Abstract
Sweet sorghum is a bioenergy crop that produces large amounts of soluble sugars in its stems (3–7 Mg ha−1) and generates significant amounts of bagasse (15–20 Mg ha−1) as a lignocellulosic feedstock. These sugars can be fermented not only to biofuels but also to bio-based chemicals. The market potential of the latter may be higher given the current prices of petroleum and natural gas. The yield and rate of production of optically pure d-(−)- and l-(+)-lactic acid as precursors for the biodegradable plastic polylactide was optimized for two thermotolerant Bacillus coagulans strains. Strain 36D1 fermented the sugars in unsterilized sweet sorghum juice at 50 °C to l-(+)-lactic acid (∼150 g L−1; productivity, 7.2 g L−1 h−1). B. coagulans strain QZ19-2 was used to ferment sorghum juice to d-(−)-lactic acid (∼125 g L−1; productivity, 5 g L−1 h−1). Carbohydrates in the sorghum bagasse were also fermented after pretreatment with 0.5 % phosphoric acid at 190 °C for 5 min. Simultaneous saccharification and co-fermentation of all the sugars (SScF) by B. coagulans resulted in a conversion of 80 % of available carbohydrates to optically pure lactic acid depending on the B. coagulans strain used as the microbial biocatalyst. Liquefaction of pretreated bagasse with cellulases before SScF (L + SScF) increased the productivity of lactic acid. These results show that B. coagulans is an effective biocatalyst for fermentation of all the sugars present in sweet sorghum juice and bagasse to optically pure lactic acid at high titer and productivity as feedstock for bio-based plastics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While much effort has been invested in microbial production of biofuels from feedstocks that do not compete with food production [1, 2], the current low price of petroleum and natural gas has raised concerns over the competitiveness of biofuels [3]. Fermentable sugars from bioenergy feedstocks can also be used for production of bio-based chemicals and polymers that are normally made from petroleum, thereby offering the same environmental benefits as the displacement of petroleum as transportation fuel [4]. Lactic acid is a commodity chemical that is used by the chemical, food, cosmetics, and pharmaceutical industries and the estimated global market of optically pure lactic acid by fermentation in 2013 was about 725,000 t. About 50 % of this lactic acid is used by the polymer industry to produce bio-based, biodegradable plastics (polylactide (PLA)), and this market is projected to grow by about 20 % per year during the next 5 years along with the demand for lactic acid at about the same rate [5].
Almost all of the lactic acid is produced by microbial fermentation of sugars, which accommodates the desire for chiral purity of the product [6]. Although l-lactic acid production is a mature industry, commercial production of d-lactic acid has only been accomplished recently. d-Lactic acid is a significant component of PLA plastics since inclusion of d-lactides during polymerization alters the thermochemical properties of the polymer leading to wider range of applications [7, 8]. Thus, a source of d-lactic acid at a competitive price is needed to expand the use of renewable PLA plastics. In addition, the sugars used in lactic acid fermentations are currently derived from food carbohydrates such as corn starch or sucrose from sugar beets or sugarcane. Use of carbohydrates derived from non-food sources, including lignocellulosic biomass, will eliminate competition with food [9] and is expected to lower the cost of lactic acid for PLA plastics [10].
Among the several sources of non-food carbohydrates, sweet sorghum (Sorghum bicolor (L.) Moench) stands out as a bioenergy crop due to its potential to generate more fermentation products, such as ethanol, per hectare than many other crops [11, 12]. Sweet sorghum stems accumulate high amounts of soluble sugar (15–20 %; 3–7 Mg ha−1) and also represent a substantial amount of lignocellulosic biomass (15–20 Mg dry biomass ha−1) [13–16]. Sorghum juice contains a mixture of sucrose, glucose, and fructose, and the sugars in the juice can be readily fermented to lactate by microbial biocatalysts [12, 17–20].
Monosaccharides derived from the cellulose and hemicellulose fraction of the bagasse obtained after extraction of juice can also be fermented to lactic acid after appropriate pretreatment [14, 20, 21]. However, hydrolysis of cellulose in the biomass to fermentable sugars requires commercial fungal cellulases that have an optimum temperature and pH of 50 °C and 5.0, respectively, for activity [22]. In cellulosic ethanol production, the cost of enzymes has been identified as one of the significant cost components and attempts are made to minimize the cellulase loading [23]. Saccharification of cellulose by cellulases and simultaneous fermentation of the released glucose to lactic acid (SSF) is expected to maintain high enzyme activity by reducing product inhibition while lowering the overall product cost by minimizing process steps [24].
