity, country of birth, BMI, duration of diabetes, medication use, CVD, musculoskeletal conditions, lung, smoking status, employment, education, baseline HbA1c, physical activity, energy intake, and dietary quality. The only results meeting our reporting threshold (p < 0.1) were for race/ethnicity (p = 0.062) and country of birth (p = 0.063).
Intervention effects (95 % CI) on percent weight loss were −1.46 (−2.32, −0.59) % and 0.90 (−1.38, 3.18) %, respectively, in Caucasians (n = 118) and non-Caucasians (n = 38), and were −1.62 (−2.59, −0.62) % and 0.02 (−1.40, 1.43) % in those born in Australia (n = 89) and elsewhere (n = 47).
Given small effect sizes, rather than formal mediation analyses, associations of potential mediators with outcomes were examined. Within the intervention group, significant associations with HbA1c improvements were seen for weight loss (Spearman’s R = 0.34; 95 % CI, 0.48, 0.18, p < .001), increased physical activity (Spearman’s R = 0.19; 95 % CI, 0.03, 0.35, p = 0.025) but not reductions in energy intake (Spearman’s R = 0.01; 95 % CI, −0.17, 0.18, p = 0.953). Similarly, a significant association with weight loss was seen for increased physical activity (Spearman’s R = 0.23; 95 % CI, 0.03, −0.19, p = 0.007) but not reduced energy intake (Pearson’s R = 0.15; 95 % CI, −0.02, 0.31 p = 0.084).
Discussion
LWWD was designed as a pragmatic trial to determine the weight loss, increased physical activity, glycemic control, and dietary-change outcomes that could be achieved when the approach used in intensive interventions—such as the landmark Diabetes Prevention Program and Look AHEAD trials—was adapted for delivery via telephone and implemented with a largely representative primary care sample of adults with type 2 diabetes. Six-month results, from the end of the intensive phase of the LWWD intervention, demonstrated small, statistically significant improvements in weight loss, objectively measured moderate-to-vigorous physical activity, and dietary outcomes in the intervention group relative to the controls. However, there were no significant intervention effects for HbA1c.
Overall, as expected given the less intensive LWWD intervention protocol, intervention effects were considerably less than those reported after 1 year in the Look AHEAD trial, which equated to a difference between intensive lifestyle intervention versus diabetes education of −7.9 % of initial bodyweight (95 % CI, −8.2, −7.6) for weight loss and −0.50 (95 % CI, −0.56, −0.44) for HbA1c. A review and meta-analysis of lifestyle and behavioral weight loss intervention studies in adults with type 2 diabetes published up until 2003 indicated that compared with usual care, lifestyle-based interventions resulted in a pooled mean weight loss of 3.1 % of initial body weight (95 % CI, −4.5, −1.7), and a pooled mean reduction in HbA1c of 0.3 (95 % CI, −0.8, 0.2) [10]. LWWD results for weight loss are closer to these, although still not as strong, but it is important to note that all of the studies in this review were delivered via face-to-face contacts, either in individual or group format, or some combination. Similar attenuation of intervention effects has been observed as intensive diabetes prevention interventions, like the US and Finnish Diabetes Prevention Programs [52, 53], have been evaluated in translational settings [54]. The weight loss achieved in LWWD (1.14 kg) was consistent with the pooled mean weight loss (relative to control) of 1.82 kg (95 % CI, −2.70, −0.99 kg) [54] reported in a recent meta-analysis of seven diabetes prevention translational trials that used randomized designs.
Six trials have used the telephone, solely or in combination with other modalities, for delivering diabetes self-management interventions in participants with type 2 diabetes [21–26], and with intervention duration ranging from 6 to 12 months. Of the four trials assessing weight loss, only one reported significant intervention effects, of −4.0 kg (95 % CI, −7.3, −0.7) [22]. The non-significant effect sizes were −0.8 kg (95 % CI, −2.3, 0.7) [23], −1.4 kg [21] and not reported [25]. Despite these weight loss outcomes being of similar magnitude to our findings, these telephone-delivered trials achieved better results for glycemic control, possibly owing to a focus on medication adherence, with all but one [25] reporting significant intervention effects for HbA1c, ranging from −0.4 (95 % CI, −0.70, −0.10; [26]) to −1.2 (95 % CI, −1.8, −0.6; [24]).
Intervention improvements in physical activity were associated with weight loss, and weight loss was, in turn, associated with improved glycemic control. However, the percentages of LWWD telephone group participants meeting the intervention targets for physical activity and diet (i.e., ≥210 min/week MVPA and at least 2 MJ/day reduction in energy intake), while tending to improve with intervention, were quite low. This may partly explain the small intervention effect for weight loss and, in turn, the lack of improvement in glycemic control. Consistently, the results for the telephone counseling participants who had received the majority of calls were more positive than the results for the telephone counseling group as a whole, with more behavioral improvement, greater weight loss, and some suggestion of a benefit in terms of glycemic control.
