Abstract
Background
Current obesity interventions use intensive behavior changes to achieve large initial weight loss. However, weight regain after treatment is common, and drop out rates are relatively high. Smaller behavioral changes could produce initial weight loss and be easier to sustain after active treatment.
Purpose
We examined the efficacy of an intervention that targeted small but cumulative participant-chosen changes in diet and physical activity (ASPIRE) and compared this treatment to standard didactic and wait-list control groups. The primary outcome measures were body weight, waist circumference, and intra-abdominal fat.
Methods
Fifty-nine overweight or obese sedentary adults were randomized to one of three groups: (1) the ASPIRE group (n = 20), (2) a standard educationally-based treatment group (n = 20), or (3) a wait list control group (n = 19) for 4 months. Active treatment groups received identical resistance and aerobic training programs.
Results
Intention-to-treat analyses showed that participants in the ASPIRE group lost significantly more weight than the standard and control groups (−4.4 vs. −1.1 and +0.1 kg, respectively), and the greater initial weight loss in the ASPIRE group was sustained 3 months after active treatment (4.1 kg). An alternative analytic strategy (0.3 kg/month weight gain for those lost to follow-up) showed continued weight loss (−0.2 kg after active treatment; −4.6 kg from baseline) at follow-up in the ASPIRE group. Similar patterns were observed for the other adiposity measures.
Conclusion
More modest behavioral changes are capable of promoting weight loss, decreasing adiposity markers and sustaining these changes over 3 months. Longer-term studies comparing this approach with traditional behavioral weight loss treatments are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Developing effective weight loss programs is imperative given the obesity epidemic and its associated health consequences [1]. Traditional behavioral therapy programs involving dietary restriction (1,000–1,200 kcals/day), physical activity (60 min most days of the week), and behavioral self-management achieve about 10% initial weight loss over 6 months [2]. However, long-term maintenance of weight loss remains elusive, as most intervention participants regain one-third of their weight during the first year and within 5 years return to pre-intervention levels [3, 4]. Even with extended ‘continuous care’ interventions, using different contact modalities and different self-regulation strategies [5–8], some weight regain is imminent. Therefore, pursuing additional treatment avenues in an attempt to achieve lasting weight loss needs to be considered.
One treatment issue concerns the amount of weight loss necessary to be considered successful. While most treatments focus on large initial weight losses, more modest, maintained weight losses (∼5%) have been shown to have clinically important health benefits for reducing disease risk [3]. Moreover, recently published population-based data showed that losing a greater percentage of maximum weight was one factor associated with greater weight regain across time [9]. As a result, some researchers have begun to question weight loss protocols, particularly the initial ‘dieting’ phase (i.e., large caloric reduction; [10]). Some recent alternative approaches include “nondieting,” “antidieting,” or “undieting” approaches [11–14]. A review of the effectiveness of non-dieting studies [10] showed that, while these approaches had favorable impacts on self-esteem, mood, and body image, they had little or no impact on weight.
The one exception to these findings was a small randomized clinical trial by Sbrocco et al. [15]. The Sbrocco et al. [15] program featured a behavioral choice model, coupled with smaller but potentially maintainable changes in eating behaviors that was compared to a traditional behavioral weight loss program. At the end of the 3-month treatment program, the traditional behavioral therapy program showed greater weight loss compared to the behavioral choice group. However, across a 9-month follow-up period, the behavioral choice program participants continued to lose weight, while the traditional program began to regain their lost weight. While these results were provocative, several notable limitations exist including: The dietary changes were fairly restricted, all participants were female, there was a significant run-in period to be eligible for randomization, and perhaps, most importantly, the findings have yet to be replicated.
