Abstract
Urban biomass from green areas is a potential resource for bioenergy recovery, which is widely unused. Different types of organic material (e.g., grass, leaf litter) usually occur in mixtures due to common collecting practice. Forty samples of grass, leaf litter (genera: Acer, Quercus, Tilia), and mixtures of both, containing one third grass or leaf litter, were investigated to evaluate the effect of the “Integrated Generation of Solid Fuel and Biogas from Biomass” (IFBB) on material and energy fluxes as well as relevant characteristics of resulting energy carriers. IFBB divides biomass into a fiber-rich press cake and a highly digestible press fluid by mashing with subsequent pressing. Ensiling of samples was successful with pH values ranging from 4.2 in grass to 4.8 in pure Tilia samples. Concentration of most minerals with exception of Ca and Mg were higher in grass than in leaf litter silage. The IFBB treatment reduced the element concentration in the press cake independently from the substrate. Linear regression models revealed high influence of the initial concentration in silage on the concentration in the press cake. The lower heating value of the press cake was nearly constant (19 MJ kg−1 DMash free) independent from mixture. Methane yields from press fluid digestion ranged from 172 (mean of leaf litter samples) to 325 lN kg−1 VS (mixture of 33 % leaf litter—66 % grass). For an evaluation of the economic and ecological potential, models of the spatial and temporal occurrence of these biomasses need to be established.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Green areas in cities are a valuable contribution to human health and well-being [1]. Additionally, various ecosystem services are provided by parks, gardens, and roadside greenings (e.g., [2, 3]). However, the maintenance of green structures is an increasing challenge for municipalities, as it is a significant cost factor in times of small public budgets. Therefore, it seems attractive to utilize urban biomass for bioenergy recovery and, thereby, to reduce management costs through the revenues from energy provision. Additionally, energetic utilization of urban biomass could lead to reduced GHG emissions, enhanced biodiversity, and an improved understanding of urban citizens for a resource-efficient lifestyle, as they can contribute with their own garden wastes to the energy provision of the city.
In municipalities, grass cuttings, leaf litter, and tree pruning occur as possible biomass for energy recovery [4]. Springer [4] assessed a potential of 164 million Mg of biomass (DM) per year harvested from urban areas in the USA. In Germany, an amount of 4.6 million Mg green waste is collected annually [5]. Current management practices are either to leave the biomass on the sites for decaying or to remove the biomass to landfills or composting, as well as waste incinerating plants [4]. Widely used energy recovery technologies as fermentation or direct combustion in heating plants are usually not suitable for these biomasses, because grass cuttings tend to cause problems with floating and abrasion during wet fermentation [6], while its high mineral content (especially chlorine and potassium) causes corrosion, slagging, and emissions during combustion [7]. Leaf litter provides only small methane yields during fermentation [8] and is contaminated with soil particles (unpublished data) possibly inducing abrasion and damage of combustion units and increases the risk of sedimentation in biogas reactors [6]. However, solid fuel quality of both, grass and leaf litter, may be improved by the “Integrated Generation of Solid Fuel and Biogas from Biomass” (IFBB) procedure. The procedure aims at dividing ligno-cellulosic biomass into a fiber-rich press cake and a press fluid, which contains major shares of minerals and easy soluble carbohydrates. The biomass is mashed with warm water and subsequently dewatered mechanically [9]. During this pretreatment, the mineral concentration (especially K and Cl) in the solid fraction is reduced significantly, enhancing the overall fuel quality. Similar effects were observed in senesced biomass of grassland swards, which were standing over winter [10], as well as in cut material, which was treated with simulated rain [11]. However, common park management usually does not allow standing stocks over winter.