Strengths of the LWWD trial include attention to rigorous trial methods, specifically appropriate randomization, the blinding of assessors, use of objective and validated measurement for all outcomes (except diet), low attrition, high accelerometer compliance, and the evaluation of the robustness of the findings to assumptions regarding missing data and accelerometer cutpoints. Further, this was a pragmatic trial that delivered an intervention feasible for uptake to what was, for the most part, a representative sample of Australian primary care patients with type 2 diabetes. Limitations were: some minor participation biases typical in trials (i.e., a slight overrepresentation of those with higher education, never smokers, and those who were heavier and more recently diagnosed with diabetes); the use of a food frequency questionnaire to measure energy intake [55], which was chosen over the preferred 24-h dietary recall method due to resource limitations; and the fact that the activity monitor primarily captures ambulatory movement while tending to underestimate participation in other activities, particularly strength training, which was encouraged as part of the intervention. Also, the way in which call attempt data were recorded in the database did not allow us to determine with certainty the extent to which low call completion related to lack of participant engagement versus non-delivery by counselors; however, examination of counselor-kept call records suggests that the vast majority of missed calls were due to participants.
While telephone-delivered lifestyle interventions show promise as a broad reach delivery modality relevant to the growing numbers of adults with type 2 diabetes, 6-month results from the LWWD trial were quite small. As a trial designed to inform translation, we sought to recruit and retain a representative sample of participants, rather than a selected group of more motivated participants. This resulted in a sizeable proportion of intervention group participants not sufficiently engaged with the intervention to derive significant benefit, despite the motivational interviewing approach, and thus with small intervention effects for the intervention group as a whole. In contrast, intervention effects for those who participated in most of the program were considerably stronger. Taken together, results suggest that if the LWWD intervention were to be delivered only to willing/motivated participants in a translational setting, the impacts on weight, behavior change, and glycemic control may be substantially stronger than is indicated by the findings from this trial. In future research, it may be advisable to screen potential participants prior to program enrollment to solicit a commitment to engage fully in all intervention activities, including all scheduled calls—perhaps not to the extent of the formal “run-in” periods implemented in the landmark intervention trials, but certainly more than the “take all comers” approach used here. The risk of such screening is that it may act to exclude the more socioeconomically disadvantaged and racial/ethnic minority groups. Notably, the only sociodemographic characteristic significantly associated with call completion was employment status (being retired). As this type of intervention research moves increasingly into translational settings, it will be important to balance the need for wide population reach and representativeness with the imperative to allocate scarce healthcare resources to those likely to benefit. Interim results from the LWWD trial suggest that consideration should be given to culturally tailored programs, and perhaps to less individually targeted approaches that might better reach those from non-Caucasian backgrounds. Subsequent reporting on end-of-intervention and maintenance outcomes, and cost-effectiveness analyses will be important to speak to the full potential to inform translation.
References
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053.
Dunstan DW, Zimmet PZ, Welborn TA, et al. The rising prevalence of diabetes mellitus and impaired glucose tolerance: The Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care. 2002;25:829-834.
Cameron AJ, Welborn TA, Zimmet PZ, et al. Overweight and obesity in Australia: The 1999–2000 Australian diabetes, obesity and lifetyle study (AusDiab). Medical Journal of Australia. 2003;178:427-432.
Centers for Disease Control and Prevention: National Diabetes Fact Sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011.
Diabetes UK: Diabetes prevalence 2011 (Oct 2011). Available at http://www.diabetes.org.uk/Professionals/Publications-reports-and-resources/Reports-statistics-and-case-studies/Reports/Diabetes-prevalence-2011-Oct-2011/. Accessibility August 7, 2012.
National Health and Medical Research Council: Clinical practice guidelines for the management of overweight and obesity in adults. Canberra: National Health and Medical Research Council, 2003.
Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycaemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. JAMA. 2001;286:1218-1227.
De Fronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281-303.
National Institutes of Health, National Heart Lung and Blood Institute, North American Association for the Study of Obesity: The practical guide: Identification, evaluation and treatment of overweight and obesity in adults. Bethesda: NIH, 2000.
Norris SL, Xuanping Z, Avenell A, et al. Long-term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: A meta-analysis. Am J Med. 2004;117:762-774.
Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610-628.
Kessler R, Glasgow RE. A proposal to speed translation of healthcare research into practice: Dramatic change is needed. American Journal of Preventive Medicine. 2011;40:637-644.
Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: Rationale and strategies. A statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care. 2004;27:2067-2073.
Eakin EG, Bull SS, Glasgow RE, Mason M. Reaching those most in need: A review of diabetes self-management interventions in disadvantaged populations. Diabetes/Metabolism Research and Reviews. 2002;18:26-35.
Eakin EG, Lawler SP, Vandelanotte C, Owen N. Telephone interventions for physical activity and dietary behavior change: A systematic review. American Journal of Preventive Medicine. 2007;32:419-434.
Goode AD, Reeves MM, Eakin EG. Telephone-delivered interventions for physical activity and dietary behavior change: An updated systematic review. American Journal of Preventive Medicine. 2012;42:81-88.
Digenio A, Mancuso J, Gerber R, Dvorak R. Comparison of methods for delivering a lifestyle modification program for obese patients: A randomized trial. Ann Intern Med. 2009;150:255-262.
Djuric Z, DiLaura NM, Jenkins I, et al. Combining weight-loss counseling with the weight watchers plan for obese breast cancer survivors. Obes Res. 2002;10:657-665.
Ely AC, Banitt A, Befort C, et al. Kansas primary care weighs in: A pilot randomized trial of a chronic care model program for obesity in 3 rural Kansas primary care practices. J Rural Heal. 2008;24:125-132.
VanWormer JJ, Benson GA, Cosentino DA. Telephone counseling and home telemonitoring: The Weigh by Day Trial. Am J Heal Behav. 2009;33:445-454.
Davis RM, Hitch AD, Salaam MM, Herman WH, Zimmer-Galler IE, Mayer-Davis EJ. TeleHealth improves diabetes self-management in an underserved community. Diabetes Care. 2010;33:1712-1717.
Franciosi M, Lucisano G, Pellegrini F, et al. ROSES: Role of self-monitoring of blood glucose and intensive education in patients with type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Diabet Med. 2011;28:789-796.
Kirkman MS, Weinberger M, Landsman PB, et al. A telephone-delivered intervention for patients with NIDDM. Effect on coronary risk factors. Diabetes Care. 1994;17:840-846.
Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber-Baldini AL. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34:1934-1942.
Sacco WP, Malone JI, Morrison AD, Friedman A, Wells K. Effect of a brief, regular telephone intervention by paraprofessionals for type 2 diabetes. Journal of Behavioral Medicine. 2009;32:349-359.
Walker EA, Shmukler C, Ullman R, Blanco E, Scollan-Koliopoulus M, Cohen HW. Results of a successful telephonic intervention to improve diabetes control in urban adults: A randomized trial. Diabetes Care. 2011;34:2-7.
Eakin EG, Reeves MM, Marshall AL, et al. Living Well with Diabetes: A randomized controlled trial of a telephone-delivered intervention for maintenance of weight loss, physical activity and glycaemic control in adults with type 2 diabetes. BMC Publ Health. 2010;10:452.
Eakin EG, Reeves MM, Lawler SP, et al. The Logan Healthy Living Program: A cluster randomized trial of a telephone-delivered physical activity and dietary behavior intervention for primary care patients with type 2 diabetes or hypertension from a socially disadvantaged community—rationale, design and recruitment. Contemporary Clinical Trials. 2008;29:439-454.
Look AHEAD, Group R, Wadden TA, West DS, et al. The Look AHEAD study: A description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring). 2006;14:737-752.
Look AHEAD Research Group: Look AHEAD—Action for Health in Diabetes: Lifestyle intervention—Year 1 materials. Available at https://www.lookaheadtrial.org/public/dspMaterials.cfm. Accessibility August 8, 2012.
Zwarenstein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: An extension of the CONSORT statement. BMJ. 2008;337:a2390.
Altman DG, Bland JM. Treatment allocation by minimisation. BMJ. 2005;330:843.
Australian Institute of Health and Welfare: The Active Australia Survey: A Guide and Manual for Implementation, Analysis and Reporting. Canberra: Australian Institute of Health and Welfare; 2003.
Cornell S: Differentiating among incretin therapies: A multiple-target approach to type 2 diabetes. J Clin Pharm Ther. 2012; 37(5):510–24
Peterson G. Current treatments and strategies for type 2 diabetes: Can we do better with GLP-1 receptor agonists? Annals of Medicine. 2012;44:338-349.
Emmons KM, Rollnick S. Motivational interviewing in health care settings. Opportunities and limitations. American Journal of Preventive Medicine. 2001;20:68-74.
Lowe M, Foster G, Kerzhnerman I, Swain R, Wadden T. Restrictive dieting vs. "undieting" effects on eating regulation in obese clinic attenders. Addict Behav. 2001;26:253-266.
