Abstract
The aim of this work is to optimize the supercritical liquefaction process of rapeseeds in order to produce a bio-oil with low viscosity. Reaction parameters, such as reaction temperature, residence time, and solvent to biomass ratio, were studied. Response surface methodology (RSM) based on central composite design (CCD) was used to determine the optimum operating conditions to minimize the bio-oil viscosity. The low bio-oil viscosity of 5.90 mPa.s was obtained at the optimal operating conditions of 280°C, 40 min, and methanol/biomass mass ratio of 5.5/1, at pressure within the batch reactor of 124.59 bars. At these optimal conditions, the bio-oil yield was high and reached around 80wt%, while its heating value was about 38.36MJ/kg. It was proved that the reaction temperature and methanol/biomass ratio were the most influencing parameters on bio-oil viscosity according to the ANOVA results. The predicted values from the RSM model was in good agreement with the experimental results. The GC-MS analysis showed that the bio-oil is mainly composed of methyl esters, which are the main components of biodiesel. This study revealed the complete supercritical transesterification of lipid into alkyl esters resulting in a low amount of triglycerides, monoglycerides, diglycerides, and glycerin, identified by GC-FID. The results will provide useful guidance for predicting other physical properties of bio-oil following a similar methodology to that used in this work. In addition, bio-oil could be used for biodiesel fuel production but after hydrodeoxygenation treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermochemical processes are potential ways to convert biomass into biofuel. Under the umbrella of thermochemical treatment, recently sub-/supercritical fluids fractionation have attracted more attention than other processes because of their low environmental impacts and required temperature levels. A fluid is named supercritical when its temperature and pressure go higher than its critical pressure (Pc) and temperature (Tc) that indicate the end of the vapor liquid coexistence curve as well as gases and liquids become indistinguishable. In fact, matter that exists in the region above the critical point, which is a new phase, is called supercritical fluid. At supercritical conditions, these fluids have liquid-like properties such as high density that means more dissolving power properties, which allow the solvation of many compounds in supercritical fluids. In addition, gas-like properties such as high diffusivity and low viscosity enhance mass transfer rates of reactants to the active biomass’s molecules and readily penetrate porous and fibrous solids, due to the dissolving power properties, allowing the solvation of many compounds in reaction medium [1].
Solvents are classified into three categories according to their polarity: dipolar aprotic, polar protic, and nonpolar ones. Protic solvents have been the most used with remarkable effects on direct liquefaction of biomass such as water and alcohols referring to compounds with a hydrogen atom-bond attached to atom-bond electronegativity [1]. Methanol (MeOH) is one of the organic solvents, which is the most selected as supercritical fluid due to its properties combined to its low cost. The most important physicochemical property of supercritical methanol is its lower critical point (239°C and 8.09 MPa) compared to that of water (374,14°C, and 22,09 MPa). In addition, the electric constant of MeOH is much lower than that of water, providing a better solvent for bio-oil production, including the high density which enhances solute-fluid interaction. On the other hand, the selection of alcohol as a solvent is related to the action of hydrogen donor and to its alkylating ability. Hydrogen donor solvents as MeOH provide an alternative to hydrogen as a reducing gas. Among the advantage of using hydrogen donor alcohol is to stabilize the free radical in the biomass liquefaction. As comparison to non-hydrogen donor solvents, hydrogen donor shows significant improvement not only in conversion and product distribution to liquid but also on the quality of bio-oil (oxygen content) due to the improvement of hydrocracking and hydrogenation reactions with inhibition of polycondensation [2]. Furthermore, a small change in pressure with respect to temperature has the ability to change the physical properties of the solvent which is unique to supercritical systems [2].
Rapeseed is known as an important energy plant. It is one of the most commonly cultivated oil plants worldwide. It was selected as biomass feedstock due to its very high oil content, rich in lignocellulosic components, easy to grow, and with a high growth potential. On the other hand, rapeseed oil is a very important source not only of edible oil technology but also of biodiesel technology. Literature findings indicate the pyrolysis of the rapeseed plant was investigated in various studies for the production of biofuel and biochar [3,4,5,6]. However, knowledge of bio-oil production from rapeseeds is limited especially by using liquefaction process.
The major bio-oil physicochemical properties related to energy use such as kinematic viscosity, higher heating value (HHV), flash point, and density are dependent on raw materials composition as well as the operating conditions applied during the process [7]. However, one of the major disadvantages of bio-oil is its high viscosity [8], surface tension coefficient, and density [9]. The later properties influence directly the spray formation process following fuel injection. Higher surface tension coefficient increases the cohesive force, which circumvents the formation of smaller drops. Moreover, the high viscosity of the fuel damps down the aerodynamic disturbance on the surface of the liquid jet injected through the nozzle and delays the breakup [9]. Our present work is limited to the study of the bio-oil viscosity. Several authors revealed the drawbacks of the high viscosity [10]; for instance, fuel could not be well-atomized during injections, resulting in poor combustion, higher engine deposits, and increased of energy requirements for fuel pumping [11]. In addition, the high fuel viscosity leads to an increased unburnt hydrocarbons and carbon monoxide emissions [12]. Furthermore, it alters injection duration and hence the quantity of injected fuel [13].