Bacillus coagulans is a sporogenic lactic acid bacterium that grows and ferments hexose and pentose sugars primarily to l-(+)-lactic acid at 50–55 °C and at pH values as low as 4.5 [25]. This bacterium offers several advantages over other mesophilic lactic acid bacteria in the fermentation of biomass-derived sugars. These include a temperature optimum of growth that matches the temperature optimum for activity of commercial fungal cellulases and thus reducing the level of enzymes needed for SSF of cellulose by at least threefold for the same level of activity [26]. In addition, the higher temperature minimizes contamination of industrial fermentations while also reducing cooling costs [27]. The inability of common environmental contaminants to grow at 50 °C may permit industrial use of raw sorghum juice directly without sterilization. While B. coagulans is ideally suited for SSF of pretreated bagasse to l-lactic acid, a d-lactic acid-producing natural isolate of B. coagulans has not been reported. We recently described the construction of B. coagulans strain QZ19 [28] as the only described d-lactic acid-producing microbial biocatalyst that grows and ferments both hexose and pentose sugars at high yield at a temperature optimum of 50–55 °C.
In this communication, we have focused on B. coagulans as a microbial biocatalyst for fermentation of raw sorghum juice at 50 °C to l-(+)- or d-(−)-lactic acid using strain 36D1 or QZ19-2, respectively. Both organisms metabolized hexose and pentose sugars to a lactic acid titer of >1 M. These strains were also used to ferment the sugars derived from pretreated sorghum bagasse to respective lactic acid isomers.
Materials and Methods
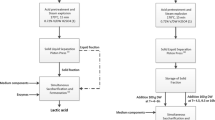
Sweet Sorghum Juice
Sweet sorghum (Sorghum bicolor (L.) Moench) cultivar M-81E was grown at the University of Florida Plant Science Research and Education Center near Citra, FL, during the summer 2013 growing season and harvested when the seed reached soft dough stage, just prior to full maturity. After removal of leaves and panicles, the stems were pressed using a roller press at 12 MPa of pressure in a grooved, two-roller electric mill, and the juice was collected and pooled. Large particles in the juice were removed by passing through two layers of cheese cloth before use. Juice was stored frozen until needed.
Sweet Sorghum Bagasse
Sweet sorghum bagasse, obtained after juice extraction, was treated with 0.5 % phosphoric acid (w/w of bagasse dry weight) and steam at 190 °C for 5 min at the University of Florida Stan Mayfield pilot biorefinery near Perry, FL, as described previously for sugarcane bagasse [29]. This process generated a slurry with a dry matter content of 25.3 % (w/w) and the following composition of free sugars (g kg−1 of slurry on a dry weight basis): cellobiose, 29.4; glucose, 30.5; xylose, 129.0; arabinose, 22.6; galactose, 6.9, as determined with HPLC using methods described previously [30] . This pretreated bagasse slurry was used directly for simultaneous saccharification and co-fermentation (SScF) of all available sugars to lactic acid. As a separate treatment, the bagasse slurry was washed twice with deionized water (three times the wet weight of slurry followed by centrifugation at 4000×g for 10 min) to remove the hemicellulose-derived sugars and inhibitors released during pretreatment before SScF. Liquefaction of pretreated bagasse slurry with a solids loading of 3.75 % (w/v) occurred in the fermentation medium supplemented with Cellic CTec2 cellulase cocktail (generously provided by Novozymes, Franklinton, NC) with an activity of 145 filter paper unit (FPU) mL−1 as determined using the procedure described previously [22]. The liquefaction was conducted at 50 °C, pH 5.0, in a 2.8-L Fernbach flask in a shaker at 100 RPM.
Bacterial Strains and Culture Conditions
B. coagulans strains 36D1 and P4-102B were described previously [25]. B. coagulans strain 2D1 was isolated from a waste container at Hollywood, FL, using a similar procedure as for isolation of strain 36D1 [25]. d-Lactic acid-producing B. coagulans strain QZ19 was described previously [28]. Strain QZ19-2 is a derivative of strain QZ19 that was selected for improved growth in mineral salts medium with biotin, folic acid, thiamine (1 mg L−1 each) and arginine (20 mg L−1). For routine use, all cultures were grown in LB medium (RPI Research Products International, Inc., Prospect, IL). Fermentation of raw sorghum juice supplemented with 0.5 % (w/v) yeast extract (Fisher Scientific, Pittsburgh, PA) or 0.5 % (v/v) corn steep liquor (50 % dry solids; Grain Processing Corp., Muscatine, IO) was conducted in a volume of 250 mL in a 500-mL vessel, at 50 °C and pH 7.0. The cultures were mixed with a magnetic stirrer operating at 250 RPM except for SScF of bagasse (300 RPM). Culture pH was maintained at 7.0 with automatic addition of 2 N KOH. Calcium carbonate (2.4 % (w/v)) was added to raw sorghum juice to support high productivity, especially during later stages of fermentation. For SScF of pretreated sorghum bagasse, a mineral salts medium supplemented with 0.5 % (w/v) corn steep liquor described previously [22] was used at 50 °C, as described previously for fermentation of crystalline cellulose [26, 25]. Cellic CTec2 cellulase cocktail was added at 15 FPU g−1 dry weight of pretreated bagasse. Since B. coagulans strains 36D1, P4-102B, and QZ19-2 lack the ability to metabolize sucrose, 0.4 % (v/v) invertase (LorAnn Oils, Lansing, MI) was added to the juice at the beginning of fermentation. Chemical analyses showed that sucrose was hydrolyzed to glucose and fructose by invertase within 30 min after addition.