Further, biomass availability in urban environments is highly depending on seasons as leaf litter occurs during autumn, while major grass is cut from April to October but not in winter. In agriculture, unsteady availability of feedstock is usually tackled by ensiling. Ensiling of common agricultural products as grasses, corn, or sorghum is well investigated [12–14]; however, the perspectives of ensiling leaf litter are widely unknown. There have been some efforts to research possibilities of ensiling leaves as fodder mainly for goats or sheep. Khan et al. [15] observed high silage quality in mixtures of 75 % maize and 25 % leaves from either Syzygium cuminii or Mangifera indica without any butyric acid odor, and Tjandraatmadja et al. [16] added 33 % of Leucaena leucocephala or Gliricidia sepium to tropical grasses receiving silages with pH values <4.4. However, these tree species do not occur in common parks of Europe or North America, and they are usually more or less evergreen. Therefore, ensiling in these studies was conducted with physiologically active leaves but not with leaf litter, whose chemical composition changes during leaf senescence [17, 18].
The general technical feasibility to process urban biomass with the IFBB technique was proven for specific input materials as grass cuttings from roadside verges [19] and leaf litter from urban park trees (unpublished data), as well as for a mixture of municipal and landscape conservation materials [20]. Piepenschneider et al. [19] put concerns about heavy metal contamination of grass from roadside green verges into perspective by showing that the concentration of various elements is in the range of agricultural grass and does therefore not hinder energetic utilization.
In practice, leaf litter and grass cuttings are often mixed together, either by collecting leaf litter with the last grass cut in autumn or by joint municipal disposal facilities. Therefore, it is important to understand possible interferences between these substrates during processing with the IFBB technique. To investigate several associated questions, we chose a replacement experimental design, which cannot determine the quantitative influence of each component on a certain outcome, but allows some valid interpretation concerning interference [21].
In this study, we addressed the following questions: (i) is it possible to conserve leaf litter and leaf litter-grass mixtures as silage, (ii) what is the chemical composition of silage from pure urban grass and leaf litter samples, as well as from mixtures of both, (iii) what are the chemical and energetic characteristics of resulting press cakes and press fluids, (iv) does the mixing of leaf litter and grass cuttings influence the reduction of minerals during the IFBB process?
Materials and Methods
Biomass
Leaf litter was sampled in a park (“Bergpark Wilhelmshöhe”) within the city of Kassel in November 2013. Four batches of three different tree genera each were taken (Acer, Quercus, Tilia) from trees, whose distance was 100 m. Batches included minor leaf proportions of other genera, but shares of intended genera were as follows (in % DM ± sd): Acer 90 ± 4, Quercus 78 ± 15, and Tilia 74 ± 13.
Grass cutting material for the study was collected from the autumn cut of a common grassland sward and chopped to 5 cm on the day of ensiling. The biomass consisted to 69 % of dry matter (DM) of Lolium perenne. About 22 % of DM was Trifolium pratense, and 9 % of DM were herbs or other grass species.
The experiment was based on a standard replacement series with constant total sample amount and varying proportions of individual components [21]. Prior to ensiling grass and leaf litter were mixed from 0 % leaf litter and 100 % grass to 100 % leaf litter and 0 % grass with mixtures of 33 % leaf litter and 66 % grass, as well as 66 % leaf litter and 33 % grass, based on fresh matter (FM). Thereby, a total of 40 silages were produced with four samples of pure grass and four samples of each mixture and leaf litter genus (24 samples) and four samples of each pure leaf litter batch (12 samples). Samples were ensiled in 60-l polyethylene barrels for 6 weeks minimum.
Pretreatment by the IFBB Procedure
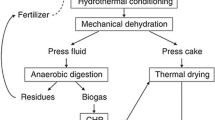
The IFBB procedure was conducted as described in the reference [22]. Silage was mixed with water in a ratio 1:4 (FM based). The mixture was mashed for 15 min at 40 °C under constant stirring and subsequently dewatered by a screw press (type AV, Anhydro Ltd.) with a pitch of 1:6 and a rotation speed of 6 revolutions per minute. The press fluid was immediately frozen at −20 °C for subsequent digestion experiments.
Chemical Analysis
Prior to ensiling, subsamples of single materials were taken for DM analysis. After ensiling, subsamples were taken for DM analysis, determination of organic acids and pH, as well as for analysis of element concentration, ash content, and concentration of neutral detergent fibers (NDF). DM, element concentration, ash content, and concentration of NDF were also determined in subsamples of the press cake after the pretreatment with the IFBB procedure.