Viscosity of bio-oil is an important physical property associated with their processing and quality control. It depends on several factors such as feedstock, operating conditions of process, phase separation efficiency, and storage time [14]. Indeed, biomass containing high lipids, proteins, cellulose, or hemicellulose fractions produces higher bio-oil yields than ones containing high lignin fractions. According to many reported studies, in terms of bio-oil yield, it was ranked in the order of lipid >> protein > cellulose > hemicellulose ≥ lignin, while the order of lignin >cellulose >hemicellulose > protein> lipid was proposed for solid residue yield [15,16,17]. In addition, feedstock with high lignin fraction leads to an increase in viscosity of bio-oil due to the average molecular weight [14]. Most of the previous research works reported that hydrocarbons straight chains have higher viscosities compared to that of branched hydrocarbons. In addition, alcohol or acid groups have more impact on viscosity opposed to ketones and esters [18, 19]. As a result of cellulose and hemicellulose reaction readily dissolved in MeOH, the production of high molecular weight products is favored due to the lower dielectric constants of solvent. A few studies about the supercritical liquefaction of biomass by using methanol as solvent were published [20]. The majority of research has been focused on bio-oil yield and conversion rate. However, the physicochemical properties have received far less attention. Literature review on MeOH chemistry shows that MeOH has higher hydrogen-bonded structures, which are destroyed to an appreciable extent with increasing the temperature [1]. Since the chemical composition of raw materials and the operating parameters of pressurized processes influence the decomposition efficiency, optimal conditions prediction becomes a useful tool that allows better management of applied thermochemical processes. In that consideration, a number of experimental studies have been made in order to predict the bio-oil production through liquefaction by optimizing its operating conditions. Zhang et al. [21] implemented response surface methodology (RSM) to predict the bio-oil yield employing lemon peel as feedstock, and a maximum bio-oil yield of 48.30% was achieved at operating settings of 336°C, 50 min, and 9.6 mass% feedstock loading. Liquefaction of Pistacia atlantica, as oleaginous seeds, has been investigated in an earlier work of the authors in order to study the temperature and pressure effects on conversion rate [22]. The high conversion rate and bio-oil yield were obtained at reaction temperature of 300°C and pressure of 46 bars with the lowest bio-oil viscosity of 6.8 mPa.s.
The chemical and physical properties of bio-oil are important in determining its characteristics. In this regard, providing relevant optimization of the properties of bio-oil can eliminate the time and cost of tests. Many abovementioned research studies have focused on bio-oil production from various sources, but there are fewer studies concerning the optimization of its chemical and physical properties. Viscosity is a very important feature of an engine fuel that plays the most important role in the injection and combustion processes [23]. Therefore, it is necessary to provide an experimental study that can figure out the variation of bio-oil viscosity with different operating conditions. However, no studies have been carried out on the impact of alcohol, under supercritical conditions, on the bio-oil properties, essentially viscosity. The objective of the present research work is to produce a biofuel with low viscosity under different supercritical operating conditions using MeOH as organic solvent. The optimization is focused on the bio-oil viscosity, determined at 40°C. The RSM, based on central composite design (CCD) method, was implemented in order to optimize the operating parameters. In addition, other physicochemical properties of bio-oil, such as higher heating value, proximate and ultimate composition, and ester content, are determined and discussed.
Materials and Methods
Reagents and Materials
All chemicals used in this study were of high purity and were employed without further treatment. The rapeseeds feedstock was received from Neunkirchen, Germany. Before supercritical liquefaction, the proximate, ultimate, and chemical analyses of rapeseeds were performed. Methanol (99.50%), acetone (99.60%), and petroleum ether (99.00%) were purchased from Fisher scientific. Heptane (99.00%) was obtained from Alfa Aesar. Triolein, diolein, monoolein, and methyl pentadecanoate (≥98.5%) standards were purchased from Sigma–Aldrich. The moisture was measured by the Karl Fischer method (870 kf Titrino plus). Biomass and bio-oil samples’ elemental compositions (C, H, N, S, O) were analyzed using a Thermo Electron Flash EA1112 elemental analyzer. Lipid content performed using the soxtherm extraction system was applied using petroleum ether as solvent. The thermogravimetric (TG) analysis were performed on a Setsys Evolution 16/18 (SETARAM) from 20 to 900°C in flow rate of 20 mL.min−1 of pure nitrogen (99.99%) and at a heating rate of 10°C/min. This analysis was used in order to determine the thermal degradation of feedstock, the fixed carbon, volatile matter, and ashes. In addition, TG analysis was used to determine the cellulose, hemicellulose, and lignin content on dry basis. Furthermore, the lignocellulosic compound contents were determined based on their degradation ranges (hemicellulose: 200<T<250°C, cellulose: 250<T<400°C, and lignin: 180<T<600°C) [24, 25]. The analysis was performed on biomass after lipids extraction. The protein content was estimated by multiplying the elemental nitrogen content according to literature [26]. The measurement of HHV was carried out using a Parr 6200 calorimeter. All the measurements were carried out in triplicate to ensure the repeatability of the results.
