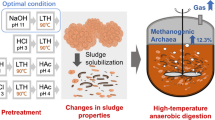
Abstract
Hydrothermal pretreatment (HTP) of sewage sludge (SS) has been shown to improve the subsequent biogas production by anaerobic digestion (AD), but the effect of catalysts on HTP performance was less explored. This study intended to investigate the SS pretreatment by wet air oxidation (WAO) with the addition of K2CO3 as a catalyst on the performance of methane production by AD. WAO was found to improve the solubilization of SS, the soluble chemical oxygen demand, dissolved organic carbon, and total dissolved nitrogen. The methane yield from WAO increased from 202 mL/gVSin with no catalyst added to 277 mL/gVSin with 10 wt% of K2CO3 added at 180 °C with 30 min of residence time. Under this pretreatment condition, the highest methane production rate could achieve 15.8 mL/gVSin day, and the percentage of methane reached 73%. The structure of the microbial community involved in the AD was affected by the residence time, working gas, and catalyst of the HTP process. The results showed that Bacteroidetes, Bacteroidia, and SC103 were the dominant phylum, class, and genus of bacteria, respectively, of almost all of the samples. In addition, the most abundant archaeal order was Methanosarcinales, while Methanosaeta was the dominant archaeal genus of most of the samples. However, Methanosarcina largely increased the relative abundance, corresponding to the amount of K2CO3 catalyst used. The findings in this study demonstrated the potential use of K2CO3 during WAO of SS and implied the link between shift of methanogen community and the enhanced methane yield in AD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sewage sludge (SS), composing of primary sludge, secondary sludge, and other sludge depending on the wastewater source and the treatment technology employed, is one of the main by-products of the activated sludge process in wastewater treatment plants [1]. SS contains a large quantity of organic compounds and has emerged as a suitable material for energy recovery [2, 3]. However, SS also includes heavy metals, microorganisms, and hazardous organic compounds [4], which can jeopardize the environment and public health if SS is not strictly treated [2, 3]. To date, agricultural activity, landfills, and incineration have been the main technologies for disposing of the sludge used in wastewater treatment plants [2, 5]. However, each of these methods carries its own advantages and limitations and may result in different environmental and economic effects [6]. Despite the longer retention time [7], anaerobic digestion (AD) is advantageous as compared with methods for stabilizing sludge due to its lower cost, lower environmental footprint, and moderated performance [8]. The biogas product of AD can be applied to cooking, heating, electricity production, and other applications. With the rapid increase in the volume of SS in recent years [9, 10], AD has proven to be an efficient technology for treating SS. This technique can transform organic wastes into green power, produce fertilizer, and reduce the environmental hazards of disposal sludges through the activity of a consortia of microorganisms, comprising both bacterial and archaeal groups [11]. The bacterial group first converts the organic compounds into hydrogen and organic acids, which are then further transmuted into methane and carbon dioxide by the archaeal group [12]. Thus, the activity of the microbial community involved in AD plays an important role in influencing the effect of biogas production.
The AD process involves four main steps, namely hydrolysis, acidogenesis, acetogenesis, and methanogenesis [1, 2, 5], and functions in the absence of oxygen. However, hydrolysis, in which complex organic matter is converted into soluble organic molecules, is the rate-limiting step for AD [2, 13, 14]. In order to enhance the performance of AD, pretreatment methods (i.e., hydrothermal [15, 16], thermal [17], chemical [18], biological [19], physical [3], and several combinations such as thermochemical [18] and physicochemical [20]) are useful ways of solving the abovementioned issue. By the application of these pretreatment methods, organic matter becomes easier for anaerobic bacteria to use, improving the digestion, enhancing the biogas production, and reducing the waste sludge released into the environment [21, 22]. Among the available pretreatment methods, hydrothermal pretreatment (HTP) is favorable by virtue of its effectiveness and simplicity of application and sanitation effects [7]. It is performed in an aqueous solvent at evaluated temperature and pressure to break down the organic and inorganic components of sludge or under inert (thermal hydrolysis (TH)) or oxidizing (wet oxidation (WO) or wet air oxidation (WAO)) atmospheres [23]. In general, the TH process, using nitrogen as the working gas, is normally applied as a pretreatment step in sludge processing, prior to AD; meanwhile, the WAO and WO processes work using air and oxygen, respectively, and can be used as the final step in the complete destruction of sludge [5, 23]. On the other hand, low-pressure WAO or WO is also used as a pretreatment step to process SS in the water phase at raised temperatures and pressures, which improves the physical and chemical properties of sludge and enhances AD [23]. In recent years, many studies have shown that water is highly reactive under the conditions of HTP; thus, this pretreatment method easily converts large molecules into smaller molecules [23,24,25] and, as a result, biogas production from sludge is improved over conventional mesophilic AD.
In an effort to enhance the effect of the anaerobic process, several conditions of HTP have been investigated. For example, Kim et al. [24] studied the effect of HTP temperature from 150 to 270 °C (1.5–3.5 MPa) on the characteristics of activated sludge and the effect of biogas production. They found that the optimum temperature for HTP was 180 °C. Donoso-Bravo et al. [21] evaluated the solubilization of macromolecular compounds and the anaerobic biodegradability of sludge from 0 to 30 min of hydrolysis time (170 °C and 8 bar). They found that the hydrolysis time in this range had a small positive effect on sludge solubilization, and a slight increase in the total biogas production was observed with increasing HTP time. Most of these studies have shown that the range of optimal temperature on HTP to enhance the effect of the AD process for SS is 160–180 °C [23, 26,27,28] with the time ranging from 20 to 60 min [29]. It is because above 180 °C, non-biodegradable compounds begin to form [21] and reduce sludge biodegradability in the AD process. Abe et al. [30] studied the influence of low-pressure WO on the thermophilic AD of secondary sludge. The highest rate of biogas production in this study was achieved at 150 °C with 40% of the theoretical oxygen required to oxidize the organic carbon content of low-pressure WO.
Alkali pretreatment of SS is a chemical technique, whereby strong alkali is used to break cell walls and membranes, thus improving the solubility of organic matter in high pH conditions [31, 32]. Kim et al. [13] showed that alkali pretreatment increased the solubilization of sludge with the effective order: NaOH > KOH > Mg(OH)2 and Ca(OH)2. Alkali pretreatment is sometimes combined with thermal treatment, which enhances the efficiency of the AD of higher biogas generation and solid reduction [10, 33,34,35]. On the other hand, in the hydrothermal liquefaction study, the weak alkali K2CO3 is often used as the catalyst to enhance the yield of oil products [36, 37], but it is not widely used in hydrothermal pretreatment for AD. Furthermore, the effect of pretreatment processes of SS on microbial communities in AD has not been well studied until recently. High-throughput sequencing technique has helped researchers to shed light on the microbial community structure of AD for the alkaline pretreatment of SS [38] and the HTP of SS [39]. Generally, previous works have primarily focused on the effect of HTP on the AD of SS on the basis of changes in temperature and time, and most study lacked comprehensive analysis of the bacterial community involved in the AD. Therefore, there is limited information regarding the microbial community structures behind the enhanced biogas production caused by different factors of HTP. In addition, most HTP used nitrogen as the working gas and did not include catalysts K2CO3 in HTP.
The current study intends to conduct evaluation of less discussed factors of HTP, including working gas and the catalyst, on the performance of the following AD process. In order to evaluate the effect of catalysts added during the HTP process on the performance of anaerobic biodegradability, K2CO3 was tested for the thermal treatment experiments in this study. Since high energy requirement is a limitation of HTP [40] and reduction of residence time will decrease energy consumption, our study plans to explore whether using catalyst in reaction will have potential to reduce the residence time of HTP. Moreover, the bacterial and archaeal communities will be further investigated to elucidate the impact of different factors of HTP on the microbial communities of the subsequent AD. Overall, this study aims to enhance our knowledge of the potential effects of HTP on the AD process and the resulting shift of microbial communities.