The present study extended this promising approach by including men and women, broadening choice by allowing participants to include different foods, target caloric amounts, and by equally increasing exercise across treatment groups. The ASPIRE (Aspiring for Lifelong Health) program represented a blending of traditional and non-dieting approaches. ASPIRE program participants were told that, to promote a negative energy balance (lose weight), only small caloric reductions and small increases in physical activity were required. Participants were provided brief instruction about nutrition and physical activity and asked each week to choose and make one small, potentially permanent change in food choices and caloric intake and one small change in physical activity. Small changes were cumulative and made within the context of healthful foods and increasing step counts [16]. Overall, the goal was to lose at least 5% of total body weight and to maintain that loss without continued treatment. It was hypothesized that the ASPIRE program would result in greater initial weight loss and improved short-term maintenance of weight loss than both a waiting-list control and a standard educational weight loss program.
Methods
Participants
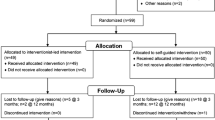
The 59 participants were recruited through newspaper articles and a radio interview featuring the ASPIRE study in the fall of 2001. A flow diagram depicting the number of eligible participants for the trial, the number randomized to each treatment group, and the number completing each assessment is shown in Fig. 1 [17]. Eligibility criteria included being overweight or obese (body mass index [BMI—weight in kilograms/height in square meters] 26–40) and having a sedentary lifestyle (<30 min of moderate to vigorous exercise per week). Participants were excluded for the following reasons: (1) presence of any cardiopulmonary or metabolic disease (i.e., diabetes, thyroid, liver, or kidney disease); (2) blood pressure > 160/100 mmHg, (3) medications that could affect body weight, other metabolic parameters, or both; (4) lack of active health insurance; (5) recent pregnancy or plans to become pregnant in the next 10 months, and (6) BMI greater than 40 kg/m2. The study population consisted mostly of middle-aged, Caucasian (94%), obese (31.66 ± 3.05%) men (41%) and women (59%). The study took place in 2002.
Design
The protocol was approved by the Institutional Review Board at Virginia Polytechnic Institute and State University. Written informed consent was obtained from all participants. After successful completion of the baseline assessment, 59 participants, stratified by gender and BMI, were randomly assigned using a random-numbers table by the first author to one of the three treatment groups: (1) A standard group focused on an educationally based, didactically delivered US Department of Agriculture (USDA) nutrition and physical activity program coupled with a center-based resistance and aerobic training program (n = 20); (2) ASPIRE, a choice-approach (no pre-set goals) focused on small, cumulative changes in physical activity and nutrition, including the same center-based resistance and aerobic training program (n = 20); or (3) a control group, who was asked to continue life as usual (n = 19). After randomization, participants that completed a 16-week initial period were reassessed and then were again followed up 3 months after the completion of treatment.
Procedures
Common Treatment Components
To minimize any differential effects of aerobic and resistance training between the two treatment groups, participants in both treatment arms completed the same progressive aerobic and resistance training protocols. Each training session was conducted with a personal trainer and included a 10- to 12-min graded exercise protocol (GXP) focusing on improving aerobic fitness using either a treadmill or cycle ergometer and a 15- to 20-min resistance training protocol with a 5-min stretching period at the end of every session. Both the aerobic and resistance training protocols have both tested in our lab and shown to be time efficient and effective protocols for increasing fitness and strength [18]. The total time for the entire aerobic and resistance training session was 40–45 min, performed twice per week. Overall, participants in both treatment groups received approximately 24 h of personal training supervision across the treatment program.
Standard Treatment
In addition to their two center-based visits per week, once a week, participants met with a nutritionist for 20 min to receive the education-based program “Dietary Guidelines for Americans” developed by the US Department of Health and Human Services [19], which included topics and handouts related to fitness, building a healthy base of food knowledge, and making nutritionally sound food choices. Consistent with these guidelines, women in the program were encouraged not to eat more than 1,600 kcal/day, and men were encouraged not to eat more than 2,000 kcal/day. Participants also were encouraged to engage in at least 30 min per day of physical activity on most days of the week. Participants received didactic behavioral counseling for approximately 5 h across the duration of the study.