Experimental Setup
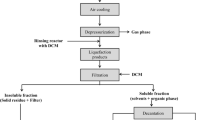
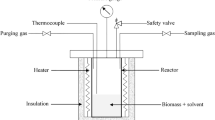
The experiments were carried out in a 998 mL batch reactor. The reactor heating capacity was designed to achieve temperatures up to 400°C. In a typical experiment, 60 g of rapeseeds with different ratios of MeOH was placed in the reactor. Then the reactor was sealed and purged with N2 during 10 min to ensure that no oxygen remained inside. Before experiments, the reactor was heated in order to reach the reaction temperature; it was hold at that temperature for the required reaction time. After cooling down the reactor to room temperature, the reactor content was transferred into a flask, using acetone as the rinsing solvent to recover all organic phase stuck in the reactor. The mixture was separated using a Büchner funnel and prior to filtration under vacuum. The solid residue was then dried at 105°C for 24 h. The organic fraction was evaporated at 50°C under a reduced pressure using a rotary evaporator to remove methanol and acetone. The obtained product is called “bio-oil.” For each tested operating condition, the experiments were conducted in triplicates. The results herein are mean values, and uncertainties are standard deviations
Product Analysis
To determine the chemical composition of the organic fraction, the bio-oil produced during supercritical liquefaction experiments was analyzed using a Perkin Elmer CLARUS 680 Mass Spectrometer coupled with a Gas Chromatograph and a flame ionization detector (GC-MS/FID) equipped with an Agilent SUPELCO SLB-5MS, 30 m×0.25 mm×0.25 μm. The bio-oil sample was dissolved in acetone, reaching a concentration of 1mg/mL. In each analysis, 1 μL of diluted sample was injected in split mode (1:10) using helium as carrier gas with a flow rate of 1 mL/min. The injection temperature was 110°C; the oven temperature was 60°C for 5 min, which rose to 110°C at the rate of 15°C/min and then to 280°C at 5°C/min and was held at this temperature for 5 min (total run time 40.67 min). The mass spectrometer was set at an ionizing voltage of 70 eV and a mass range of m/z 30–450. The identification of organic compounds was determined based on the retention time matching with National Institute of Standards and Technology’s (NIST) library. The fatty acids methyl ester esters (FAME) content was determined using EN 14103 norm [27].
Agilent technologies 7820A Gas Chromatography coupled with a Flame Ionization Detector (GC-FID) was used for the determination of the concentrations triglyceride, diglyceride, monoglyceride, and glycerin in bio-oil. The column used is Agilent containing 5% phenyl polydimethyl-siloxane (15 m in length and 0.32 m internal diameter, the thickness of the film being 0.10 μm) used with a helium flow rate of 3 mL/min. For calibration of the GC-FID chromatograph, ASTM D 6584 norm [28] was applied for the standard solutions preparation in order to determine the triglyceride, diglyceride, monoglyceride, and glycerin concentrations. An AND vibro-viscometer was used to measure the dynamic viscosity of the produced bio-oil in each experiment at 40°C according to EN 3104 test methods.
The bio-oil yield was expressed in wt.% and calculated as follows:
Experimental Design
The study was performed with a CCD experimental design using five levels and three variables. A total of 17 experimental runs were carried out along with three replicates of the center point. The process variables were the reaction temperature (°C, X1), reaction time (min, X2), and MeOH/biomass ratio (g/g, X3). The selection of the factors in the present work is based on screening experiments and previous publications reported in the literature [21, 22, 29, 30]. The selected response variable (Y) is the viscosity of bio-oil (mPa.s).
RSM with CCD was employed to study the effects of three independent variables and their interactions by finding the combination of three factors, X1, X2, and X3 levels on the selected response, and to determine the optimum operating conditions for lowest bio-oil viscosity production. Three independent variables, i.e., X1, X2, and X3, at five coded levels (-α, -1, 0, +1, +α) were chosen. The alpha (α) value depends on the variables number (n) in the factorial experiments design and is determined by Eq. 2:
In the present study n = 3, therefore α = 1.682. The temperature was ranged between 266 and 334°C, the time was set from 13 to 47 min, and the MeOH/biomass mass ratio was varied from 1/1 to 7/1, as shown in Table 1.
To optimize the reaction conditions and determine the response surface, Design-Expert 12.0.1.0 (Stat-Ease, Minneapolis, USA) software was used as statistical software. A second-order polynomial equation was obtained from response surface analysis by a complete analysis of results using analysis of variance (ANOVA). The model overall fitness was validated by examining the coefficient of determination (R2) value. The development of empirical second-order polynomial equation which describes the relationships between the response and independent factors is shown in Eq. (3).
where i and j are the linear and quadratic coefficients, respectively; Y is the response function (viscosity of bio-oil); Xi and Xj are the coded independent variables; and b0, bi, bii, and bij represent the coefficients of intercept, linear, quadratic, and interaction terms, respectively. The data of all bio-oil viscosity measurement were means of triplicate determinations. Reproducibility of the viscosity data was excellent, with a standard deviation less than 5%.
Results and Discussion
Characterization of Feedstock
Calorific value, proximate analysis, and ultimate analysis are generally used to describe the properties of feedstock. The characterization of feedstock was performed and results are illustrated in Table 2. High carbon and hydrogen contents have a beneficial impact on calorific value, whereas high oxygen content decreases the HHV. In addition, it is reported that the presence of fixed carbon contributes to the formation of char, whereas the high content of volatile matter results in high volatility and reactivity and consequently favors the production of gas and liquid products. Compared to the other oleaginous seeds [22, 31], rapeseeds are rich in carbon and lipid contents. In addition, their high content with cellulose and hemicellulose contributes to the production of bio-oil. From Table 2, rapeseeds have a high HHV, an absence of sulfur, and low amount of nitrogen.