Materials and Methods
Materials
The SS and anaerobic sludge (AS) used were respectively collected from the concentrated sludge and anaerobic digester of Dihua Wastewater Treatment Plant in Taipei, Taiwan. Table 1 shows the properties of these sludges. After the samples were obtained from this plant, they were stored at 4 °C if not immediately used in experimental analyses.
Methods
Hydrothermal Pretreatment Process
The HTP process was performed in a reactor (HP/HT pressure reactor, 4563 Bench Top Reactor, Parr Instrument, Moline, 1 L), which was equipped with a temperature controller and pressure indicator. After adding 200 mL of SS, nitrogen or air was fed into reactor at 345 kPa for 2 min in order to replace prior gas before the HTP reaction occurred. In each test, the sample was mixed by an agitator at 100 rpm. The temperature for the HTP reactions was 180 °C at 648.1 kPa (94 psi) while the reaction time for the constant temperature reaction period was 30, 60, or 90 min. On the other hand, K2CO3 was added at 0, 5, or 10 wt% of total solids (TS) in the samples before HTP, which then checked the effect of the catalyst on the performance of the process. During the HTP process, the temperature of the samples increased from 25 to 180 °C at which it remained during the residence time of 30, 60, or 90 min before being cooled down to 50 °C by air and then to ambient temperature by cold water to end the experiment. The time for the increasing of the temperature to 180 °C was 15 min, but 110 min was recorded for the period for reducing the temperature from that to ambient temperature. The HTP process was conducted following the schematic diagram for the experimental apparatus required for HTP presented by Chang et al. [37]. The reactions of the HTP are shown in Table 2.
AD Assays
The batch AD of the SS was conducted at a mesophilic temperature (35 °C) in a water bath. To maintain the constant temperature, the water level in the bath was adjusted to the upper level of the sludge height in the bottles. In this process, 800 mL of the raw (as control) or treated SS or de-ionized (DI) water (as background) and 200 mL of the AS were added to the batch reactors (1-L glass bottles). This inoculum/substrate ratio was approximately 0.2, which was also within the ratios of previous study [41]. One-hundred milliliter bottles containing 80 mL of the treated SS and 20 mL of the AS were also set up for the confirmation of the trend of biogas production (Fig. S1 in Supplementary Information, SI). SS was the substrate of this process, while AS was the inoculum of the AD. The nitrogen gas was supplied to the bottles for 5 min to remove air from the head space and ensure the presence of anaerobic conditions. In order to minimize the effects of settling during AD, the bottles were manually mixed twice a day. The biogas was collected in a 1-L gas bag, which was then measured in terms of the volume and concentration of methane and carbon dioxide, once or two times per week. The volume of biogas gas was measured manually using the 50-mL Hamilton syringe. After 35 days of AD, the cumulative methane and carbon dioxide generation, as well as volatile solids reduction (VSR), were quantified.
Analytical Methods
Chemical oxygen demand (COD) and soluble chemical oxygen demand (SCOD) were measured with a COD kit (DR3900, HACH, USA) while the dissolved organic carbon (DOC) and total dissolved nitrogen (TDN) were measured using a TOC analyzer (1080 TOC analyzer, O∙I∙Analytical, USA). The samples were then filtered through a 0.45-μm pore size membrane filter and used for the analysis of DOC, SCOD, and TDN. The TS, volatile solids (VS), and pH were then measured in accordance with the Standard Methods [42]. All of the analyses were determined three times in order to assess the precision and data errors. The results were then presented as mean values. The contents of methane (CH4) and carbon dioxide (CO2) in the biogas were analyzed by a gas chromatography/thermal conductivity detector (GC/TCD, 8900, China Chromatography Co., Taipei, Taiwan) with a packing column (60/80 Carbonxen-1000, 4 ft. × 1/8 in SS, Sigma-Aldrich, Saint Louis, MO, USA). The pH of the control and pretreated samples was measured by pH meter (HQ40d, HACH, USA) and then adjusted to 7.0 prior to AD.
In order to exhibit the transfer of COD from the particulate form to the soluble one of sludge, the solubilization COD was determined by the following formula [43]:
where SCODT is the soluble COD of sludge after HTP (mg/L), and SCOD0 and COD0 are the soluble COD and COD of the sludge before HTP (mg/L).
The VSR was determined by following equation [24]:
where VS0/TS0 is the VS/TS before AD and VST/TST is the VS/TS after AD.
The COD mass balance was determined according to Riau et al. [44] with the following formula:
where PCODI and SCODI are particulate and soluble COD (%) before anaerobic digestion, PCODF and SCODF are particulate and soluble COD (%) after anaerobic digestion, and B is biogas converted from particulate and soluble COD in anaerobic digestion (%).
DNA Extraction and 16S rRNA Gene Amplification and Sequencing
The microbial community structures of the AD samples (35 days) were analyzed using 16S rRNA gene amplicon high-throughput sequencing. The DNA was extracted from SS by the DNeasy PowerSoil Kit (QIAGEN, Germany). The hypervariable V3 and V4 regions of microbial 16S rRNA genes were amplified using the universal primers: 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′) in the conditions of an initial denaturation at 95 °C for 3 min, followed by 30 cycles at 95 °C for 30 s, annealing at 57 °C for 30 s, with elongation at 72 °C for 30 s and a final extension at 72 °C for 5 min.
The polymerase chain reaction (PCR) products were mixed equally and then checked by means of agarose (2.0%) gel electrophoresis. The 16S rRNA gene amplicons were sent to a sequencing company (Genomics, Taiwan), and the library sequencing was carried out on an Illumina Miseq platform (Illumina Inc., San Diego, CA, USA), generating 300 bp paired-end reads. UIIME is the main tool of the workflow [45]. The raw reads were quality trimmed by Trimmomatic with PEAR. OTU (Operational Taxonomic Unit) was analyzed by mothur v.1.39.5 with 97% [46]. Chimeric sequences were identified by UCHIME v4.2 [47].
Results and Discussion
Effect of Residence Time in HTP Process on the Performance of AD
Characteristics of Pretreated Sewage Sludge by the HTP
The reaction time (or the residence time “RT”) for the constant temperature reaction is an important factor in the HTP of SS, which results in some influence on the energy balance and operating costs of the system. Therefore, this study first investigated the effect of residence time in the range of 30 to 90 min for HTP on the performance of AD at the previous suggested optimal temperature of 180 °C, under 648.1 kPa and nitrogen as the working gas.
Figure 1 shows the changes in the characteristics of the SS following HTP (values are available in Table S1 in SI). According to Fig. 1a, the value of TS and VS was nearly unchanging, and the value of VS/TS remained constant around 80% with increasing time in the pretreatment stage. However, Fig. 1b exhibits the increase in the TDN, which increased from 61 mg/L in the raw sludge to 374, 384, and 475 mg/L in the treated SS in 30, 60, and 90 min. Figure 1b also shows that the value of the DOC is 113 mg/L under untreated conditions, followed by a substantial increase to 2136, 2266, and 2670 mg/L after pretreatment at 180 °C during the residence times of 30, 60, and 90 min, respectively. Like DOC, the value of SCOD increased from 350 mg/L in the control to 7050 (RT30), 7115 (RT60), and 8450 mg/L (RT90) in Fig. 1c. Figure 1d reveals the decrease in pH when the reaction time increases during the HTP process. The value of the pH in the raw SS sample was 6.72; however, this value dropped considerably to 6.17, 6.01, and 5.95 after the HTP reaction at 180 °C in 30, 60, and 90 min of residence times, respectively. The pH decrease is considered due to the formation of the volatile fatty acids (VFA) as shown in the previous study [48, 49].