For DM analysis in all fractions, subsamples were dried at 105 °C for 48 h. For the determination of the ash content, a subsample was dried at 105 °C and subsequently incinerated in a muffle oven at 550 °C. Organic acids (acetic, propionic, iso butyric, butyric, iso valeric, valeric, and caproic acid) were analyzed with gas chromatography with flame ionization detector (GC-FID), and the pH was tested in an aqueous solution. The amount of organic acids was used to correct the previously determined DM and ash content figures.
Element concentration was measured in subsamples, which were dried at 65 °C and grinded with a cutting mill (SM 1, Retsch) with a 5-mm sieve followed by a sample mill (1093 Cyclotec, Foss) to pass a 1-mm sieve. Concentrations of C, H, and N were determined by an elemental analyzer (Vario MAX CHN Elementar Analysensysteme GmbH) using 150 mg of dried material. Concentrations of Al, Ca, Cl, K, Mg, P, S, and Si were determined with inductively coupled plasma optical emission spectrometry (ICP-OES) and concentrations of Na with inductively coupled plasma mass spectrometry (ICP-MS).
For determination of NDF concentration, half of the samples (both silage and press cake) were analyzed with a fiber analyzer (ANKOM A220). A subsample of about 0.5 g was sealed in a filter bag and boiled with neutral detergent solution and alpha-amylase without use of Na2SO3, as this regularly leads to loss of lignin [23] and thereby to an underestimation of NDF. Filter bags were subsequently rinsed three times with alpha-amylase solution for 5 min and then with acetone for 3 to 5 min. After drying, NDF concentration was calculated as
where m1 is the mass of empty filter bag, m2 is the mass of sample, m3 is the mass of organic matter after boiling and incineration, and C is the “blank-bag correction” factor. The determined NDF concentration in 40 of a total of 80 samples was used for near-infrared calibration of NDF concentrations. A near-infrared spectroscope (XDS Rapid Content Analyser; FOSS NIRSystems Inc.) was used, and NDF values for the 80 samples were predicted after cross-validation (R 2 = 91.4, RSC = 3.3; RSC defined as the ratio of standard deviation and standard error of cross-validation).
Heating Value
The higher heating value was calculated based on C, H, and N concentrations using the formula of the following [24]:
Lower heating value (LHV) was calculated from the higher heating value (HHV) taking the enthalpy of water vaporization into account:
Digestion Experiments with Press Liquids
For measuring methane yields from the press fluid, we followed the methodology and experimental setup of Bühle et al. [25] and Zerr [26] taking the German standard [27] into account. Three replicates of 4-kg press fluid mixed with 8 kg inoculum were digested in gas-proof polyethylene containers at mesophilic temperature (37 ± 1 °C). Digestion of pure inoculum served as a control and allowed identifying the proportion of methane originating from press fluid and inoculum. Fermentation time was 14 days, as the daily biogas production is below 1 % of total biogas production at this time. At days 1, 2, 3, 4, 7, 9, 11, and 14 of digestion, amount of biogas and methane concentration in the biogas was analyzed with a wet drum gas meter (Ritter TG5) and a gas analyzer (GS IRM100), respectively. Current air pressure and temperature were recorded to calculate the methane yield under normalized conditions (273.15 K, 1013.25 hPa). Methane yields were referred to volatile solids in order to present the feedstock-specific methane yield [6]. These were determined by incineration of a subsample of press fluid at 550 °C in a muffle furnace after drying at 105 °C in a drying oven. To compensate partly for the loss of highly volatile organics during drying, we added the amount of organic acids, for which we assumed a mass flow into the press fluid similar to water, as they are highly water soluble. The chemical oxygen demand was measured with a cell test (LCK 514, Hach-Lange).