ASPIRE Treatment
Participants randomized to the ASPIRE treatment received the same center-based aerobic- and resistance-training program as people randomized to the Standard group. In addition, participants met weekly, one on one, with a lifestyle coach for approximately 20 min. Participants set challenging, yet achievable goals in relation to nutrition and physical activity involving small changes that were presented as choices each week. For the nutrition portion, participants were asked to complete a 1-week baseline record to serve as a basis for subsequent goals. Energy intake range/calorie goals were individualized based on each participant’s resting energy expenditure using the Harris–Benedict equation [20], total energy expenditure (activity level based on participants’ step counts at baseline), and total energy intake at baseline from food records. Based on these guidelines, daily energy intake goals in the ASPIRE ranged from 1,500 to 2,200 kcal/day for women (−200 to 500 kcal/day from baseline) and from 1,900 to 2,600 kcal/day for men (−200 to 600 kcal/day from baseline). The nutrition program stressed smaller, healthful targeted changes and substitutions such as increasing fruits and vegetables and whole grains, decreasing high fat dairy and meat products, soft drinks, higher calorie snacks, and portion sizes, and maintaining consistent kcal/day consumption [16]. For physical activity, all participants were given a pedometer to first assess their current volume of physical activity. After the baseline period, participants were asked to set weekly goals to slowly increase steps to eventually reach 3,000 steps/day greater than baseline or a total of 10,000 steps/day. Participants kept a detailed daily step count and calorie log. Participants also received guidance in selecting and using a venue to continue exercising after the intervention ended. This choice-oriented program lasted approximately 5 h across the duration of the study.
Control
Control participants were asked to continue life as usual during the first 16-week study period, to attend an assessment, and then to continue life as usual for an additional 12-weeks. Participants were told they would then receive the most effective treatment program.
Measures
Anthropometrics and Body Composition
Total body weight, waist circumference, and intra-abdominal fat (%) were the primary outcome variables. An experienced investigator with extensive assessment experience, blind to treatment condition, performed all body composition assessments with the exception of waist measurements. Weight (without shoes) was measured to the nearest 0.25 lb using a calibrated balance-beam scale. Height (without shoes) was measured to the nearest 0.5 cm using a calibrated stadiometer. Waist measurements were taken to the nearest 0.1 cm using a Gulick anthropometric tape (Perform Better, Cranston, RI, USA). Intra-abdominal fat was measured by a dual energy X-ray absorptiometry (DXA; Hologic QDR 4500A, Hologic, Inc., Bedford, MA, USA).
Manipulation Checks
To more precisely partition the adiposity differences between the ASPIRE and standard treatment groups, both groups engaged in structured aerobic and resistance training activities. We used markers of these activities to determine whether the groups were indeed comparable on these dimensions after active treatment.
Cardiorespiratory Fitness
Cardiorespiratory fitness was determined by predicting aerobic capacity (VO2max) from heart rate and VO2 responses obtained in a progressive multistage sub-maximal exercise test, performed on a stationary cycle ergometer (Monark 818E, Varberg, Sweden) and using an automated respiratory gas analysis system (MedGraphics® CPX-D, Minneapolis, MN, USA). Because a strong linear relation exists between heart rate and VO2 response in exercise, even in overweight adults, VO2 was regressed on heart rate responses and extrapolated to the level of predicted maximal heart rate to obtain a VO2max value [21].
Strength
Total body strength (total kilograms lifted/number of machines) was assessed by trainers using eight air-powered Keiser resistance exercise machines. Participants completed a full strength test on each machine, and the total was then divided by eight (the number of machines) to obtain their total strength at each testing period.
Physical Activity
Participants used step counter pedometers (Accusplit 120E, Accusplit, San Jose, CA, USA) to log the number of steps taken each day for 1 week. The pedometer was worn on the waistband directly above the knee.