Effects of HTP time on the characteristics of SS at 180 °C and N2 as working gas. a TS and VS. b DOC and TDN. c COD, SCOD, and solubilization COD. d pH. Control, RT30, RT60, and RT90 present the characteristics of the untreated SS and treated SS at 180 °C, 648.1 kPa, and with nitrogen as the working gas during the residence time: 30, 60, and 90 min, respectively
The main purposes of HTP are the solubilization and cell disruption of substrates [24]. Accordingly, as is shown in Fig. 1, there was a rapid increase in the value of TDN, DOC, and SCOD after the HTP process, and the COD solubilization was revealed to increase with the increase in the reaction time: 25.52% (RT30), 25.9% (RT60), and 30.86% (RT90). The results illustrate the positive effect of HTP on the characteristics of treated sludge. Qiao et al. [50] also showed that organic carbon in the solid phase was converted into the liquid phase after increasing the temperature and pressure of HTP. Previous reports showed that HTP changed the physical characteristics of the sludge [24] and broke up its long carbon chain to improve the solubilization of organic matter [51]. The longer time the HTP was carried out, the more the solubilization of sludge was improved.
AD of Substrates
The VSR is an important value in AD and can be used to assess the performance of the AD process. Figure 2a illustrates the VSR of pretreated and non-pretreated samples following AD. The increase in the VSR value when the residence time increased, shown in Fig. 2a, revealed the positive effect of the residence time on the performance of the AD process. The percentage of VSR in the raw SS was 28.8%; however, this increased rapidly to the following: 44.2%, 47.7%, and finally peaked at 50.3% after 30, 60, and 90 min HTP, respectively. In addition, according to COD mass balance in Fig. 2c, only 18% of COD was converted into biogas in the raw SS; however, this percentage increased sharply in pretreated samples to 38%, 42%, and 43% after 30, 60, and 90 min HTP, respectively.
VS reduction after AD (a); volume and percentage of methane and carbon dioxide (b); COD mass balances in AD (c). Control, RT30, RT60, and RT90 represent the untreated SS and treated SS at 180 °C, 648.1 kPa, and with nitrogen as the working gas during the residence time: 30, 60, and 90 min, respectively
The volumes of methane and carbon dioxide in the AD of control and pretreated sludge are shown in Fig. 2b. In the control sample, the volumes of CH4 and CO2 were 1626 and 771 mL, which accounted, respectively, for 58% and 28% of the volume of the biogas. Besides CH4 and CO2, the rest of the biogas volume might include nitrogen, water vapor, hydrogen sulfide, ammonia, and hydrocarbons [15, 52]. However, the volumes of CH4 and CO2 were largely improved after the HTP process. When the residence time was 30 min, the volume of CH4 and CO2 was increased to 3699 mL (70% of biogas) and 1384 mL (26% of biogas), respectively. Moreover, Fig. 2b showed that the percentage of CH4 and CO2 in the biogas of the treated samples after 30, 60, and 90 min of residence time was almost the same value. The highest volumes of CH4 and CO2 were 4172 mL and 1544 mL in the sample treated for 90 min, followed by 4027 mL (CH4) and 1491 mL (CO2) of the sample pretreated for 60 min, respectively. Compared with the sample pretreated for 30 min, the percentage of CH4 increased by 8.9% and 12.8%, respectively, for samples pretreated for 60 and 90 min. According to Fig. S2a, the CH4 yield of the pretreated sludge was 220, 242, and 256 mL/gVSin, corresponding to 30, 60, and 90 min of residence time, while the yield of the raw SS was 96 mL/gVSin. Moreover, the value of CO2 was 82 mL/gVSin (30 min), 89 mL/gVSin (60 min), 95 mL/gVSin (90 min), and 45 mL/gVSin (raw sludge). The highest CH4 production rates were all observed in the second week of AD, with the values of 5.5 mL/gVSin day, 14.4 mL/gVSin day, 15.3 mL/gVSin day, and 15.8 mL/gVSin day for raw SS, 30, 60, and 90 min of residence time, respectively (Fig. S3a–d).
According to the above results, the 90 min of residence time had higher CH4 yield than that of 30 min, but they had similar percentage of CH4 around 70% of the biogas. Since the following study intends to compare the effect of working gas and the catalyst of HTP, in order to save input energy and time in the laboratory, 30 min of residence time will be chosen for the experiments afterwards to test the two factors of HTP. This time was also used for the HTP in order to enhance the yield of biogas with other substrates. For example, Huang et al. [7] carried out HTP after 30 min of residence time to improve the biogas yield of manure waste. Carrère et al. [33], meanwhile, reviewed HTP technologies to enhance AD for activated sludge. Their results showed an optimal temperature being between 160 and 180 °C following a treated time of 30–60 min of TH.
Effect of Working Gas in the HTP Process on the Performance of AD
Characteristics of Pretreated Sewage Sludge by the HTP
By employing nitrogen and air as the working gases for HTP, this study evaluated the effect of TH and WAO on the efficiency of the AD process. The characteristics of the raw SS and pretreated SS are depicted in Fig. 3. The HTP was carried out at 180 °C, 648.1 kPa, and with 30 min of residence time. As shown in Fig. 3, while the value of TS, VS and COD remained almost constant in all of the samples, the value of DOC, TDN, and SCOD changed substantially after the hydrothermal reactions were carried out. Compared with the control, there was a considerable increase of, respectively, 18.9, 6.1, and 20.1 times in the value of the DOC, TDN, and SCOD of pretreated SS using nitrogen as the working gas. In addition, with the pretreated SS using air as the working gas, the concentration of DOC, TDN, and SCOD rose rapidly to 20.2, 6.4, and 20.3 times, respectively, by comparison with the raw SS. It is worth noting that the values of the DOC, TDN, and SCOD of pretreated SS using air increased and were comparable with the values of pretreated SS using nitrogen.
AD of Substrates
As is shown in Fig. 4a, there was a substantial increase in the percentage of VSR, from 28.8% (control) to 44.2% (TH), and 39.0% (WAO); thus, the methane yield increased to 220 and 202 mL/gVSin after HTP using nitrogen and air as the working gases, respectively, as depicted in Fig. S2b. Meanwhile, the yield of carbon dioxide increased slightly, from 45 mg/L (control) to 82 mL/gVSin (nitrogen) and 87 mL/gVSin (air) after HTP. The highest CH4 production rates were all observed in the second week of AD, with the values of 5.5 mL/gVSin day, 14.4 mL/gVSin day, and 13.6 mL/gVSin day for raw SS, TH, and WAO, respectively (Fig. S3a, b, e).
Figure 4b shows the volume of biogas after the HTP was conducted with the TH and WAO processes. After the AD stage, the volume of biogas was 2793 mL for the control sample, with 5293 and 4982 mL for the pretreated sludge using TH and WAO, respectively. The amount of biogas of pretreated SS by TH was higher, by 89.5%, compared with untreated SS, and it was 6.24% higher than the amount of biogas in the pretreated SS that used WAO. Although WAO showed slightly better COD solubilization of SS than TH, TH could achieve higher VSR, which might result in the larger amount of biogas production from pretreated SS by TH. The COD mass balance (Fig. 4c) also supports the results, since 38% of COD was converted into biogas in the pretreated sample by TH, while it was 36% in the pretreated sample by WAO. It is notable that the percentage of methane and carbon dioxide in TH and WAO presented similar results. The percentage of methane in biogas was 70% using TH and 68% using WAO, respectively; meanwhile, the percentage of carbon dioxide in the two cases was 26%. From what has been shown above, HTP using nitrogen and air as the working gases effectively improved the biogas yield. At the temperature of 180 °C, 648.1 kPa, and 30 min of residence time, the amount of biogas produced from SS after HTP using nitrogen was slightly higher than that which used air. Although TH is more widely used as a pretreatment technology to enhance the performance of AD, and WAO is more often used for final sludge destruction [23], our results showed the effective use of WAO pretreatment to improve AD.