Thermogravimetric Analysis of Feedstock
The thermal degradation behaviors of the raw material are studied by thermogravimetric analysis (TGA) in inert condition within the range of degradation temperature of 25 to 900°C. As illustrated in Fig. 1, the mass loss of rapeseeds starts at approximately 100°C, and then a rapid decomposition occurred between 200 and 550°C. From differential thermogravimetric (DTG) curve, rapeseeds had the largest mass loss rate with a peak approximately −0.540%/min at about 407.31°C with a mass loss of 79.43%. The feedstock exhibited three stages during the thermal decomposition process. The first weight loss was observed from 100 to 300°C, possibly caused by the removal of light volatile compounds. The second stage and the third stage from 324 to 600°C account for the majority of weight loss, which can be attributed to the degradation of major biomass biomolecules. The TG and DTG curves suggest that the rapeseeds were much easier to decompose during liquefaction due to the onset degradation of seeds at the low temperatures. Therefore, the supercritical liquefaction process is commonly processed at low temperature (<400°C) which explains the choice of reaction temperature range (266–334°C), resulting in a high conversion rate of feedstock.
Temperature and Pressure Profiles
Fig. 2 illustrates the pressure and temperature profiles with respect to time within the liquefaction reactor during an experiment. There are three steps to produce a biofuel: heating time, reaction time (40min), and cooling time. Fig. 3 shows the variation of pressure against temperature of pure methanol. When the curve exceeds the critical point (239.35°C, 80.8 bars), the methanol properties (density, specific weight, kinematic viscosity, and specific heat capacity) change by moving into the supercritical state; in this case, the operating parameters conditions were at a reaction temperature of 280°C±2°C and a pressure of 124.59±1bar.
Statistical Analysis Model
The ANOVA of the response surface quadratic model was depicted in Table 3. It aimed to explain the independent variables effects (X1, X2, and X3) on the obtained response at a significance level of 0.05 in terms of their interaction and linear and quadratic contributions. It demonstrated that the fitted model was valid, with R2 = 0.9857 and p-value of 0.0001<<0.05. On the other hand, the significant terms in prediction model do not cause much difference in the value of R2adjusted (as shown in Table 3). The quadratic model for bio-oil viscosity in terms of coded factors is represented in Eq. (3).
The values from Table 3 show that residence time did not have significant effect, while the interaction between temperature, time, and MeOH/biomass ratio had a positive effect on the bio-oil viscosity (p-value < 0.001). The quadratic terms of temperature and biomass/solvent ratio (\({X}_1^2\) and \({X}_3^2\)) were significant, with p-values lower than 0.05, while other terms with p-values higher than 0.05 were statistically insignificant on the response model at the 98.57% confidence level.
The comparison between the predicted viscosity response values using the quadratic model and the actual values obtained from experiments. The predicted values are close to experimental ones, thereby validating the reliability of the developed model for establishing a correlation between the process variables and the bio-oil viscosity with a maximum relative error of 9% and an average error value of 3%.
Effects of Operating Variables on the Bio-oil Viscosity
The effects of the three factors on the bio-oil viscosity were studied using the different interaction between parameters. One factor was kept constant in each plot, which allows to understand how the other two factors interact. The third non-target variable was maintained at its mean value, i.e., temperature, X1 = 300 °C, time X2 = 30 min, and MeOH/ biomass ratio X3 = 4/1 (g/g).
The interaction between temperature and reaction time on the bio-oil viscosity at fixed MeOH/biomass mass ratio of 4/1 was studied. The production of bio-oil has been carried out at different temperatures ranging from 266 to 334°C. The lowest viscosities were obtained at 280°C and 300°C. On the other hand, the viscosity of bio-oil reduces with increasing reaction times up to 30 min, probably due to an increase in the concentration of low molecular weight compounds and short-chain molecules by further decomposition of reagents, leading to the lowest viscosities.
The combined effect of reaction time and MeOH/biomass ratio on the bio-oil viscosity response. According to Table 3, there is no interaction between both parameters resulting in no synergistic effect on bio-oil viscosity. However, when the MeOH/biomass mass ratio varies, time has no significant effect on bio-oil viscosity.
Viscosity significantly decreases by increasing MeOH/biomass mass ratio and then increases slowly after a mass ratio of 5.8/1. It can be explained by the role of alcohol involving in the depolymerization reaction. In fact, higher MeOH/biomass mass ratios resulted in higher solubility of small molecular products or intermediates in methanol and favored the formation of low molecular weight compounds. As a result, the increase of MeOH concentration (as free radicals) to a certain threshold value has promoted the breakdown of lignocellulosic and lipids bonds to form a variety of small molecules. Beyond this threshold, the bio-oil viscosity increased and is related to an increase in molecular weight explaining by self-condensation reactions and the reduction in free radical reaction [32, 33].
In fact, the action of methanol as solvent can be related to the hydrogen donor and to its alkylating ability. Hydrogen atoms act to prevent the repolymerization as a result of biomass decomposition by providing a dual-function to donate hydrogen for fragments stabilization and assisting thermal cleavage [34]. During biomass liquefaction, the hydrogen transfer from the hydrogen donor solvents stabilizes the free radical of fragmented biomass. The free radical mechanism takes place when the heated biomass cleaves into free radical and seeks stabilization depending on the energy requirements [2].
The viscosity of bio-oil decreases significantly with increasing temperature (below 295°C) and MeOH/biomass mass ratio (below 5.8/1). Afterwards an increase in bio-oil viscosity was observed. Both parameters had a synergistic effect of bio-oil viscosity.