Statistical Analysis
Statistical analysis as well as generation of figures was conducted with the software R [28]. Samples were insofar independent as they were stored and processed in single batches after mixing. However, single replacement series were established by mixing grass from one sward with a distinct leaf litter batch (three genera in four independent repetitions each). Thus, the requirements of multiplicity and independence [29] were fulfilled by single batches taken in the field but no longer within and among each repetition series. Thereby, the naturally occurring variance was reduced. However, the aim of this study was not to show the variance of parameters in biomass but to focus on possible interactions of biomasses during processing, which might influence the quality of the solid fuel. To avoid that natural variance masks these interactions, it was necessary to reduce it to a minimum level. For statistical analysis, we therefore renounced the comparison among levels of leaf litter share (trends are visible from tables) and focused on linear regression analysis to predict the element concentration in the press cake from the element concentration in the silage. Models were visually checked for homoscedasticity and normal distribution of residuals. Few occurring outliers were removed from the analysis if Cook’s distance was >0.5. For linear regression analysis, the car package was used taking single samples (40 results for each factor) into account [30].
Results and Discussion
DM of Untreated Material and Silage
DM content of grass was 14.9 ± 0.9 %, while the DM content (±sd, in %) of leaf litter was 26.8 ± 2.6 for Acer, 28.8 ± 1.6 for Quercus, and 23.7 ± 2.4 for Tilia. After ensiling, the mean DM content of all samples (mixed and pure) was 21.9 ± 4.1 % with a minimum value of 14.1 % in grass silage and a maximum value of 31.9 % in pure Quercus leaf litter silage.
pH and Organic Acids in Silages
Lowest pH values were measured for pure grass silages with a mean of 4.2 ± 0.0, and highest values were detected for pure Tilia with a mean of 4.8 ± 0.5 (Table 1). Cherney and Cherney [31] suggested a pH value <4.2 for low DM silage and a pH value <5.2 for high DM silage as guiding values for a proper fermentation process. pH values detected in this study, rising with increasing DM content, were in accordance with these guiding values. Mixing leaves of Syzygium cuminii or Mangifera indica with maize or grass resulted in pH values of silage between 4.6 and 4.9 with 25, 50, and 75 % contributions of leaves [15] and pH values <4.5 were detected in mixtures of grass and 33 % leaves of Leucana laucocephala or Glidricidia sepium [16].
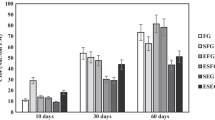
In 65 % of the samples, the concentration of butyric acid in the DM was below 0.1 %, indicating a successful ensiling process in low DM silage [31]. However, 13 % of samples, which were mainly derived from pure Quercus and Tilia leaf litter, had a butyric acid concentration between 0.5 and 2 % of DM. For silage with rather high DM content, a guiding limiting value of 0.5 % DM was suggested [31], which was exceeded by those samples. However, for a poorly fermented silage significant higher values of >2.5 %, DM are given in literature [13]. Increased concentrations of butyric acid may occur due to a lack of or an inefficient utilization of fermentable carbohydrates, whereby acidification is slow and clostridia may ferment the already formed lactic or acetic acids into butyric acid [32]. However, for Tilia samples, the concentration of acetic acids remained stable independently from leaf proportion (Fig. 1), while for Acer and Quercus mixtures, the acetic acid concentrations decreased significantly according to Pearson’s product moment correlation (p = 0.01 for both correlations) with decreasing grass share. Thus, highest acetic acid concentrations in the DM were measured in pure grass samples with a mean value of 1.5 ± 0.2 %, which is rather low considering an acetic acid concentration of 2 to 5 % in well-preserved silage [13]. The question, if this is due to a lactic acid-based fermentation or if this is rather an indicator of an overall reduced production of acids with increasing leaf litter share, has to remain open, as lactic acid concentrations were not determined. In summary, ensiling of pure tree leaf litter seems to be possible in terms of achieving anaerobic stability, as long as the existing guidelines given for fodder production are carefully obeyed.