The Effect of the Catalyst Added to the HTP Process on the Performance of AD
Characteristics of Sewage Sludge Pretreated by HTP
Figure 5 shows the change in the characteristics of SS after reactions without and with the catalyst (K2CO3). Mouneimne et al. [53] reported that too high a concentration of K+ may result in the inhibition of AD. Therefore, in this study, K2CO3 was added to the reaction at 0, 5, and 10 wt% of solids in the SS. The pH values of SS samples after spiking 5 and 10 wt% K2CO3 were 8.50 and 9.56, respectively. The contribution of alkaline sludge disintegration in our experiments could be relatively minor, since according to the previous study, the alkaline pretreatment of sludge will result in limited SCOD when the pH values were below 10 [54]. The ratio of VS/TS of samples in Fig. 5a remained nearly unchanged and was the same with the above two cases; by contrast, the value of pH in Fig. 5d varied. Specifically, there was an apparent decrease in the pH, from 6.72 in the raw sample to 6.09 in the pretreated sample without the catalyst. Nevertheless, this value rose considerably, to 7.37 and 7.78, after K2CO3 was added at 5 and 10 wt% of the TS in the sample in the WAO reaction, respectively. As is shown in Fig. 5b, the DOC quickly increased by 20.2 times, from 113 mg/L of raw sludge to 2281 mg/L of WAO pretreated SS without the use of K2CO3. The concentration of the DOC also rapidly increased by approximately 28.1 times and 35.1 times, respectively, after using the catalyst corresponding to 5 and 10 wt% of TS of the SS sample. Figure 5b shows that there was a large increase in the value of TDN following WAO. This value was only 61 mg/L in the raw SS but increased to 389 mg/L after WAO pretreatment at 180 °C during the residence time of 30 min without the catalyst. In comparison with the WAO pretreated SS without the catalyst, the value of TDN increased by 38.56% and 60.92% when K2CO3 was added, corresponding to 5 and 10 wt% of the sample’s TS.
Effects of HTP catalyst on the characteristics of SS at 180 °C, 30 min, and air as the working gas. a TS and VS. b DOC and TDN. c COD, SCOD, and solubilization COD. d pH. C0, C5, and C10 represent the values of pretreated SS that K2CO3 was added to the reaction at 0, 5, and 10 wt% of the TS of the sample, respectively
As is depicted in Fig. 5c, the value of the COD in all of the samples remained unchanged; nevertheless, the concentration of SCOD in the WAO pretreated sludge improved sharply without or with the K2CO3 catalyst. The value of the SCOD jumped from 350 mg/L in the raw SS to 7100 mg/L during the WAO without the K2CO3. However, when the K2CO3 was added in the amount of 5% and 10 wt% of the TS of the sample, the value of the SCOD rose considerably by 67.6% and 102.81%, respectively. Furthermore, the increase in the SCOD was connected to the increase in the COD solubilization, and this value is shown in Fig. 5c. The value of the COD solubilization was improved to 25.71% after the WAO reaction without the catalyst and then increased to 44% and 53.52% when K2CO3 was added in the amount of 5% and 10 wt% of TS of the sample.
AD of Substrates
After AD, with a VSR of 28.78% (Fig. 6a), the yields of CH4 and CO2 in the raw SS were found to be 96 mL/gVSin and 45 mL/gVSin, respectively, which is shown in Fig. S2c. Again, the yield of methane and carbon dioxide increased rapidly when HTP was applied. In the WAO pretreated SS without the catalyst (K2CO3), the methane yield was improved to 202 mL/gVSin, with a VS removal of 38.99%. The results also show that using the catalyst (K2CO3) in WAO had a positive effect on AD. After K2CO3 was added in the WAO pretreatment reaction at the ratio of 5 wt% and 10 wt% of TS in the sample, the methane yield increased dramatically, to 247 mL/gVSin and 277 mL/gVSin, corresponding to a VSR of 45.6% and 51.4%, respectively. Nevertheless, the yield of carbon dioxide in the WAO pretreated samples was 77, 86, and 94 mL/gVSin, respectively, when the K2CO3 was added in the quantities of 0%, 5%, and 10% of the TS in the sample. The highest CH4 production rates also increased from13.6 mL/gVSin day (without K2CO3) to 15.7 mL/gVSin day (5 wt% K2CO3) and 15.8 mL/gVSin day (10 wt% K2CO3) (Fig. S3e-g). These results demonstrate that using K2CO3 in HTP with air as the working gas was very effective to enhance COD solubilization and further improve methane yield in the subsequent AD. Since the methane yield 277 mL/gVSin is the highest value observed among all treatments, especially higher than that from 90 min of residence time in HTP with nitrogen as the working gas, using K2CO3 in HTP with air as the working gas can reduce the residence time compared with traditional HTP.
The volume of methane, carbon dioxide, and biogas is presented in Fig. 6b. The volume of biogas in the untreated sludge was found to be 2793 mL after AD. However, the volume of biogas in the samples after the hydrothermal process was increased by 78.4% (without K2CO3), 101.4% (5 wt% K2CO3), and 119.8% (10 wt% K2CO3). The COD mass balance (Fig. 6c) also supports the results, since 18% of COD was converted into biogas in the untreated sludge, while it increased to 36% (without K2CO3), 41% (5 wt% K2CO3), and 44% (10 wt% K2CO3). Similar to this trend, the percentage of methane in the biogas was 58% with raw sludge; however, the percentage of methane increased to 68%, 72%, and 73% in the pretreated samples when K2CO3 was added in the amount of 0%, 5%, and 10% of the TS, respectively. It should be noted that the percentage of carbon dioxide in biogas is also similar to the above two cases, amounting to approximately 26% with and without the addition of K2CO3.
Microbial Community Analysis
AD is a biological decomposition technology involving groups of microorganisms in the absence of oxygen. The taxonomic classification of the microbial community in AD samples is shown in Fig. 7. In order to understand the bacterial and archaeal communities in AD for raw and pretreated SS, the bacterial phylum, class, and genus and the archaeal order and genus were identified after sequencing for all of the samples. The phylum, class, order, and genus are shown in Fig. 7 in terms of the percentage of the amount of archaea or bacteria present. The treatment method considerably affected the microbial community structure, and therefore, in order to evaluate the effect of the HTP conditions on the microbial communities, Fig. 7 is separated into Figs. S4, S5, and S6, which correspond to samples of different HTP residence times, different working gases, and different amounts of the catalyst in the following sections. In those figures, M1 depicts the AD of raw SS; M2, M3, and M4 represent the AD of pretreated SS under 180 °C, at 648.1 kPa, with no catalyst, N2 as the working gas, and 30, 60, and 90 min of residence time, respectively; M5, M6, and M7 show the microorganisms in AD of pretreated SS under 180 °C, 648.1 kPa, and 30 min of residence time, air as working gas and K2CO3 added 0, 5, and 10 wt% of TS, respectively; M8 shows the microbial community of AS; and M9 shows the bacterial and archaeal communities of background AD (20% AS and 80% DI water).