Lactate Dehydrogenase Assay
For determination of lactate dehydrogenase activity, cells were cultured in1 L of LB supplemented with glucose (5 g L−1) and HEPES buffer (100 mM; pH 7.0) using 10 mL of an aerobic exponential phase of growth culture in LB with glucose (5 g L−1) as inoculum. The 2-L Erlenmeyer flasks with the cultures were incubated at 50 °C on a shaker with gentle mixing (120 RPM). Cells were harvested at mid-exponential phase of growth (0.6 g dry weight cells L−1) by centrifugation at 8000×g for 20 min at room temperature. Cells were washed once with 25 mL of phosphate-buffered saline (PBS) (phosphate, 12 mM; NaCl, 137 mM; KCl, 2.7 mM; pH 7.4) and resuspended in 2 mL of PBS. DNase and protease inhibitor cocktail (Sigma Chemical Co., St. Louis, MO) were added to the cell suspension before breaking the cells by passing through a French pressure cell operating at 138 MPa. The cell extract was centrifuged at 27,000×g for 20 min at 4 °C to remove cell debris. The supernatant was filtered through a 0.45-μm filter, and the membrane vesicles were removed by centrifugation at 150,000×g for 75 min at 4 °C. The supernatant was used for LDH assay.
LDH activity in the extract was determined by following the forward reaction with pyruvate (30 mM) and NADH (0.225 mM) as substrates in MES-Tricine buffer (50 mM; pH 6.5) [28]. The initial rate of reduction in A360 nm, due to NADH oxidation, was used to calculate the enzyme activity and is expressed as μmol min−1 mg protein−1.
Analytical Methods
Sugar and organic acid concentrations were determined by HPLC [30]. Optical purity of lactic acid was determined using an Agilent HPLC (1090) equipped with Chirex 3126(d)-penicillamine column (150 × 4.6 mm; Phenomenex, Torrance, CA) and variable wavelength detector. The eluent was 2 mM CuSO4 at 0.6 mL min−1. Product yield is presented as gram of product produced per gram of calculated amount of sugars that can be derived from the carbohydrates in the biomass (cellulose, 40 % (w/w), and hemicellulose, 20 % (w/w)) or in the case of juice, the amount of sugar consumed.
Results and Discussion
Fermentation of Raw Sweet Sorghum Juice to l-Lactic Acid
Raw sweet sorghum juice contains readily fermentable sucrose, glucose, and fructose as carbon sources, and initial attempts to evaluate the potential of B. coagulans for l-lactate production were conducted with raw juice and pH control. At 50 °C, and after a lag of 24 h, B. coagulans strain 36D1 fermented the glucose and fructose in the raw sorghum juice to a final titer of 0.3 M l-lactic acid with a significant amount of sugars left behind. This could be due to limiting micronutrients present in the juice. Supplementing the juice with vitamins (biotin, folic acid, and thiamine at 1 mg L−1) and histidine at 20 mg L−1, the components that improved aerobic growth of strain 36D1 in glucose mineral salts medium (unpublished data) did not significantly alter the fermentation profile or product titer (data not shown). Addition of ammonium sulfate (2 g L−1) as N source to the juice did increase the lactic acid titer to about 0.6 M after 144 h (Fig. S1; online resource). Sucrose was not fermented by strain 36D1 and required supplementation of the juice with invertase at the start of the experiment to hydrolyze the sugar to glucose and fructose. Fermentation of the sugars in the juice was severely affected when NH4OH was used to maintain pH as lactic acid accumulated, although it not only controls pH but also serves as a nitrogen source (Fig. S2; online resource). KOH was determined to be a more effective base for neutralization of the lactic acid produced by the culture. Based on these results, NH4OH was used to adjust the initial pH of the juice to 7.0 to provide a N source for growth of the bacterium and the pH was maintained with KOH during fermentation.