Statistical Analyses
For the primary outcome variables (weight, waist circumference, and percent abdominal fat), all randomized participants were analyzed regardless of completion. Specifically, we replaced any posttreatment or follow-up missing values with the baseline value. We used separate regression analyses to evaluate changes in body weight, waist circumference, and intra-abdominal fat. Although no baseline differences in the primary outcome measures were detected across treatment groups (see Table 1), we included baseline (or the posttreatment measurement for the 3-month follow-up comparisons) values as covariates in our estimation of treatment effects. Because these analyses were similar to those using simple change scores, we report unadjusted means in the tables. Test statistics reflect focused treatment group contrasts that compared either active treatment groups versus the control group or the ASPIRE group versus the standard treatment group. Treatment maintenance after 3 months was examined using within groups t-tests.
Given that the test statistics for treatment effects are extremely conservative, particularly for the 3-month follow-up analyses, we also analyzed a primary outcome variable (weight) using an alternative operational definition of weight gain across time (0.3 kg per month) based on a recent meta-analysis [4] and published studies [8] of treatment non-completers.
In addition to p values, we also report Cohen’s [22] standardized effect sizes (d) for simple change scores. Analyses were performed using the Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA) for Windows version 15 and Stata 10.0 (Stata Corp, College Station, TX, USA). Post hoc power analysis for our highly correlated repeated measure (r = .97) revealed that the trial had power > 0.90 to detect a 6-lb posttreatment weight change between the active treatment groups.
Results
Baseline Comparisons and Attendance
Participants in the three conditions did not differ on any demographic or outcome measures at baseline (see Table 1). Participants in each group adhered well to their in-person behavioral and exercise sessions, 98% for ASPIRE and 91% for standard (z = −0.97, P = 0.33).
Effectiveness of Fitness Matching
To evaluate the posttreatment similarity of the intervention groups, we compared strength, cardiorespiratory fitness, and step counts for those with complete data. As shown in Table 2, the active treatment groups had similar increases in strength and cardiorespiratory fitness [F(1,33) = 3.28, p = 0.08; F(1,34) = .21, p = 0.65, respectively], increases that were significantly greater than the control group [F(1,51) = 143, p < 0.001; F(1,51) = 9.94, p = 0.003, respectively]. Finally, while participants in ASPIRE increased their pedometer steps/day by 68% from baseline, compared to participants in the Standard treatment who increased their steps/day by 40% and to the 21% increase in steps observed among control participants, only the difference between ASPIRE and control group was significant, F(1,20) = 7.30, p < 0.05.
Immediate Posttreatment Effects on Adiposity Measures
As shown in Table 2, both active treatment groups showed improvements in adiposity measures at the end of Phase 1, while the control group showed no change. In addition, changes in the ASPIRE group were significantly greater than the standard treatment condition for all three primary outcome measures. Both active treatment groups lost more weight than the control group [F(1,56) = 10.70, p = 0.002, d = 0.84], and participants in the ASPIRE group lost more weight than the standard participants [F(1,37) = 11.78, p = 0.002, d = 0.96]. The control group experienced a nonsignificant increase in weight [t(18) = −0.26, p = 0.80, d = 0.01]. Active treatment groups lost more intra-abdominal fat at the end of Phase 1 [F(1, 55) = 7.68, p = 0.008, d = 0.73], while the ASPIRE group lost more intra-abdominal fat than the standard group participants [F(1, 36) = 6.01, p = 0.02, d = 0.76], and the control participants showed no change [t(18) = .11, p = 0.91, d = 0.01]. For waist circumference, active treatment groups had significantly greater reductions [F(1,56) = 7.71, p = 0.008, d = 0.71], and the ASPIRE group reduced waist circumference more than the standard condition [F(1,37) = 29.60, p < 0.001, d = 1.26], while the control group was unchanged for fat and waist circumference [t’s(18) = 0.11 and 0.20, p’s > 0.84, respectively].
At the end of the 4 months, participants in both treatment groups were told that they would have no contact for 3 months and then would be asked to return for follow-up testing at that time. Participants in the control condition were provided with an abbreviated ASPIRE program at that time.