Taxonomic classification of microbial community. a Order (archaea). b Genus (archaea). c Phylum (bacteria). d Class (bacteria). e Genus (bacteria). M1 depicts the AD of raw SS. M2, M3, and M4 represent the AD of pretreated SS under 180 °C, at 94 PSI, with no catalyst, N2 as the working gas, and 30, 60, and 90 min of residence time, respectively. M5, M6, and M7 show the microorganisms in AD of pretreated SS under 180 °C, 94 PSI, 30 min of residence time, air as working gas, and K2CO3 added 0, 5, and 10 wt% of TS of sample, respectively. M8 shows the microbial community of AS; M9 shows the bacterial and archaeal communities of background (20% AS and 80% DI water)
Effect of the HTP Residence Time on the Microbial Community in AD
The microbial communities of archaea and bacteria in AD with the SS of the control, 30 min, 60 min and 90 min of the HTL residence times are shown in Fig. 7 and Fig. S4. The results of the taxonomic assignment demonstrate that Methanosarcinales was the most abundant order of archaea, accounting for 79.3, 43.6, 57.0, and 71.3% of the archaeal communities under these four respective conditions. The relative abundance values of Methanomicrobiales are 15.9, 44.1, 24.9, and 18.6% under these conditions, respectively (Fig. S4a). In Fig. S4b, the two dominant genera under these four conditions were Methanolinea (order Methanomicrobiales) and Methanosaeta (order Methanosarcinales), accounting for 15.2% and 79.2%, 39.5% and 41.4%, 22.2% and 55.2%, and 17.2% and 69.9% of the relative abundance values. In the short pretreatment time (30 min), the relative abundance of Methanolinea was increased considerably from 15.2 to 39.5%. Compared with Methanolinea, Methanosaeta decreased from 79.2 to 41.4%. Furthermore, when the residence time of the HTP increased, the percentage of Methanolinea decreased to 22.2% and 17.2% after 60 and 90 min. Unlike Methanolinea, the percentage of Methanosaeta increased to 55.2 and 69.9%, corresponding to a residence time increase of 2 and 3 times, respectively.
The relative abundance of bacterial communities is shown in Fig. S4c-e, in which the phylum, class, and genus levels for all of the samples were chosen for the analysis. Proteobacteria made up 32.7% of the bacterial community, which was the most abundant phylum of the control sample. Gammaproteobacteria was the most abundant class in this phylum, which made up 22.5% of the total community in the untreated sample (Fig. S4c, d). The most abundant bacterial phylum was Bacteroidetes in the three pretreated samples, which constitutes 22.7, 23.8, and 22.7% of the relative abundance values for the 30, 60, and 90 min pretreating times, respectively. Moreover, Bacteroidia was the dominant class in this phylum in the 30, 60, and 90 min residence times, which accounted for 22.3%, 23.3%, and 22.2% of the relative abundance values. The most abundant bacterial population at the genus level was SC103 (within class and phylum: Thermotogae), which constituted 4.1, 7.7, and 9.3% of the relative abundance values for the control, 60, and 90 min pretreating times. Exilispira (within phylum Spirochaetes and class Brachyspirae) was predominant for 30 min of residence time, which was 5.0% of the total community.
Methanogenesis is the final step in the AD process, by which methane is produced from three main methanogenic pathways, namely acetate by acetoclastic, methyl compounds by methylotrophic and hydrogen/carbon dioxide, and formate by hydrogenotrophic methanogens [55]. The genus Methanolinea was shown to be the major hydrogenotrophic pathway [56] while Methanosaeta constituted the major acetoclastic pathway [55]. These experimental results show that the acetoclastic pathway was the main pathway of biogas production in the AD of control or pretreated samples when the residence time was extended to 60 and 90 min. Previous kinetic values found for Methanosaeta suggest that Methanosaeta will dominate at low acetate concentrations [57]. On the other hand, although Methanosarcina can use different pathways for methane production, high acetate concentrations will favor the dominance of Methanosarcina. The predominance of Methanosaeta suggests the low acetate concentrations in our AD. In addition, the hydrogenotrophic pathway became equally important as the acetoclastic pathway in the sample with 30 min of pretreating time, implying more hydrogen or formate could be in the specific AD system. Moreover, a novel genus of methanogens Candidatus Methanofastidiosum was found increasing after HTP even though it is present as a minor methanogen. Since Candidatus Methanofastidiosum performs methanogenesis only through methylated thiol reduction [58], the result implied more methylated thiol was formed in AD of the SS after HTP with 30–90 min of residence time. Therefore, changing the residence time in HTP could change the archaeal and bacterial community of the samples.
Effect of HTP Working Gas on the Microbial Community in AD
The microbial communities of the archaea and bacteria are shown in Fig. 7 and Fig. S5. Methanomicrobiales and Methanosarcinales were the dominant archaeal orders in the control and pretreatment with TH and WAO, making up 95.2, 87.6, and 78.8% of the relative abundance, respectively (Fig. S5a). The relative abundance of the genus Methanosaeta (order: Methanosarcinales) largely dropped, from 79.2% in the control sample to 41.4% in that which was pretreated with TH and 55.0% in that pretreated with WAO. The genus Methanosarcina (order: Methanosarcinales) slightly increased from 0.12% in the control to 2.2 and 6.8% with TH and WAO pretreatment. The relative abundance of Methanolinea (order: Methanomicrobiales) increased to 39.5% of the TH pretreatment but slightly decreased to 15.03% of the WAO pretreatment in comparison with 15.2% of the control (Fig. S5b).
The most abundant phylum in the bacterial community in the untreated sample was Proteobacteria, which had a relative abundance of 32.7% (Fig. S5c). The dominant class of this sample was Gammaproteobacteria (phylum: Proteobacteria), which amounted to 22.5% (Fig. S5d). As can be seen in Fig. S5e, the genus SC103, belonging to the class and phylum Thermotogae, was the most abundant genus of bacteria in the control sample (4.1%). Furthermore, the relative abundance of Bacteroidetes was 22.7 and 29.1% with TH and WAO pretreatment and was the most abundant phylum of the bacterial community in these two AD samples (Fig. S5c). Moreover, Bacteroidia (phylum: Bacteroidetes) was the predominant class, which accounted for 22.29 and 28.50% of the bacterial community with TH and WAO pretreatment (Fig. S5d). In Fig. S5e, the relative abundance value of SC103 was 9.7% for the sample with WAO pretreatment. The relative abundance of Exilispira (phylum: Spirochaetes and class: Brachyspirae) was 5.0%, which was the most abundant genus of the sample with TH pretreatment.
From the results outlined above, it can be seen that the gas conditions of HTP play important roles for the bacterial and archaeal communities. Methane was mainly formed from both the acetoclastic and hydrogenotrophic pathways with HTP using nitrogen as the working gas and the acetoclastic pathway with HTP using air as the working gas. In addition, Methanosarcina and Candidatus Methanofastidiosum were both found increasing in AD of SS following HTP using air as the working gases, suggesting more acetate and methylated thiol could be present in this AD system.