Using raw juice supplemented with corn steep liquor and invertase, and a mix of NH4OH and KOH to maintain the pH at 7.0, fermentation of the sugars present in sorghum juice by strain 36D1 at 50 °C resulted in a maximum volumetric productivity of 4.2 g l-(+)-lactic acid L−1 h−1 and the titer was 1.4 M after 144-h fermentation (Fig. 1; Table 1). Significant amounts of acetate, ethanol, and formate were also detected in the broth. Presence of these co-products reduced the l-lactate yield (g g−1 of sugars) to 86 % of the theoretical value. Since formate is an indicator of pyruvate formate-lyase (PFL) activity and the acetate and ethanol are generated from the acetyl-CoA to support redox balance, these co-products are apparently derived from the activity of PFL in strain 36D1 grown in sorghum juice. It is known that the PFL activity of facultative anaerobes is higher under poor nutritional condition compared to growth in a nutrient-rich medium [31–33]. These results suggest that the sweet sorghum juice supplemented with 0.5 % corn steep liquor is apparently not supporting the highest growth rate B. coagulans strain 36D1 can achieve in an optimal medium. To improve the l-lactate productivity of strain 36D1, yeast extract was added to the juice.
Fermentation of raw sorghum juice supplemented with corn steep liquor to l-lactic acid by B. coagulans strain 36D1. Raw sorghum juice was supplemented with 0.5 % (w/v) corn steep liquor, 0.4 % (v/v) invertase, and 2.4 % (w/v) CaCO3. Fermentation temperature was 50 °C at pH 7.0. Average of the results from three independent experiments are presented
Supplementing raw sorghum juice with yeast extract (0.5 %, w/v) increased the volumetric productivity to 7.2 g L−1 h−1 and the l-lactic acid titer reached 1.7 M after all the sugars in the juice were fermented in about 60 h (Table 1; Fig. 2). With yeast extract supplementation, co-product concentration in the broth was significantly lower compared to the fermentations with corn steep liquor. Consequently, this increased the lactic acid yield to 101 % of the sugar consumed. The slightly higher than 100 % lactic acid yield is apparently due to the small amount of sugars present in the yeast extract. Although glucose and fructose were fermented together, a preference for glucose was apparent (Fig. 2). The minimal amount of yeast extract required to support the highest productivity and lactic acid titer was determined to be 0.5 % (w/v). Since supplementation of sorghum juice with yeast extract provided the highest volumetric productivity observed, further experiments on sweet sorghum juice fermentation to lactic acid included 0.5 % (w/v) yeast extract as a nutritional supplement.
Fermentation of raw sorghum juice with added yeast extract by B. coagulans strain 36D1 to l-lactic acid. Raw sorghum juice was supplemented with 0.5 % (w/v) yeast extract, 0.4 % (v/v) invertase, and 2.4 % (w/v) CaCO3. Fermentation temperature was 50 °C at pH 7.0. Mean from the results of three independent experiments are presented with standard deviation
Since strain 36D1 required invertase for hydrolysis of sucrose before fermentation, our collection of B. coagulans isolates was screened for their ability to ferment sucrose. Of the 25 isolates tested, only four fermented sucrose to lactic acid. One of those isolates, strain 2D1, fermented raw sorghum juice (without invertase) to l-lactic acid at a volumetric productivity of 6.35 g L−1 h−1 in the presence of yeast extract to a yield of 84 % (Table 1; Fig. 3). The lactic acid titer was 1.2 M. Although the average volumetric productivity of strain 2D1 was intermediate between strains 36D1 and QZ19-2 (see next section), significant amount of sucrose (48 mM) was left unfermented by this strain at the end of 72 h of fermentation. This is in contrast to strain 36D1, which fermented all the sugars in sorghum juice within 72 h (Fig. 2). Apparently, the accumulation of 1.25 M lactic acid is inhibiting fermentation by this strain.
Fermentation of raw sorghum juice by sucrose-utilizing B. coagulans strain 2D1 to l-lactic acid. Raw sorghum juice was supplemented with 0.5 % (w/v) yeast extract and 2.4 % (w/v) CaCO3. Fermentation temperature was 50 °C at pH 7.0. Mean from the results of three independent experiments are presented with standard deviation
The lactic acid produced by strains 36D1 and 2D1 was determined to be l-(+)-lactic acid using Chiral-HPLC. The optical purity was higher than 99 % since the d-isomer was undetectable.
Fermentation of Raw Sorghum Juice to d-Lactic Acid
Among the microorganisms that produce d-lactic acid as a fermentation product, only B. coagulans strain QZ19 has been reported to ferment both hexoses and pentoses to d-lactic acid optimally at 50 °C and higher [28]. Strain QZ19-2 fermented sugars in raw sweet sorghum juice to d-lactic acid at a volumetric productivity of 5 g L−1 h−1 (Table 1; Fig. 4a). The product titer was 1.4 M with significant amount (total of ∼0.35 M) of co-products formate, acetate, and ethanol, even with yeast extract supplementation. The optical purity of d-lactic acid in this fermentation was higher than 99 % as determined by Chiral-HPLC.