The supercritical liquefaction of seeds with higher concentration of organic solvent requires a high temperature. This means that a high temperature could lead to a higher degree of biomass decomposition, leading to better bio-oil yield with moderate viscosities. Consequently, the supercritical fluids have properties similar to those of liquids, such as high density, which lead to more dissolving power, by allowing many compounds solvation in supercritical conditions. Therefore, the reactions that are limited by diffusion rates rather than chemical kinetics will occur more rapidly in supercritical conditions than in the subcritical conditions [1]. On the other hand, beyond a certain threshold, further increase in reaction temperature produces the opposite results due to repolymerization reactions of bio-oil molecules producing a high molecular weight [35].
The different behaviors of bio-oil viscosity against the three operating parameters are suggesting that alcohols play an important role in the change in bio-oil viscosity. According to ANOVA analysis, the linear and quadratic terms of MeOH/biomass ratio had significant effects on the response, which explains the positive influence on viscosity.
The supercritical conditions allow the transesterification of lipids at a high temperature which provides a higher reaction rates, decreases mass transfer limitations, and improves phase solubility.
The bio-oil yield from supercritical liquefaction of seeds feedstock was extremely high ranging from 55.92 to 79.62wt% along with a low solid residue yield and gaseous products. According to literature, liquefaction of carbohydrates and lignin tended to produce less bio-oil and more solid residue compared to liquefaction of lipid [36].
Process Optimization
The optimization was carried out in order to identify the operating conditions leading to the lowest viscosity. The desired purpose of bio-oil viscosity as dependent factor was defined as “minimize,” and the selected operational variables remained the value range to achieve best treatment execution. The lowest viscosity corresponds to the following optimal conditions: 282°C, 40min, and a ratio of 5.5/1, giving a viscosity of 5.90 mPa.s with low percentage of relative error (<5%) between experimental and predicted values indicating the accuracy of the optimization process. In addition, these conditions are nearly similar to the experiment run where temperature was 280°C, a time of 40min, and MeOH/biomass ratio of 5.5/1 producing a bio-oil with low viscosity of 5.94mPas.s.
Characterization of Bio-oils
The bio-oil is the target product of the supercritical liquefaction of rapeseeds. It was produced from the macromolecules decomposition by the depolymerization reaction of biomass resulting in a reactive micromolecular organic molecules combined in order to form new compounds [37]. The physicochemical characterization of the biofuel according to international standards has been carried out (as presented in the “physicochemical properties” section), thereby concluding, from an energy point of view, the quality of the product and therefore its use in an internal combustion engine.
Physicochemical Properties
The produced bio-oil at the optimal conditions was characterized following the international standards analysis. The physicochemical properties of bio-oil produced from rapeseeds were compared to biodiesel from rapeseeds oil, as shown in Table 4. The viscosity of bio-oil was measured at a temperature of 40°C. The bio-oil viscosity is slightly higher than biodiesel produced from rapeseeds oil, due to the chemical composition of biofuel, which is highly associated to the composition of feedstock (carbohydrate, lignin, protein, and lipids).
The density value of bio-oil obtained is close to the European norm EN14214 but lower than the rapeseeds oil-based biodiesel. The HHV of bio-oil is slightly lower than that of biodiesel, which may be due to the content of oxygenated components leading to a slightly higher oxygen content. Triglycerides, monoglycerides, and diglycerides were formed as intermediate products and are present in traces in bio-oil from rapeseeds. In addition, the glycerin was not separated from bio-oil which explained its presence compared to the low content in biodiesel. In fact, the production of glycerin was explained by the three-step process transesterification of lipids at methanol supercritical conditions.
To reach critical methanol conditions, the reaction mixture had to be performed at high temperature and pressure leading to the conversion of feedstock into bio-oil with a significant amount of esters. Biodiesel had a higher esters content compared to that of bio-oil. Indeed, the rapeseeds contain 42.72% of lipids which were converted into monoglycerides (1.99%), diglycerides (0.06%), esters (38.41%), glycerin (2.06%), and non-converted triglycerides (1.90%) by supercritical transesterification. In addition, a small part of esters could be produced from the lignocellulosic matrix. As esters are dissolved into bio-oil, feedstock was depolymerized leading to the production of a variety of compounds due to the interaction of different molecules (lipids, carbohydrates, lignin, and protein). In contrast to rapeseeds oil, 100% of lipids are converted into esters as major compounds (90.99%) and the other byproducts.
The O/C and H/C ratios are low compared to the feedstock, and this can be attributed to the deoxygenation and dehydration reactions that occurred at high temperature, which is in agreement with literature [38]. The advantage of using raw oleaginous seeds might enhance the bio-oil yield via the interaction between the biochemical compounds by supercritical liquefaction and transesterification. In addition, the byproducts like biochar could be used for chemical application. In order to obtain a biodiesel for engine test, the bio-oil should be upgraded through hydrodeoxygenation pathway for the conversion of oxygenated compounds, increasing the content of hydrocarbons, reducing viscosity, and increasing the calorific value.
GC-MS Analysis
Table 5 presented the major compounds of bio-oil analyzed by GC-MS, which is produced from rapeseeds at optimal operating conditions. The chemical composition is expressed as a percentage peak area (%). This value was assigned as the proportion of the peak area of each compound on the total area of the selected peaks in the chromatograms. The viscosity of bio-oil is related to its chemical composition. A few studies investigate the effect of chemical composition of viscosity of bio-oil. However, GC-MS analysis allows to understand the variation of bio-oil viscosity.