Chemical Composition of Silage
NDF concentration in grass silage (35.2 % of DM) was rather low but within the range of values detected in silage from L. perenne (42.6 % of DM) and T. repens (30.0 % of DM) at first cutting date [14]. Element concentrations in ensiled grass were mostly in line with findings of previous studies. Ca, Mg, and K concentrations (1.40, 0.25, 2.66 % of DM, respectively, Table 2) were similar to levels detected by Hopkins et al. [33] in fresh material from a sown sward of L. perenne and T. repens at the fourth cut (mean values of years 2–4: 1.12, 0.25, 2.60 % of DM, respectively), also when taking a slight DM loss (e.g., 4 % measured by the reference [34]) during ensiling into account. N concentration in grass (2.36 % of DM, on average) was close to concentrations of 2.5 % found in silage from L. perenne cut in May [35]. Concentrations of P (0.3 % of DM) and S (0.18 % of DM) were close to or in the range of literature values (0.37 % of DM [33], 0.32 % of DM [19] and 0.37 % DM [33], 0.06 % DM [35], respectively). In contrast, Na and Cl concentrations were rather high (0.33 % of DM and 1.16 % of DM, respectively) compared to values found in fresh ryegrass-clover mixture (Na, 0.17 % of DM, [33]) and in silage from perennial ryegrass (Cl, 0.43 % of DM, [35]) or in silage from roadside verges with high L. perenne share (Na, 0.01 % of DM; Cl, 0.51 % of DM [19]). Similarly, concentrations of Al and Si were higher than expected with 0.97 and 5.98 % of DM, respectively, in comparison with previously detected concentrations in the mentioned material from roadside verges (Al, 0.03 % of DM; Si, 1.07 % of DM [19]). However, this deviation is explainable by the high ash content of 31.37 % of DM, which indicates a significant amount of soil adherence to the leaf litter collected. Silicon is the second frequent element in soil (after oxygen) and aluminum the most frequent metal [36]. An ash content of about 10 % of DM can be regularly assumed for such material [35, 19]).
Only concentrations of C, H, Ca, Mg, and NDF were higher in the collected leaf litter (42.49, 5.37, 2.61, 0.47, and 51.85 % of DM, respectively) than in grass. However, C and H concentrations were low in grass, as well as in leaf litter in comparison to previous studies involving leaf litter (unpublished data) and a variety of 18 European grassland sites [37]. For Ca and Mg, the values found for leaf litter exceeded literature values [18]. However, during leaf senescence, both elements tend to increase in concentration [38, 18] depending on several abiotic factors as soil fertility, leaf nutrient status, or summer temperature [39]. NDF concentrations of fresh leaves were found to be extremely variable with 9.6 % of DM for leaves of Sesbania sesban [40], 29.8 and 31.1 % of DM for leaves of Prunus persica and Prunus domestica, respectively [41], 40.6 % of DM for olive leaves [42], and 66.1 % of DM for leaves of Quercus incana [43]. A high NDF concentration indicates a reduced degradability of biomass during fermentation as degradation of hemi-cellulose and lignin are reported to be challenging [6], whereas fibers are favorable in combustion as lignin in particular increases the heating value [44].
Concentrations of Cl, K, N, Na, and P were lower in leaf litter than in grass but in a range, which was also observed in previous studies on leaf litter [45, 19]. Further, ash contents were similar to values detected in the above-mentioned studies, and the authors assume that these high values were caused by contamination through soil adherence. The actual ash content of leaf litter is assumed to be well below 10 % [19] without any soil adherence. Si concentrations of 1.1 % of DM and Al concentrations of 0.07 % of DM have been measured in freshly fallen leaf litter of Fagus sylvatica [46], which are lower than concentrations identified in this study (Si, 3.09 ± 0.54; Al, 0.31 ± 0.04 % of DM). Like for the grass samples, the elevated concentrations are probably due to soil adherence, as indicated by the high ash content. Mixtures of grass and leaf litter showed intermediate concentrations for all elements, as well as for ash and NDF. Values were continuously increasing (Ca, Mg, NDF) or decreasing (Al, Cl, K, N, Na, P, S, Si, ash) according to the share of both components in the mixtures.