Fermentation of raw sorghum juice to d-lactic acid by B. coagulans strain QZ19-2 to d-lactic acid and its wild-type parent strain P4-102B to l-lactic acid. a Strain QZ19-2; b wild-type strain P4-102B. Raw sorghum juice was supplemented with 0.5 % (w/v) yeast extract, 0.4 % (v/v) invertase, and 2.4 % (w/v) CaCO3. Fermentation temperature was 50 °C at pH 7.0. Mean from the results of three independent experiments are presented with standard deviation
The co-product fraction of about 0.2 with strain QZ19-2 is in contrast to that of strain 36D1, in which the co-product fraction was less than 0.05 under the same fermentation condition (Table 1). However, this co-product ratio of strain QZ19-2 was comparable to the fermentation profile of strain 36D1 with corn steep liquor (0.22) or to that of its wild-type parent, strain P4-102B (0.3). The fermentation profile of strain QZ19-2 was essentially the same irrespective of yeast extract or corn steep liquor supplementation of raw sorghum juice (Table 1). These results suggest that in strain QZ19-2, the glycolytic flux is limited by the d-lactate dehydrogenase activity. In support of this possibility, the cell extracts of strain QZ19-2 had a d-LDH activity of 0.012 μmol min−1 mg protein−1 compared to almost twice the level of l-LDH activity detected in the extracts of its wild-type parent strain P4-102B (0.021 μmol min−1 mg protein−1) cultured under similar condition. Yield of d-lactic acid in the sorghum juice fermentation can be increased by using a pflB mutant or by lowering the culture pH to minimize expression and activity of PFL as observed with strain QZ19 [28]. However, these conditions that minimize or remove PFL activity are expected to lower productivity due to a lower growth rate and cell yield in a non-ideal fermentation condition as observed with an E. coli pflB mutant [32].
It is also interesting to note that strain QZ19-2 utilized fructose preferentially over glucose, although the maximum rate of utilization of either sugar was comparable (Fig. 4a). This is in contrast to the preferential utilization of glucose over fructose by strains 36D1 and 2D1 (Fig. 1, 2, and 3). In order to evaluate whether this is a property of the wild-type parent of strain QZ19-2, the fermentation profile of strain P4-102B was determined. B. coagulans strain P4-102B fermented the mixture of glucose and fructose in the sorghum juice with glucose preference (Fig. 4b). The l-(+)-lactic acid titer of the wild-type parent was 1.1 M after 120 h of fermentation when all the sugars were exhausted. Based on these results, it is apparent that the metabolic evolution that led to strain QZ19 [28] altered the sugar metabolism. Genome sequence analysis that is in progress is expected to provide a mechanistic and genetic explanation for the inversion in sugar utilization by strain QZ19-2.
Strain P4-102B also produced significant amount of formate as a fermentation product, suggesting that the l-LDH activity is not matching the glycolytic flux and the excess pyruvate is channeled to pyruvate formate-lyase, as seen with strain QZ19-2. The lower specific activity of l-LDH in the cell extract from strain P4-102B (0.021 μmol min−1 mg protein−1) compared to that of strain 36D1 (0.13 μmol min−1 mg protein−1) is in agreement with this possibility.
Fermentation of Carbohydrates from Sweet Sorghum Bagasse
In addition to the juice, the sorghum bagasse also contains a significant amount of carbohydrates in the form of cellulose and hemicellulose (about 60–70 % by dry weight) [14] that can be fermented to lactic acid after appropriate pretreatment and saccharification to release the constituent sugars. Sorghum bagasse was treated with phosphoric acid and steam at 190 °C for 5 min as described previously [29]. In a simultaneous saccharification and co-fermentation (SScF) of this pretreated bagasse at 3.75 % solids loading (dry weight) with Cellic CTec2 cellulase (15 FPU g−1 dry weight acid-treated bagasse), lactic acid production started after about 24 h and reached a maximum of 170 mM in 120 h at 50 °C and pH 5.0 (Fig. 5), conditions that are also optimal for the activity of fungal cellulases [22]. The lag before appearance of lactate in the broth is most likely due to poor mixing of bagasse with the enzymes at this solids loading under our small-scale fermentation condition.