Diabetes Australia, The Royal Australian College of General Practitioners: Diabetes Management in General Practice—Guidelines for Type 2 Diabetes—2011/12. Available at http://www.racgp.org.au//NavigationMenu/ClinicalResources/RACGPGuidelines/Diabetesmanagement/201107diabetesmanagementingeneralpractice.pdf. Accessibility August 8, 2012.
Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Medicine and Science in Sports and Exercise. 2009;41:459-471.
American Diabetes Association, Bantle JP, Wylie-Rosett J, et al. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl 1):61-78.
Dunstan DW, Vulikh E, Owen N, Jolley D, Shaw JE, Zimmet PZ. Community center-based resistance training for the maintenance of glycemic control in adults with type 2 diabetes. Diabetes Care. 2006;29:2586-2591.
Franz MJ, Bantle JP, Beebe CA, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2003;26(Suppl 1):51-61.
Heart Foundation: Position statement on dietary fats and dietary sterols for cardiovascular health. Available at http://www.heartfoundation.org.au/SiteCollectionDocuments/Dietary-fats-position-statement-LR.pdf. Accessed August 8, 2012.
Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Medicine and Science in Sports and Exercise. 1998;30:777-781.
Hodge A, Patterson A, Brown W, Ireland P, Giles G. The Anti Cancer Council of Victoria FFQ: Relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Australian and New Zealand Journal of Public Health. 2000;24:576-583.
Lewis J, Milligan G, Hunt A. NUTTAB95 Nutrient Data Table for Use in Australia. Canberra: Australian Government Publishing Service; 1995.
Haines PS, Siega-Riz AM, Popkin BM. The Diet Quality Index revised: A measurement instrument for populations. J Am Diet Assoc. 1999;99:697-704.
Newby PK, Hu FB, Rimm EB, et al. Reproducibility and validity of the Diet Quality Index Revised as assessed by use of a food-frequency questionnaire. Am J Clin Nutr. 2003;78:941-949.
Australian Government Department of Health and Ageing, National Health and Medical Research Council: Food for health: Dietary guidelines for Australians—A guide to healthy eating. Canberra: Australian Government Department of Health and Ageing; 2005.
Australian Government Department of Health and Ageing, National Health and Medical Research Council, New Zealand Ministry of Health: Nutrient reference values for Australia and New Zealand: Executive summary. Canberra: National Health and Medical Research Council; 2006.
Matthew CE. Calibration of accelerometer output for adults. Medicine and Science in Sports and Exercise. 2005;37(Suppl 11):512-522.
Diabetes Prevention Program Research Group, Knowler WC, Barrett-Connor E, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350.
Cardona-Morrell M, Rychetnik L, Morrell SL, Espinel PT, Bauman A. Reduction of diabetes risk in routine clinical practice: Are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC Publ Health. 2010;10:653.
Rutishauser IH. Dietary intake measurements. Public Health Nutrition. 2005;8:1100-1107.
Acknowledgments
We wish to thank the patients, general practitioners, practice and program staff of the Greater Metro South Brisbane Medicare Local (Logan, Australia) for their study participation and support, and Diabetes Australia Queensland for their endorsement and provision of materials for the usual care group. We would also like to thank project staff for their integrity and commitment: Kym Spathonis, Charlotte Brakenridge, Erin Robson, Amy Chatwin, Jennifer Job, Fiona Heys, Natalie Doyle, Jodie Jetann, Ellen Baker, Lisa Ulyate, Fiona Porter, and Charani Kiriwandeniya. This study was supported by a National Health and Medical Research Council (NHMRC) project grant and a Diabetes Australia Research Trust grant. Eakin is supported by a NHMRC Senior Research Fellowship; Reeves is supported by a NHMRC Postdoctoral Training Fellowship; Winkler is supported by Queensland Health core infrastructure funding; Healy is supported by a NHMRC/National Heart Foundation of Australia Postdoctoral Fellowship; Dunstan is supported by a VicHealth Public Health Research Fellowship; Owen is supported by a NHMRC Senior Principal Research Fellowship; Marshall is supported by a NHMRC Career Development Award. The trial is registered with the Australian Clinical Trials Registry # ACTRN12608000203358.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Eakin, E.G., Reeves, M.M., Winkler, E. et al. Six-Month Outcomes from Living Well with Diabetes: A Randomized Trial of a Telephone-Delivered Weight Loss and Physical Activity Intervention to Improve Glycemic Control. ann. behav. med. 46, 193–203 (2013). https://doi.org/10.1007/s12160-013-9498-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12160-013-9498-2