The high saturation of the FAME results in a high viscosity. Viscosity is also known to increase with increasing chain length [40]. It was noted that the bio-oil sample contains low saturated and high unsaturated FAMEs. Indeed, the feedstock is composed mainly from lipids (42.72 %) that was converted into FAME at supercritical conditions due to the complete miscibility between MeOH and oil, as reported in literature [41]. The multicomponent mixtures (carbohydrates, lignin, protein, and lipids) are fragmented and depolymerized to produce variety of compounds with different of molecular weight.
Generally, lipids are insoluble in methanol at low temperatures because they are majorly nonpolar compounds, particularly triglycerides (immiscible/hydrophobic). However, they gradually tend to become polar while heated to high temperatures. The transesterification of rapeseeds oil at supercritical conditions leads to the production of diglycerides, monoglycerides, and glycerol, which were detected by GC-FID (as shown in Table 4). The conversion of triglycerides into esters was complete which is explained by the low presence of diglycerides and monoglycerides in bio-oil. During supercritical liquefaction, the solvent undergoes a change in its polarity and dielectric constant making it capable to dissolve lipids, thus forming a single organic phase [42]. The reaction mechanism of supercritical transesterification involves the attack of the carbonyl group of the triglycerides by hydroxyl alcohol group to form FAME and diglyceride. Then, the diglyceride is attacked to form monoglyceride (and FAME) which subsequently esterifies to FAME and glycerol. This reaction is a three-step process that is highly dependent on the reaction operating conditions. According to literature, the supercritical transesterification below the temperature of 300°C is sufficient for FAME formation [43], which is in agreement with our result. Nevertheless, in the case of some unsaturated fatty acids, like those present in algae and in view of performing liquefaction during the reaction mechanism, the use of high temperatures is necessary to ensure a complete conversion of components to organic phase [42]. On the other hand, bio-oil contains high unsaturated fatty acid methyl esters which lead to a low viscosity. In fact, the intermolecular interactions are weaker in unsaturated than saturated molecules. However, the viscosity of unsaturated fatty compounds strongly depends on the nature and number of double bonds with double bond position affecting viscosity less. According to literature, the variation of the double bond position towards the middle of the chain has comparatively little effect on viscosity of FAME, so that double bond isomerization within this region should not be of significance for biofuel [44]. The presence of aromatic, oxygenated, and nitrogenated compounds, such as benzene,1,2,3-trimethoxy-5-methyl-, pyridine, 3-methoxy-, and 9-Octadecenamide,(Z), are produced from the degradation of protein and from the depolymerization of cellulose to simple sugars [45]. The high content of oleic acid and methyl ester (9-Octadecenoic acid (Z)-, methyl ester) is due to the high amount of oleic acid in lipid of rapeseeds. The synergetic effect between the different compounds of rapeseeds leads to the production of bio-oil with low molecular weight with low viscosity and good yield.
Conclusion
In this experimental study, RSM was used to determine the optimum reaction conditions for the production of bio-oil from rapeseeds with low viscosity. According to surface methodology experiments, the lowest bio-oil viscosity was 5.90 mPa.s. This result was obtained for the optimal conditions, which are reaction temperature of 280°C, reaction time of 40min, and a mass ratio MeOH/biomass of 5.5/1. Moreover, the good performance of supercritical methanol liquefaction was revealed by a high bio-oil yield of 79.62wt% containing 38.41% of FAME. Physicochemical properties of bio-oil under optimal conditions were close to that of biodiesel from rapeseeds oil. From GC-MS analysis, it was concluded that the bio-oil composition is dominated by alkyl ester compounds. GC-FID analysis has shown the low presence of triglycerides, monoglycerides, diglycerides, and glycerin content in bio-oil produced from the supercritical transesterification of lipids. Finally, rapeseeds bio-oil could be a potential alternative to diesel in addition to being an environmentally friendly fuel.