Effect of the HTP Catalyst on the Microbial Community in AD
The microbial communities of the batch AD samples after WAO with and without K2CO3 were analyzed (Fig. 7; Fig. S6). Methanosarcinales was predominant in the archaeal order, which amounted to 79.3% in the control sample, 62.1% for WAO pretreatment without added K2CO3, 73.5% for WAO pretreatment with 5 wt% added K2CO3, and 74.4% for WAO pretreatment with 10 wt% added K2CO3 (Fig. S6a). Pretreatment and the adding of K2CO3 increased the relative abundance value of the genus Methanosarcina (order: Methanosarcinales), which increased from 0.12% in the control sample to 6.8% after WAO pretreatment without K2CO3, 21.8% of WAO pretreatment with 5 wt% K2CO3, and 39.5% of WAO pretreatment with 10 wt% K2CO3, respectively (Fig. S6b). Meanwhile, the relative abundance value of the genus Methanosaeta (order: Methanosarcinales) decreased when the samples were pretreated and more K2CO3 was added, dropping from 79.2% in the control sample to 55.0% in pretreatment without K2CO3, 51.1% in pretreatment with 5 wt% K2CO3, and 34.3% in pretreatment with 10 wt% K2CO3. As with Methanosaeta, the value of Methanolinea (order: Methanosarcina) also went down from 15.2% of the control to 15.0% in pretreatment without K2CO3, 6.6% in pretreatment with 5 wt% K2CO3 and 6.0% in pretreatment with 10 wt% K2CO3 (Fig. S6b).
Proteobacteria was the predominant bacterial phylum in the control sample, which had a relative abundance value of 32.7%. Moreover, Gammaproteobacteria was the abundant class in this phylum with 22.5% of the relative abundance value. The genus SC103 of the class and phylum Thermotogae was predominant in the control and WAO pretreatment without K2CO3, making up 4.1 and 9.7% of the relative abundance, respectively. The most abundant phylum of bacteria in all of the pretreated samples was Bacteroidetes, which constituted 29.1, 31.4 and 30.2% of the bacteria community in WAO pretreatment with 0, 5, and 10 wt% K2CO3, respectively (Fig. S6c). Nevertheless, Bacteroidia (phylum Bacteroidetes) was the predominant class and amounted 28.5% in WAO pretreatment without K2CO3, 30.8% in WAO pretreatment with 5 wt% K2CO3, and 29.7% in WAO pretreatment with 10 wt% K2CO3 (Fig. S6d). However, Macellibacteroides (phylum: Bacteroidetes and class: Bacteroidia) was the most abundant genus of bacteria under higher ratios of K2CO3 addition, showing 7.2 and 9.5% in the 5 and 10 wt% K2CO3 conditions (Fig. S6e).
The results show that K2CO3 addition influenced the bacterial and archaeal community structures in AD. Methane was formed mainly from the acetoclastic pathway if the amount of K2CO3 was equal to and less than 10 wt% of the TS in the WAO. However, Methanosarcina population gradually increased as we increased the K2CO3 addition in WAO and exceeded Methanosaeta population when 10 wt% K2CO3 was in WAO. As reported in the previous literature, high acetate concentrations will favor the dominance of Methanosarcina [57], this result implied more acetate was produced in the AD. Volatile fatty acid (VFA) generation has been reported during thermal pretreatment of sludge with oxygen [30]. A previous study has reported that VFA yield could be improved in the anaerobic fermentation of heat-alkaline pretreated SS, and acetate was detected as the major VFA [59]. Therefore, higher concentrations of acetate could be formed after WAO or during AD of pretreated sludge. In addition, spiking K2CO3 in WAO would increase salt concentration, and the elevated osmotic pressure due to high salt concentrations will affect archaeal community and likely selectively enrich Methanosarcina, since the species of Methanosarcina were reported tolerant to high levels of salt [60]. Further study is still needed to elucidate the mechanisms of the increasing Methanosarcina, since currently, there is limited information regarding the possible reactions for HTP when air is the working gas and K2CO3 is added.
Conclusions
In this study, HTP was demonstrated to enhance the efficiency of the AD of SS. HTP changed the characteristics of the sludge, increasing the values of SCOD, TDN, DOC, and COD solubilization. The WAO process at low pressure served as an effective pretreatment to improve the yield of methane in AD, but the yield of methane from WAO alone was less than TH. However, this process largely improved the yield of methane to 277 mL/gVSin and percentage of methane to 73% at 180 °C and 30 min of residence time when K2CO3 was added during WAO in the amount of 10 wt% of the TS in the sample. Furthermore, this study shows that the residence time, working gas, and catalyst of HTP had an effect on the structure of the microbial community in AD. Bacteroidetes, Bacteroidia, and SC103 were, respectively, the dominant phylum, class, and genus of bacteria in most of the samples. The most abundant archaeal order was Methanosarcinales, while Methanosaeta was the most abundant archaea genus in almost all of the samples. In addition, the relative abundance of the order Methanosarcinales and genus Methanosarcina increased when the amount of K2CO3 rose during the WAO process. Our results warrant the need to further understand the mechanisms behind the enhanced methane yield and shift of methanogen community in AD when K2CO3 was added during HTP of SS using air as the working gas.
Abbreviations
- AD:
-
Anaerobic digestion
- AS:
-
Anaerobic sludge
- CH4 :
-
Methane
- COD:
-
Chemical oxygen demand
- CO2 :
-
Carbon dioxide
- DI:
-
De-ionized
- DGGE:
-
Denaturing gradient gel electrophoresis
- DOC:
-
Dissolved organic carbon
- FISH:
-
Fluorescent in situ hybridization
- HTP:
-
Hydrothermal pretreatment
- N2 :
-
Nitrogen
- OTU:
-
Operational taxonomic units
- PCR:
-
Polymerase chain reaction
- RT:
-
Reaction time
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- SCOD:
-
Soluble chemical oxygen demand
- SS:
-
Sewage sludge
- TDN:
-
Total dissolved nitrogen
- TH:
-
Thermal hydrolysis
- TS:
-
Total solids
- VFAs:
-
Volatile fatty acids
- VS:
-
Volatile solids
- VSR:
-
Volatile solids reduction
- WAO:
-
Wet air oxidation
- WO:
-
Wet oxidation
References
Wang L, Chang Y, Li A (2019) Hydrothermal carbonization for energy-efficient processing of sewage sludge: a review. Renew Sust Energ Rev 108:423–440. https://doi.org/10.1016/j.rser.2019.04.011
Zhen G, Lu X, Kato H, Zhao Y, Li YY (2017) Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: current advances, full-scale application and future perspectives. Renew Sust Energ Rev 69:559–577. https://doi.org/10.1016/j.rser.2016.11.187
Le T, Vo P, Do T, Tran L, Truong H, Le T, Chen YH, Chang CC, Chang C, Tran Q, Tran T, Manh D (2019) Effect of assisted ultrasonication and ozone pretreatments on sludge characteristics and yield of biogas production. Processes 7(10):743. https://doi.org/10.3390/pr7100743
Escala M, Zumbühl T, Koller C, Junge R, Krebs R (2013) Hydrothermal carbonization as an energy-efficient alternative to established drying technologies for sewage sludge: a feasibility study on a laboratory scale. Energy Fuel 27(1):454–460. https://doi.org/10.1021/ef3015266