Maintenance of Treatment Effects
Generally, both active treatment groups were able to maintain the positive changes achieved after active treatment (see Table 2). Based on the intention-to-treat model, the ASPIRE group maintained their weight loss [t(19) = −0.41, p = 0.67], waist circumference decreases [t(19) = .21, p = 0.83], and abdominal fat loss [t(19) = −1.44, p = 0.16]. Although the initial change was significantly smaller, those in the standard condition were also able to maintain their more modest posttreatment changes [t(19) = 0.73, p = 0.48; t(19) = −0.52, p = 0.61; t(18) = −1.45, p = 0.17 for weight, waist, and abdominal fat, respectively]. The abdominal fat measurements appeared to regress the most, but this was because of the relatively large number of baseline values (n = 5 in each group) carried over for those who were unable to complete this assessment at follow-up. We detected no differences in the rate of change after active treatment between the two active treatment arms for any of the three primary outcome measures (F’s from 0.18–1.17, p’s > 0.29).
We also examined weight change across time using an alternative, empirically justified estimate of weight regain for non-completers (0.3 kg per month [4]). According to this analysis, participants in the ASPIRE group exhibited significantly greater weight loss across treatment compared to either the standard or control groups [−4.5 ± 3.5 vs. −1.1 ± 2.9 and +.1 ± 2.4 kg, respectively; F(1,37) = 11.27, p < 0.001, d = 0.97 for the ASPIRE vs. standard group] and continued to lose weight across the 3-month follow-up (−0.2 ± 3.7 vs. −0.1 ± 1.2). From baseline to 3-month follow-up, participants in the ASPIRE group lost significantly more weight compared to the standard group [−4.6 ± 5.7 vs. −1.2 ± 2.7 kg; F(1,37) = 6.12, p = 0.02, d = 0.97].
Discussion
The present study hypothesized that having participants choose small but cumulative changes in nutrition, caloric consumption, and physical activity would result in modest but sustainable weight loss. This treatment blended traditional behavioral therapy with a non-dieting behavioral choice approach. Participants in this ASPIRE program achieved statistically and clinically significant reductions in total body weight (4.62 kg; 5% of body weight), intra-abdominal fat, and waist circumference. Perhaps more notably, across a 3-month follow-up period, these participants were able to maintain all treatment-based improvements.
The present study had several strengths. First, the study was a randomized controlled trial with both treatment and wait-list comparison groups. Second, we matched resistance and aerobic training across standard and ASPIRE groups to control for adiposity changes unrelated to the behavioral treatment element. These groups were also matched on treatment contact time to more clearly partition the unique effects of the ASPIRE approach. Thus, we can reasonably assume that the favorable adiposity changes in the ASPIRE group after treatment and the maintenance of these changes 3 months after treatment were attributable to the intervention rather than nonspecific attention effects or changes in fitness. Finally, the ASPIRE effects were robust despite a very conservative analytic approach, one that carried the pretreatment values forward for those participants who did not complete the study.
Despite these strengths, limitations should be considered. First, the follow-up was only 3 months rather than at least 1 year [23]. Second, although the standard USDA comparison group was useful because of the similar caloric and activity goals relative to ASPIRE, it would be desirable to compare ASPIRE to more traditional behavioral weight loss programs that emphasize greater caloric restriction and more rapid weight loss. Regarding the repeatability of the ASPIRE approach, it is notable that our control group, who received a 12-week version of the ASPIRE program after the other active treatments ended, achieved and maintained (3 months) improvements in adiposity that were comparable to the original ASPIRE group (data not shown).
While no one treatment approach can address the obesity epidemic, the development and testing of novel approaches, such as the ‘small changes’ approach, will provide an evidence base for effective and enduring risk reduction interventions.
References
Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old: body mass and the risk of death from any cause. New Engl J Med. 2006; 355: 763–778.
Wing RR. Behavioral weight control. In: Wadden TA, Stunkard AJ, eds. Handbook of Obesity Treatment. New York: Guilford; 2002: 301–316.