References
Mazaheri H, Lee KT, Bhatia S, Mohamed AR (2010) Sub/supercritical liquefaction of oil palm fruit press fiber for the production of bio-oil: effect of solvents. Bioresour Technol 101:7641–7647. https://doi.org/10.1016/j.biortech.2010.04.072
Isa KM, Abdullah TAT, Ali UFM (2018) Hydrogen donor solvents in liquefaction of biomass: a review. Renew Sustain Energy Rev 81:1259–1268. https://doi.org/10.1016/j.rser.2017.04.006
Karaosmanoglu F, Tetik E, Göllü E (1999) Biofuel production using slow pyrolysis of the straw and stalk of the rapeseed plant. Fuel Process Technol 59:1–12. https://doi.org/10.1016/S0378-3820(99)00004-1
Şensöz S, Angin D, Yorgun S (2000) Influence of particle size on the pyrolysis of rapeseed (Brassica napus L.): fuel properties of bio-oil. Biomass Bioenergy 19:271–279. https://doi.org/10.1016/S0961-9534(00)00041-6
Özçimen D, Karaosmanoǧlu F (2004) Production and characterization of bio-oil and biochar from rapeseed cake. Renew Energy 29:779–787. https://doi.org/10.1016/j.renene.2003.09.006
Onay O, Koçkar OM (2006) Pyrolysis of rapeseed in a free fall reactor for production of bio-oil. Fuel 85:1921–1928. https://doi.org/10.1016/j.fuel.2006.03.009
Hadhoum L, Balistrou M, Burnens G et al (2016) Hydrothermal liquefaction of oil mill wastewater for bio-oil production in subcritical conditions. Bioresour Technol 218:9–17. https://doi.org/10.1016/j.biortech.2016.06.054
Dernotte J, Hespel C, Foucher F et al (2012) Influence of physical fuel properties on the injection rate in a Diesel injector. Fuel 96:153–160. https://doi.org/10.1016/j.fuel.2011.11.073
Das M, Sarkar M, Datta A, Santra AK (2018) Study on viscosity and surface tension properties of biodiesel-diesel blends and their effects on spray parameters for CI engines. Fuel 220:769–779. https://doi.org/10.1016/j.fuel.2018.02.021
Jena U, Das KC, Kastner JR (2011) Effect of operating conditions of thermochemical liquefaction on biocrude production from Spirulina platensis. Bioresour Technol 102:6221–6229. https://doi.org/10.1016/j.biortech.2011.02.057
Alptekin E, Canakci M (2008) Determination of the density and the viscosities of biodiesel-diesel fuel blends. Renew Energy 33:2623–2630. https://doi.org/10.1016/j.renene.2008.02.020
Ng JH, Ng HK, Gan S (2012) Development of emissions predictor equations for a light-duty diesel engine using biodiesel fuel properties. Fuel 95:544–552. https://doi.org/10.1016/j.fuel.2011.12.049
Dernotte J, Hespel C, Houille S, Foucher F, Mounaim-Rousselle C (2012) Influence of fuel properties on the diesel injection process in nonvaporizing conditions. At Sprays 22:461–492. https://doi.org/10.1615/AtomizSpr.2012004401
Lalit Kumar Singh GC (2019) Liquid Biofuel Production. John Wiley and Sons, Incorporated
Teri G, Luo L, Savage PE (2014) Hydrothermal treatment of protein, polysaccharide, and lipids alone and in mixtures. Energy Fuels 28:7501–7509. https://doi.org/10.1021/ef501760d
Déniel M, Haarlemmer G, Roubaud A et al (2017) Modelling and predictive study of hydrothermal liquefaction: application to food processing residues. Waste Biomass Valorization 8:2087–2107. https://doi.org/10.1007/s12649-016-9726-7
Biller P, Ross AB (2011) Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour Technol 102:215–225. https://doi.org/10.1016/j.biortech.2010.06.028
Hadhoum L, Burnens G, Loubar K, Balistrou M, Tazerout M (2019) Bio-oil recovery from olive mill wastewater in sub-/supercritical alcohol-water system. Fuel 252:360–370. https://doi.org/10.1016/j.fuel.2019.04.133
Boelhouwer JWM, Nederbragt GW, Verberg G (1951) Viscosity data of organic liquid. Appl Sci Res 2:249–268. https://doi.org/10.1007/BF00411987
Zhou D, Zhang S, Fu H, Chen J (2012) Liquefaction of macroalgae Enteromorpha prolifera in sub-/supercritical alcohols: direct production of ester compounds. Energy Fuels 26:2342–2351. https://doi.org/10.1021/ef201966w
Zhang B, Chen J, He Z, Chen H, Kandasamy S (2019) Hydrothermal liquefaction of fresh lemon-peel: Parameter optimisation and product chemistry. Renew Energy 143:512–519. https://doi.org/10.1016/j.renene.2019.05.003
Kassargy C, Awad S, Kahine K, Khiari K, Loubar K, Tazerout M (2016) Study on the simultaneous lipids transesterification and cellulosic matter liquefaction of oleaginous seeds of Pistacia atlantica. Energy Convers Manag 124:369–376. https://doi.org/10.1016/j.enconman.2016.07.034
Yadav C, Saini A, Bera M, Maji PK (2017) Thermo-analytical characterizations of biodiesel produced from edible and non-edible oils. Fuel Process Technol 167:395–403. https://doi.org/10.1016/j.fuproc.2017.07.026
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Stefanidis SD, Kalogiannis KG, Iliopoulou EF, Michailof CM, Pilavachi PA, Lappas AA (2014) A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J Anal Appl Pyrolysis 105:143–150. https://doi.org/10.1016/j.jaap.2013.10.013
Boisen S, Bech-Andersen S, Eggum BO (1987) A critical view on the conversion factor 6.25 from total nitrogen to protein. Acta Agric Scand 37:299–304. https://doi.org/10.1080/00015128709436560
Ruppel T, Huybrighs T, Shelton CT (2008) Fatty acid methyl esters in B100 biodiesel by gas chromatography (Modified EN 14103). Perkin Elmer’s Appl note Shelton CT USA
6584–07 D (2007) Determination of free and total glycerin in B-100 biodiesel methyl esters by gas chromatography. United States ASTM Stand
Hu Y, Qi L, Feng S, Bassi A, Xu CC (2019) Comparative studies on liquefaction of low-lipid microalgae into bio-crude oil using varying reaction media. Fuel 238:240–247. https://doi.org/10.1016/j.fuel.2018.10.124