Effect of liquefaction on SScF of acid and steam-treated sorghum bagasse to l-lactic acid. Phosphoric acid and steam pretreated sorghum bagasse slurry (3.75 % solids, dry weight) suspended in mineral salts medium with 0.5 % (w/v) corn steep liquor was mixed with 15 FPU (per gram dry weight of solids) Cellic CTec2 cellulases and incubated at 50 °C (pH 5.0) in a shaker. The slurry was transferred to a fermentation vessel after 3 or 6 h of incubation and inoculated with B. coagulans strain 36D1. Fermentation temperature was 50 °C at pH 5.0. In the control without liquefaction, cellulases were added at the time of inoculation and start of fermentation. Results from a typical experiment are presented (see “Materials and Methods” section for other details)
In previous studies, it was observed that a liquefaction step prior to SScF of glucose, xylose, and other sugars (L + SScF) increased the rate of fermentation of pretreated sugarcane bagasse to ethanol by reducing viscosity and increasing flow characteristics [34, 35]. Since the added enzymes are expected to be active optimally at the fermentation temperature of 50 °C and pH 5.0, continued release of glucose from cellulose is projected to support simultaneous saccharification and fermentation of cellulose to lactic acid by B. coagulans. To minimize the lag in SScF of sorghum bagasse by B. coagulans, pretreated slurry with a solids loading of 3.75 % (w/v) in mineral salts medium at pH 5.0 was mixed with cellulases (15 FPU g−1 dry weight of bagasse) and incubated in a shaker at 50 °C for either 3 or 6 h. After this liquefaction step, the slurry was inoculated with strain 36D1 and the fermentation of the released sugars was monitored (Fig. 5). The liquefaction step eliminated the lag in fermentation. The release of higher concentration of glucose and xylose during the 6-h liquefaction step (75 and 14 mM, respectively) compared to 3 h of liquefaction (65 and 7 mM, respectively), apparently accounted for the slightly higher level of lactate titer during the first 24 h of fermentation. At 48 h, lactate titer of both treatments was comparable, indicating SScF of the glucans in the slurry. In all further experiments, the time of liquefaction was 3 h.
Strain 36D1 fermented the released sugars to about 210 mM of l-lactate (Fig. 6) and the l-lactate yield was about 75 % of the theoretical yield. In these experiments, the hemicellulose hydrolysate generated during the acid and steam treatment was not removed from the slurry. However, under the same condition, strain QZ19-2 failed to ferment the sugars present in the acid-treated sorghum bagasse post-liquefaction, although significant amount of sugars were present in the medium. Strain QZ19-2 did ferment the sugars from the acid-treated bagasse to d-lactic acid when the hemicellulose hydrolysate released during pretreatment was washed off with water. The hemicellulose hydrolysate of biomass is known to contain several inhibitory compounds in addition to pentose sugars [36]. The average concentration of the major inhibitory compounds—furfural, hydroxymethyl furfural, acetic, and formic acids—in the pretreated sweet sorghum bagasse slurry was 6.1, 2.0, 15.0, and 2.3 g kg−1 of slurry, respectively. Apparently, the genetically modified and metabolically evolved strain QZ19-2 is extremely sensitive to these and possibly other minor inhibitors. It is interesting to note that strain QZ19-2 acquired this sensitivity during metabolic evolution for d-lactic acid production, since the wild-type parent of strain QZ19-2, P4-102B, was not affected by the products of acid treatment at this solids loading (Fig. 6). The l-lactic acid titer of strain P4-102B was 225 mM in this fermentation, and the yield of l-lactic acid was 83 % of the theoretical value. Analysis of the genome sequence of P4-102B and QZ19-2, currently in progress, is expected to yield unique insights into the genes/proteins responsible for sensitivity/tolerance to hemicellulose hydrolysate derived inhibitors in B. coagulans. Further metabolic evolution focused on inhibitor tolerance is expected to yield derivatives of d-lactate-producing strain QZ19-2, as observed with ethanologenic E. coli [37, 38].
L + SScF of pretreated sorghum bagasse to optically pure lactic acid. Phosphoric acid and steam pretreated sorghum bagasse slurry (3.75 % solids, dry weight) in mineral salts medium with 0.5 % (w/v) corn steep liquor was fermented to l-lactic acid with B. coagulans strain 36D1 and P4-102B or to d-lactic acid by strain QZ19-2 at 50 °C and pH 5.0. Liquefaction with Cellic CTec2 at 15 FPU g−1 dry weight of pretreated bagasse was for 3 h before SScF of the sugars to optically pure lactic acid. Mean from the results of three independent experiments are presented with standard deviation (see “Materials and Methods” section for other details)
Using strain 36D1 as the microbial biocatalyst, a direct correlation between the solids loading and lactic acid titer can be observed up to about 15 % solids (fresh weight) in this L + SScF process with a lactic acid yield of 80 % of the theoretical value (data not shown). Better mixing and other process conditions that can be achieved at the industrial scale are expected to increase the solids loading to higher level without compromising the lactic acid titer and yield.