Tyagi VK, Lo SL (2013) Sludge: a waste or renewable source for energy and resources recovery? Renew Sust Energ Rev 25:708–728. https://doi.org/10.1016/j.rser.2013.05.029
Fytili D, Zabaniotou A (2008) Utilization of sewage sludge in EU application of old and new methods—a review. Renew Sust Energ Rev 12(1):116–140. https://doi.org/10.1016/j.rser.2006.05.014
Huang W, Zhao Z, Yuan T, Huang W, Lei Z, Zhang Z (2017) Low-temperature hydrothermal pretreatment followed by dry anaerobic digestion: a sustainable strategy for manure waste management regarding energy recovery and nutrients availability. Waste Manag 70:255–262. https://doi.org/10.1016/j.wasman.2017.09.011
Appels L, Baeyens J, Degrève J, Dewil R (2008) Principles and potential of the anaerobic digestion of waste-activated sludge. Prog Energy Combust Sci 34(6):755–781. https://doi.org/10.1016/j.pecs.2008.06.002
Li X, Chen L, Mei Q, Dong B, Dai X, Ding G, Zeng EY (2018) Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res 142:75–85. https://doi.org/10.1016/j.watres.2018.05.034
Chen H, Yi H, Li H, Guo X, Xiao B (2020) Effects of thermal and thermal-alkaline pretreatments on continuous anaerobic sludge digestion: performance, energy balance and, enhancement mechanism. Renew Energy 147(1):2409–2416. https://doi.org/10.1016/j.renene.2019.10.051
Anukam A, Mohammadi A, Naqvi M, Granström K (2019) A review of the chemistry of anaerobic digestion: methods of accelerating and optimizing process efficiency. Processes 7(8):504. https://doi.org/10.3390/pr7080504
Lim JW, Chen CL, Ho IJR, Wang JY (2013) Study of microbial community and biodegradation efficiency for single- and two-phase anaerobic co-digestion of brown water and food waste. Bioresour Technol 147:193–201. https://doi.org/10.1016/j.biortech.2013.08.038
Kim J, Park C, Kim TH, Lee M, Kim S, Kim SW, Lee J (2003) Effects of various pretreatments for enhanced anaerobic digestion with waste activated sludge. J Biosci Bioeng 95(3):271–275. https://doi.org/10.1016/S1389-1723(03)80028-2
Antwi E, Engler N, Nelles M, Schüch A (2019) Anaerobic digestion and the effect of hydrothermal pretreatment on the biogas yield of cocoa pods residues. Waste Manag 88:131–140. https://doi.org/10.1016/j.wasman.2019.03.034
Ferrentino R, Merzari F, Fiori L, Andreottola G (2019) Biochemical methane potential tests to evaluate anaerobic digestion enhancement by thermal hydrolysis pretreatment. Bioenergy Res 12(3):722–732. https://doi.org/10.1007/s12155-019-10017-6
Aragón-Briceño C, Ross AB, Camargo-Valero MA (2017) Evaluation and comparison of product yields and bio-methane potential in sewage digestate following hydrothermal treatment. Appl Energy 208:1357–1369. https://doi.org/10.1016/j.apenergy.2017.09.019
Ennouri H, Miladi B, Diaz SZ, Güelfo LAF, Solera R, Hamdi M, Bouallagui H (2016) Effect of thermal pretreatment on the biogas production and microbial communities balance during anaerobic digestion of urban and industrial waste activated sludge. Bioresour Technol 214:184–191. https://doi.org/10.1016/j.biortech.2016.04.076
Rafique R, Poulsen TG, Nizami AS, Asam ZZ, Murphy JD, Kiely G (2010) Effect of thermal, chemical and thermo-chemical pre-treatments to enhance methane production. J Energy 35(12):4556–4561. https://doi.org/10.1016/j.energy.2010.07.011
Melamane X, Tandlich R, Burgess J (2007) Anaerobic digestion of fungally pre-treated wine distillery wastewater. Afr J Biotechnol 6(17):1990–1993. https://doi.org/10.5897/AJB2007.000-2305
Chiu YC, Chang CN, Lin JG, Huang SJ (1997) Alkaline and ultrasonic pretreatment of sludge before anaerobic digestion. Water Sci Technol 36(11):155–162. https://doi.org/10.1016/S0273-1223(97)00681-1
Donoso-Bravo A, Pérez-Elvira S, Aymerich E, Fdz-Polanco F (2011) Assessment of the influence of thermal pre-treatment time on the macromolecular composition and anaerobic biodegradability of sewage sludge. Bioresour Technol 102(2):660–666. https://doi.org/10.1016/j.biortech.2010.08.035
Gong L, Yang X, Wang Z, Zhou J, You X (2019) Impact of hydrothermal pre-treatment on the anaerobic digestion of different solid–liquid ratio sludges and kinetic analysis. RSC Adv 9(33):19104–19113. https://doi.org/10.1039/C9RA01662G
Hii K, Baroutian S, Parthasarathy R, Gapes DJ, Eshtiaghi N (2014) A review of wet air oxidation and thermal hydrolysis technologies in sludge treatment. Bioresour Technol 155:289–299. https://doi.org/10.1016/j.biortech.2013.12.066
Kim D, Lee K, Park KY (2015) Enhancement of biogas production from anaerobic digestion of waste activated sludge by hydrothermal pre-treatment. Int Biodeterior Biodegrad 101:42–46. https://doi.org/10.1016/j.ibiod.2015.03.025
Mustafa AM, Li H, Radwan AA, Sheng K, Chen X (2018) Effect of hydrothermal and Ca(OH)2 pretreatments on anaerobic digestion of sugarcane bagasse for biogas production. Bioresour Technol 259:54–60. https://doi.org/10.1016/j.biortech.2018.03.028
Jeong SY, Chang SW, Ngo HH, Guo W, Nghiem LD, Banu JR, Jeon BH, Nguyen DD (2019) Influence of thermal hydrolysis pretreatment on physicochemical properties and anaerobic biodegradability of waste activated sludge with different solids content. Waste Manag 85:214–221. https://doi.org/10.1016/j.wasman.2018.12.026
Choi JM, Han SK, Lee CY (2018) Enhancement of methane production in anaerobic digestion of sewage sludge by thermal hydrolysis pretreatment. Bioresour Technol 259:207–213. https://doi.org/10.1016/j.biortech.2018.02.123
Kepp U, Machenbach I, Weisz N, Solheim OE (2000) Enhanced stabilisation of sewage sludge through thermal hydrolysis - three years of experience with full scale plant. Water Sci Technol 42(9):89–96. https://doi.org/10.2166/wst.2000.0178
Sapkaite I, Barrado E, Fdz-Polanco F, Pérez-Elvira SI (2017) Optimization of a thermal hydrolysis process for sludge pre-treatment. J Environ Manag 192:25–30. https://doi.org/10.1016/j.jenvman.2017.01.043
Abe N, Tang YQ, Iwamura M, Morimura S, Kida K (2013) Pretreatment followed by anaerobic digestion of secondary sludge for reduction of sewage sludge volume. Water Sci Technol 67(11):2527–2533. https://doi.org/10.2166/wst.2013.154
Tyagi VK, Lo SL (2011) Application of physico-chemical pretreatment methods to enhance the sludge disintegration and subsequent anaerobic digestion: an up to date review. Rev Environ Sci Biotechnol 10(3):215–242. https://doi.org/10.1007/s11157-011-9244-9
Tulun Ş, Bilgin M (2019) Enhancement of anaerobic digestion of waste activated sludge by chemical pretreatment. J Fuel 254:115671. https://doi.org/10.1016/j.fuel.2019.115671
Carrère H, Dumas C, Battimelli A, Batstone DJ, Delgenès JP, Steyer JP, Ferrer I (2010) Pretreatment methods to improve sludge anaerobic degradability: a review. J Hazard Mater 183(1):1–15. https://doi.org/10.1016/j.jhazmat.2010.06.129
Liu J, Dong L, Dai Q, Liu Y, Tang X, Liu J, Xiao B (2019a) Enhanced anaerobic digestion of sewage sludge by thermal or alkaline-thermal pretreatments: influence of hydraulic retention time reduction. Int J Hydrog Energy 45(4):2655–2667. https://doi.org/10.1016/j.ijhydene.2019.11.198