Kim JY, Lee HW, Lee SM, Jae J, Park YK (2019) Overview of the recent advances in lignocellulose liquefaction for producing biofuels, bio-based materials and chemicals. Bioresour Technol 279:373–384. https://doi.org/10.1016/j.biortech.2019.01.055
Aysu T, Durak H (2015) Assessment of avocado seeds (Persea americana) to produce bio-oil through supercritical liquefaction. Biofuels, Bioprod Biorefining 9:231–257. https://doi.org/10.1002/bbb
Hafez I, Hassan EB (2015) Rapid liquefaction of giant Miscanthus feedstock in ethanol – water system for production of biofuels. 91:219–224. https://doi.org/10.1016/j.enconman.2014.12.016
Peng X, Ma X, Lin Y, Wang X, Zhang X, Yang C (2016) Effect of process parameters on solvolysis liquefaction of Chlorella pyrenoidosa in ethanol-water system and energy evaluation. Energy Convers Manag 117:43–53. https://doi.org/10.1016/j.enconman.2016.03.029
Kuznetsov PN, Bimer J, Salbut PD, Korniyets ED, Kuznetsova LI, Snape CE (1997) The nature of the synergistic effect of binary tetralin-alcohol solvents in Kansk-Achinsk brown coal liquefaction. Fuel Process Technol 50:139–152. https://doi.org/10.1016/S0378-3820(96)01047-8
De Caprariis B, De Filippis P, Petrullo A, Scarsella M (2017) Hydrothermal liquefaction of biomass : influence of temperature and biomass composition on the bio-oil production. Fuel 208:618–625. https://doi.org/10.1016/j.fuel.2017.07.054
Yang J, Niu H, Corscadden K, Astatkie T (2018) Hydrothermal liquefaction of biomass model components for product yield prediction and reaction pathways exploration. Appl Energy 228:1618–1628. https://doi.org/10.1016/j.apenergy.2018.06.142
Durak H, Genel Y (2018) Hydrothermal conversion of biomass (Xanthium strumarium) to energetic materials and comparison with other thermochemical methods. J Supercrit Fluids 140:290–301. https://doi.org/10.1016/j.supflu.2018.07.005
Zeb H, Park J, Riaz A, Ryu C, Kim J (2017) High-yield bio-oil production from macroalgae (Saccharina japonica) in supercritical ethanol and its combustion behavior. Chem Eng J 327:79–90. https://doi.org/10.1016/j.cej.2017.06.078
Boukhalkhal AL, Kadi MEA, Lasbet Y, Loubar K, Awad S, Makhlouf M, Tazerout M (2020) A continuous biodiesel production process using a chaotic mixer-reactor. Waste Biomass Valorization 11:6159–6168. https://doi.org/10.1007/s12649-019-00880-x
Ramírez-Verduzco LF, Rodríguez-Rodríguez JE, Jaramillo-Jacob ADR (2012) Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 91:102–111. https://doi.org/10.1016/j.fuel.2011.06.070
Glišić S, Montoya O, Orlović A, Skala D (2007) Vapor-liquid equilibria of triglycerides-methanol mixtures and their influence on the biodiesel synthesis under supercritical conditions of methanol. J Serbian Chem Soc 72:13–27. https://doi.org/10.2298/JSC0701013G
Patel B, Hellgardt K (2016) Hydrothermal liquefaction and: in situ supercritical transesterification of algae paste. RSC Adv 6:86560–86568. https://doi.org/10.1039/c6ra11376a
Han H, Cao W, Zhang J (2005) Preparation of biodiesel from soybean oil using supercritical methanol and CO2 as co-solvent. Process Biochem 40:3148–3151. https://doi.org/10.1016/j.procbio.2005.03.014
Knothe G, Steidley KR (2005) Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 84:1059–1065. https://doi.org/10.1016/j.fuel.2005.01.016
Galebach PH, Soeherman JK, Wittrig AM, Lanci MP, Huber GW (2019) Supercritical methanol depolymerization and hydrodeoxygenation of maple wood and biomass-derived oxygenates into renewable alcohols in a continuous flow reactor. ACS Sustain Chem Eng 7:15361–15372. https://doi.org/10.1021/acssuschemeng.9b02704
Acknowledgements
The authors warmly thank the technical staff of the Energy Systems and Environment Department of IMT Atlantique for their guidance and support.
Availability of Data and Materials
All data generated or analyzed during this work are included in this published paper.
Funding
This work was supported by HaloSYS project (http://halosys.eu/#) funded by the national French research agency (ANR), in the framework of FACCE SURPLUS Program.
Author information
Authors and Affiliations
Contributions
Loubna Hadhoum: Experiments design, conducting the experiments, results interpretation, and drafting the manuscript
Say Awad: Supervision, experiments design, results interpretation, and writing
Gaëtan Burnens: Results interpretation
Maria Paraschiv: Manuscript revision
Khaled Loubar: Supervision, manuscript revision, and validation
Mohand Tazerout: Supervision and manuscript revision
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable
Consent for Publication
All the authors consent to publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• CCD design was used to model viscosity of bio-oil using supercritical liquefaction.
• Optimum conditions were 280°C during 40 min for mass ratio of 5.5/1 using RSM.
• A bio-oil viscosity of 5.90 mPa.s was obtained at optimal conditions.
• An optimal bio-oil yield 79.62wt% was achieved containing 38.41% of FAME.
• High correlation R2 = 0.9857 between the experimental data and the predicted values.
Rights and permissions
About this article
Cite this article
Hadhoum, L., Awad, S., Burnens, G. et al. Experimental Investigation on the Supercritical Rapeseed Methanolysis for Biofuel Production: Effects of the Operating Conditions on the Bio-oil Viscosity. Bioenerg. Res. 15, 1304–1315 (2022). https://doi.org/10.1007/s12155-021-10330-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10330-z