Conclusion
Sweet sorghum, an annual bioenergy crop with a short life cycle (4 months), offers the potential for multiple crops per year in warm climates such as the Southeastern USA. The crop can also be produced on low-productivity lands. We have shown here that all the sugars in the unsterilized sweet sorghum juice can be fermented to d- or l-lactic acid at concentrations of 1.4 and 1.7 M, respectively, representing a yield of 90–100 %. After phosphoric acid pretreatment, the carbohydrates derived from the bagasse can also be fermented to lactic acid using the L + SScF process at a lactic acid yield of over 80 % of the theoretical value. These results show that optically pure lactic acid, a feedstock for biodegradable plastics, can be readily derived from the sugars and carbohydrates in sweet sorghum as a sustainable alternative to petroleum-derived plastics.
References
Chakravorty U, Hubert M-H, Nostbakken L (2009) Fuel versus food. Ann Rev Resour Econ 1:645–663
Rosegrant MW, Msangi S (2014) Consensus and contention in the food-versus-fuel debate. Ann Rev Environ Resour 39:18.11–18.24
Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA, Blanch HW (2012) The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioeng 109:1083–1087
Ten E, Vermerris W (2013) Functionalized polymers from lignocellulosic biomass: state of the art. Polymers 5:600–642
GrandViewResearch (2014) Lactic acid and poly lactic acid (PLA) market analysis by application (packaging, agriculture, transport, electronics, textiles) and segment forecasts to 2020. http://wwwgrandviewresearchcom/industry-analysis/lactic-acid-and-poly-lactic-acid-market
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31:877–902
Auras R, Lim L, Selke SEM, Tsuji H (2010) Poly(lactic acid): synthesis, structures, properties, processing and applications. Wiley series on polymer engineering and technology. Wiley, New York
Tsuji H (2005) Poly(lactide) stereocomplexes: formation, structure, properties, degradation, and applications. Macromol Biosci 5:569–597
Tenenbaum DJ (2008) Food vs. fuel: Diversion of crops could cause more hunger. Environ Health Perspect 116:A254–A257
Wang Y, Tashiro Y, Sonomoto K (2015) Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J Biosci Bioeng 119:10–18
Regassa TH, Wortmann CS (2014) Sweet sorghum as a bioenergy crop: literature review. Biomass Bioenergy 64:348–355
Whitfield MB, Chinn MS, Veal MW (2012) Processing of materials derived from sweet sorghum for biobased products. Ind Crop Prod 37:362–375
Erickson JE, Helsel ZR, Woodard KR, Vendramini JMB, Wang Y, Sollenberger LE, Gilbert RA (2011) Planting date affects biomass and brix of sweet sorghum grown for biofuel across Florida. Agron J 103:1827–1833
Kim M, Day DF (2011) Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J Ind Microbiol Biotechnol 38:803–807
Venuto B, Kindiger B (2008) Forage and biomass feedstock production from hybrid forage sorghum and sorghum-sudan grass hybrids. Grassland Sci 54:189–196
Vermerris W, Erickson J, Wright D, Newman Y, Rainbolt C (2011) Production of biofuel crops in Florida: sweet sorghum. University of Florida
Cao W, Liu R (2013) Screening and optimization of trce elements supplement in sweet sorghum juice for ethanol production. Biomass Bioenergy 50:45–51
Liu Q, Wang S, Zhi JF, Ming H, Teng D (2013) Efficient production of lactic acid from sweet sorghum juice by a newly isolated Lactobacillus salivarius CGMCC 7.75. Indian J Microbiol 53:332–336
Yadav AK, Bipinraj NK, Chaudhari AB, Kothari RM (2011) Production of L(+)lactic acid from sweet sorghum, date palm, and golden syrup as alternative carbon sources. Starch/Starke 63:632–636
Yu J, Zhang T, Zhong J, Zhang X, Tan T (2012) Biorefinery of sweet sorghum stem. Biotech Adv 30:811–816
Cai D, Zhang T, Zheng J, Chang Z, Wang Z, Qin PY, Tan TW (2013) Biobutanol from sweet sorghum bagasse hydrolysate by a hybrid pervaporation process. Bioresour Technol 145:97–102
Patel MA, Ou M, Ingram LO, Shanmugam KT (2005) Simultaneous saccharification and co-fermentation of crystalline cellulose and sugar cane bagasse hemicellulose hydrolysate to lactate by a thermotolerant acidophilic Bacillus sp. Biotech Prog 21:1453–1460
Energy-EERE U-Do (2015) Multiyear program Plan March 2015 update. http://www.energy.gov/sites/prod/files/2015/03/f20/mypp_beto_march2015.pdf