Liu X, Xu Q, Wang D, Yang Q, Wu Y, Li Y, Fu Q, Yang F, Liu Y, Ni BJ, Wang Q, Li X (2019b) Thermal-alkaline pretreatment of polyacrylamide flocculated waste activated sludge: process optimization and effects on anaerobic digestion and polyacrylamide degradation. Bioresour Technol 281:158–167. https://doi.org/10.1016/j.biortech.2019.02.095
Karagöz S, Bhaskar T, Muto A, Sakata Y (2006) Hydrothermal upgrading of biomass: effect of K2CO3 concentration and biomass/water ratio on products distribution. Bioresour Technol 97(1):90–98. https://doi.org/10.1016/j.biortech.2005.02.051
Chang CC, Chen CP, Yang CS, Chen YH, Huang M, Chang CY, Shie JL, Yuan MH, Chen YH, Ho C, Li K, Yang MT (2016) Conversion of waste bamboo chopsticks to bio-oil via catalytic hydrothermal liquefaction using K2CO3. Sustain Environ Res 26(6):262–267. https://doi.org/10.1016/j.serj.2016.08.002
Sun R, Xing D, Jia J, Zhou A, Zhang L, Ren N (2014) Methane production and microbial community structure for alkaline pretreated waste activated sludge. Bioresour Technol 169:496–501. https://doi.org/10.1016/j.biortech.2014.07.032
Li W, Guo J, Cheng H, Wang W, Dong R (2017) Two-phase anaerobic digestion of municipal solid wastes enhanced by hydrothermal pretreatment: viability, performance and microbial community evaluation. Appl Energy 189:613–622. https://doi.org/10.1016/j.apenergy.2016.12.101
Ruiz HA, Conrad M, Sun SN, Sanchez A, Rocha GJM, Romaní A, Castro E, Torres A, Rodríguez-Jasso RM, Andrade LP, Smirnova I, Sun RC, Meyer AS (2020) Engineering aspects of hydrothermal pretreatment: from batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour Technol 299:122685. https://doi.org/10.1016/j.biortech.2019.122685
Boulanger A, Pinet E, Bouix M, Bouchez T, Mansour AA (2012) Effect of inoculum to substrate ratio (I/S) on municipal solid waste anaerobic degradation kinetics and potential. Waste Manag 32(12):2258–2265. https://doi.org/10.1016/j.wasman.2012.07.024
American Public Health Association (APHA) (1998) Standard methods for the examination of water and wastewater, Washington DC
Benabdallah El-Hadj T, Dosta J, Márquez-Serrano R, Mata-Álvarez J (2007) Effect of ultrasound pretreatment in mesophilic and thermophilic anaerobic digestion with emphasis on naphthalene and pyrene removal. Water Res 41(1):87–94. https://doi.org/10.1016/j.watres.2006.08.002
Riau V, De la Rubia MA, Pérez M (2015) Upgrading the temperature-phased anaerobic digestion of waste activated sludge by ultrasonic pretreatment. Chem Eng J 259:672–681. https://doi.org/10.1016/j.cej.2014.08.032
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Keley ST, Knoghts D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allow analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Blaxter M, Mann J, Chapman T, Thomas F, Whitton C, Floyd R, Abebe E (2005) Defining operational taxonomic units using DNA barcode data. Philos Trans R Soc London, Ser B 360:1935–1943. https://doi.org/10.1098/rstb.2005.1725
Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, Ciulla D, Tabbaa D, Highlander SK, Sodergren E, Methé B, DeSanits TZ, Human MC, Petrosino JF, Knight R, Birren BW (2011) Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res 21:494–504. https://doi.org/10.1101/gr.112730.110
Urrea JL, García M, Collado S, Oulego P, Díaz M (2018) Sludge hydrothermal treatments. Oxidising atmosphere effects on biopolymers and physical properties. J Environ Manage 206:284–290. https://doi.org/10.1016/j.jenvman.2017.10.043
Eskicioglu C, Kennedy KJ, Droste RL (2006) Characterization of soluble organic matter of waste activated sludge before and after thermal pretreatment. Water Res 40(20):3725–3736. https://doi.org/10.1016/j.watres.2006.08.017
Qiao W, Yan X, Ye J, Sun Y, Wang W, Zhang Z (2011) Evaluation of biogas production from different biomass wastes with/without hydrothermal pretreatment. Renew Energy 36(12):3313–3318. https://doi.org/10.1016/j.renene.2011.05.002
Vlyssides AG, Karlis PK (2004) Thermal-alkaline solubilization of waste activated sludge as a pre-treatment stage for anaerobic digestion. Bioresour Technol 91(2):201–206. https://doi.org/10.1016/S0960-8524(03)00176-7
Angelidaki I, Treu L, Tsapekos P, Luo G, Campanaro S, Wenzel H, Kougias PG (2018) Biogas upgrading and utilization: current status and perspectives. Biotechnol Adv 36(2):452–466. https://doi.org/10.1016/j.biotechadv.2018.01.011
Mouneimne AH, Carrère H, Bernet N, Delgenès JP (2003) Effect of saponification on the anaerobic digestion of solid fatty residues. Bioresour Technol 90(1):89–94. https://doi.org/10.1016/S0960-8524(03)00091-9
Li H, Li C, Liu W, Zou S (2012) Optimized alkaline pretreatment of sludge before anaerobic digestion. Bioresour Technol 123:189–194. https://doi.org/10.1016/j.biortech.2012.08.017
Li N, He J, Yan H, Chen S, Dai X (2017) Pathways in bacterial and archaeal communities dictated by ammonium stress in a high solid anaerobic digester with dewatered sludge. Bioresour Technol 241:95–102. https://doi.org/10.1016/j.biortech.2017.05.094
Kim J, Kim W, Lee C (2013) Absolute dominance of hydrogenotrophic methanogens in full-scale anaerobic sewage sludge digesters. J Environ Sci 25(11):2272–2280. https://doi.org/10.1016/S1001-0742(12)60299-X
Conklin A, Stensel HD, Ferguson J (2006) Growth kinetics and competition between Methanosarcina and Methanosaeta in mesophilic anaerobic digestion. Water Environ Res 78:486–496. https://doi.org/10.2175/106143006X95393
Nobu M, Narihiro T, Kuroda K, Mei R, Liu WT (2016) Chasing the elusive Euryarchaeota class WSA2: genomes reveal a uniquely fastidious methyl-reducing methanogen. ISME J 10:2478–2487. https://doi.org/10.1038/ismej.2016.33
Ma H, Chen X, Liu H, Liu H, Bo F (2016) Improved volatile fatty acids anaerobic production from waste activated sludge by pH regulation: alkaline or neutral pH? Waste Manag 48:397–403. https://doi.org/10.1016/j.wasman.2015.11.029
Vrieze JD, Hennebel T, Boon N, Verstraete W (2012) Methanosarcina: the rediscovered methanogen for heavy duty biomethanation. Bioresour Technol 112:1–9. https://doi.org/10.1016/j.biortech.2012.02.079
Acknowledgments
The authors would also like to thank Dihua Wastewater Treatment Plant (Taipei, Taiwan) for providing the sludge used in the experiments.
Funding
This research was supported by the Ministry of Science and Technology, Taiwan, under grants MOST 107-2218-E-197-003 and National Taiwan University from Excellence Research Program—Core Consortiums under Higher Education Sprout Project, Ministry of Education, Taiwan (NTUCCP-109L891203). This article was also subsidized by National Taiwan University under the Excellence Improvement Program for Doctoral Students. We would like to thank the Ministry of Science and Technology, Taiwan, for providing V. T. Pham the Ph.D. scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 4470 kb)
Rights and permissions
About this article
Cite this article
Pham, V.T., Wu, PH., Guan, CY. et al. Biogas Production and Microbial Communities in the Anaerobic Digestion of Sewage Sludge Under Hydrothermal Pretreatment with Air and a Catalyst. Bioenerg. Res. 14, 828–843 (2021). https://doi.org/10.1007/s12155-020-10199-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10199-4