Katz DL. Competing dietary claims for weight loss: Finding the forest through truculent trees. Annu Rev Public Health. 2005; 26: 61–86.
Dansinger ML, Tatsioni AM, Wong JB, Chung M, Balk EM. Meta-analysis: The effects of dietary counseling for weight loss. Ann Intern Med. 2007; 147: 41–50.
Perri MG, Sears SF Jr, Clark JE. Strategies for improving maintenance of weight loss. Toward a continuous care model of obesity management. Diabetes Care. 1993; 161: 200–209.
Perri MG, Corisca JA. Improving the maintenance of weight lost in behavioral treatment of obesity. In: Wadden TA, Stunkard AJ, eds. Handbook of Obesity Treatment. New York: Guilford; 2002: 357–375.
Perri MG, Durning PE, Janicke DM, et al. (2007, April) Treatment of obesity in underserved rural settings (TOURS): 18-month findings. 28th annual meeting for Society of Behavioral Medicine. Washington, DC: 2007.
Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation approach for maintenance of weight loss. New Engl J Med. 2006; 355: 1563–1571.
Weiss EC, Galuska DA, Khan LK, Gillespie C, Serdula MK. Weight regain in US adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007; 331: 34–40.
Foster GD, McGuckin BG. Nondieting approches: principles, practices, and evidence. In: Wadden TA, Stunkard AJ, eds. Handbook of Obesity Treatment. New York: Guilford; 2002: 494–512.
Berg FM. Health risks associated with weight loss and obesity treatment programs. J Soc Issues. 1999; 55: 277–297.
Garner DM, Wooley SC. Confronting the failure of behavioral and dietary treatments for obesity. Clin Psychol Rev. 1991; 11: 729–780.
Goodrick GK, Foreyt JP. Why treatments for obesity don’t last. J Am Diet Assoc. 1991; 91: 1243–1247.
McFarlane T, Polivy J, McCabe RE. Help, not harm: Psychological foundation for a nondieting approach toward health. J Soc Issues. 1999; 55: 261–276.
Sbrocco T, Nedegaard RC, Stone JM, Lewis EL. behavioral choice treatment promotes continuing weight loss: Preliminary results of a cognitive-behavioral decision-based treatment for obesity. J Consult Clin Psychol. 1999; 672: 260–266.
Winett RA, Anderson ES, Wojcik JR, Winett SG, Bowden T. Guide to Health: Nutrition and physical activity outcomes of a Group-Randomized Trial of an Internet-Based Intervention in Churches. Annals Behav Med. 2007; 33: 245–255.
Altman RA, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: Explanation and Elaboration. Ann Inter Med. 2001; 1348: 665–694.
Winett RA, Wojcik JR, Fox LD, et al. The effects of very brief duration, infrequent cardiovascular training and resistance training protocols on the aerobic capacity and strength of unfit men and women: A demonstration of the threshold model. J Behav Med. 2003; 26: 183–195.
US Department of Health and Human Services. Nutrition and Your Health: Dietary Guidelines for Americans. (http://www.health.gov/dietary guidelines/) 2004.
Harris J, Benedict F. A biometric study of basal metabolism in man. Washington DC: Carnegie Institute of Washington; 1919.
Jakicic JM, Donnelly JE, Pronk NP, Jawad AF, Jacobsen DJ. Prescription of exercise intensity for the obese patient: the relationship between heart rate, VO2 and perceived exertion. Int J Obes. 1995; 19: 382–387.
Cohen J. A power primer. Psychol Bull. 1992; 112: 155–159.
Wing RR, Hill JO. Successful weight loss maintenance. Ann Rev Nutr. 2001; 21: 323–341.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lutes, L.D., Winett, R.A., Barger, S.D. et al. Small Changes in Nutrition and Physical Activity Promote Weight Loss and Maintenance: 3-Month Evidence from the ASPIRE Randomized Trial. ann. behav. med. 35, 351–357 (2008). https://doi.org/10.1007/s12160-008-9033-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12160-008-9033-z