Abe S, Takagi M (1991) Simultaneous saccharification and fermentation of cellulose to lactic acid. Biotech Bioeng 37:93–96
Patel MA, Ou MS, Harbrucker R, Aldrich HC, Buszko ML, Ingram LO, Shanmugam KT (2006) Isolation and characterization of acid-tolerant, thermophilic bacteria for effective fermentation of biomass-derived sugars to lactic acid. Appl Environ Microbiol 72:3228–3235
Ou MS, Mohammed N, Ingram LO, Shanmugam KT (2009) Thermophilic Bacillus coagulans requires less cellulases for simultaneous saccharification and fermentation of cellulose to products than mesophilic microbial biocatalysts. Appl Biochem Biotechnol 155:379–385
Abdel-Banat BMA, Hoshida H, Ano A, Nonklang S, Akada R (2010) High-temperature fermentation: how can processs for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl Microbiol Biotechnol 85:861–867
Wang Q, Ingram LO, Shanmugam KT (2011) Evolution of D-lactate dehydrogenase activity from glycerol dehydrogenase and its utility for D-lactate production from lignocellulose. Proc Natl Acad Sci U S A 108:18920–18925
Geddes CC, Mullinnix MT, Nieves IU, Peterson JJ, Hoffman RW, York SW, Yomano LP, Miller EN, Shanmugam KT, Ingram LO (2011) Simplified process for ethanol production from sugarcane bagasse using hydrolysate-resistant Escherichia coli strain MM160. Bioresour Technol 102:2702–2711
Underwood SA, Buszko ML, Shanmugam KT, Ingram LO (2002) Flux through citrate synthase limits the growth of ethanologenic Escherichia coli KO11 during xylose fermentation. Appl Environ Microbiol 68:1071–1081
Garrigues C, Loubiere P, Lindley ND, Cocaign-Bousquet M (1997) Control of the shift from homolactic acid to mixed-acid fermentation in Lactococcus lactis: predominant role of the NADH/NAD+ ratio. J Bacteriol 179:5282–5287
Hasona A, Kim Y, Healy FG, Ingram LO, Shanmugam KT (2004) Pyruvate formate lyase and acetate kinase are essential for anaerobic growth of Escherichia coli on xylose. J Bacteriol 186:7593–7600
Melchiorsen CR, Jokumsen KV, Villadsen J, Israelsen H, Arnau J (2002) The level of pyruvate-formate lyase controls the shift from homolactic to mixed-acid product formation in Lactococcus lactis. Appl Microbiol Biotechnol 58:338–344
Geddes CC, Mullinnix MT, Nieves IU, Hoffman RW, Sagues WJ, York SW, Shanmugam KT, Erickson JE, Vermerris WE, Ingram LO (2013) Seed train development for the fermentation of bagasse from sweet sorghum and sugarcane using a simplified fermentation process. Bioresour Technol 128:716–724
Geddes CC, Peterson JJ, Mullinnix MT, Svoronos SA, Shanmugam KT, Ingram LO (2010) Optimizing cellulase usage for improved mixing and rheological properties of acid-pretreated sugarcane bagasse. Bioresour Technol 101:9128–9136
Martinez A, Rodriguez ME, Wells ML, York SW, Preston JF, Ingram LO (2001) Detoxification of dilute acid hydrolysates of lignocellulose with lime. Biotechnol Prog 17:287–293
Miller EN, Jarboe LR, Yomano LP, York SW, Shanmugam KT, Ingram LO (2009) Silencing of NADPH-dependent oxidoreductase genes (yqhD and dkgA) in furfural-resistant ethanologenic Escherichia coli. Appl Environ Microbiol 75:4315–4323
Wang X, Yomano LP, Lee JY, York SW, Zheng H, Mullinnix MT, Shanmugam KT, Ingram LO (2013) Engineering furfural tolerance in Escherichia coli improves the fermentation of lignocellulosic sugars into renewable chemicals. Proc Natl Acad Sci U S A 110:4021–4026
Acknowledgments
This work was supported by grants from the US Department of Agriculture (USDA) (2012-67009-19596; LOI) and Biomass Research and Development Initiative Competitive Grant (2011-10006-30358) from the USDA National Institute of Food and Agriculture. This work was also supported by funding from the US Department of Energy’s Office of Energy Efficiency and Renewable Energy, Bioenergy Technologies Office, and sponsored by the US Department of Energy’s International Affairs under award number DE-PI0000031 and the Florida Department of Agriculture and Consumer Services.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PPTX 77 kb)
Rights and permissions
About this article
Cite this article
Ou, M.S., Awasthi, D., Nieves, I. et al. Sweet Sorghum Juice and Bagasse as Feedstocks for the Production of Optically Pure Lactic Acid by Native and Engineered Bacillus coagulans Strains. Bioenerg. Res. 9, 123–131 (2016). https://doi.org/10.1007/s12155-015-9670-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-015-9670-6