Characteristics of IFBB Products Press Cake and Press Fluid
In the press cake, concentrations of C were increasing with increasing share of leaf litter (Table 3). Thus, highest C concentrations with a mean value of 50.5 ± 1.8 % of DMash free were detected in the samples from pure leaf litter. This is in accordance with unpublished results, which revealed mean C concentrations of 50.8 % of DMash free in the press cake of urban leaf litter from five tree genera (Acer, Aesculus, Fagus, Quercus, Tilia). H and N concentrations in the press cake from pure leaf litter in this study (6.4 and 1.0 % of DMash free, respectively) were also similar to the unpublished data (6.34 and 0.96 % of DMash free, respectively). In comparison to the composition of the press cake from leaf litter, C concentrations in press cake of pure grass samples were lower (48.6 % of DMash free), while N concentrations were higher (2.6 % of DMash free). However, the lower heating values of press cakes (18.6 to 18.9 MJ kg−1 DMash free) were very similar for the mixtures. Referring to DM including ash, the lower heating value was higher in pure leaf litter because the ash content was lower. However, the lower ash content was partly caused by lower soil adherence and thus, cannot be further interpreted. Beside the heating value, the K2O/CaO index is an important quality parameter for solid fuels, as it indicates the level of risk for ash slagging [47]. For press cake from pure grass, the K2O/CaO index accounted for 0.6, which was higher than in press cake produced from seminatural grassland (0.5), however low in comparison to untreated grass silage (1.5) [37]. The index values decreased with increasing share of leaf litter, which indicates a lower risk of slagging. Press cake from pure leaf litter had an K2O/CaO index of 0.1 in accordance with previous research, which detected an index of 0.15 in press cake from leaf litter of a variety of different tree genera and a corresponding ash softening temperature of well above 1200 °C (unpublished data).
Press fluids derived from grass samples had a DM concentration of 1.3 % of FM with a tendency to decrease to 1 % of FM with increasing leaf litter share (Table 3). The COD follows this pattern in general (14.5 in press fluid from pure grass to 10.8 g l−1 in press fluid from pure leaf litter), though the highest concentrations were detected in the press fluid of the mixture with 33 % leaf litter (15.0 g l−1). This pattern is also valid for methane yield derived from press fluids, which was highest in press fluid of the 33 % leaf litter—66 % grass mixture with a value of 325 ± 24 lN kg−1 VS. However, the concentration of volatile solids was lowest in this mixture (64.5 % of DM). Possibly, leaf litter provides a more structured matrix, which allows minerals to rinse out during dewatering more easily than through a highly compacted mass of grass biomass, and thereby may alter the relative share of volatile solids. However, also, the quality of volatile solids seems to be influenced by the mixing ratio, as indicated by highest COD and methane yield at this mixture level. The exact underlying mechanisms cannot be clarified in this study. In particular, the applied method for determining volatile solids does not comprise all compounds as some are already lost during drying. Although we corrected the figures for organic acids, we do not know the amount of lost alcohols. However, methane yield data seem to be valid, as the mean coefficient of variation of laboratory repetitions was small with 0.1 for both the whole data set and the given mixture. Additionally, repetitions were treated independently, being digested in different subunits, while the same standardized inoculum was used for all repetitions.
Impact of IFBB Procedure on Element Concentrations
The concentration of investigated elements in the press cake was generally highly depending on their concentration in the silage (Fig. 2). However, the slope and intercept were variable possibly due to different tendencies to dissolve in water from plant material. For example, the concentrations of Cl and K are known to leach into the press fluid very easily [20] leading to a small increase in concentration in the press cake even when the concentration in the silage increased substantially. This resulted in a relative reduction of Cl and K concentration in the press cake of 94 % for Cl and of 69 % for K compared to the concentrations in the silage. In contrast, the regression models predicting Ca and Mg concentrations in the press cake in dependency on their concentrations in the silage showed a parallel shift from the intersecting line indicating a certain amount of soluble ions. However, parts of the Ca and Mg ions seem to be fixed to fibrous molecules and are therefore not available for dissolving. For Ca, it is known that it can be found dissolved in the vacuole and as structural element in cell membrane and cell wall. Magnesium also occurs in the vacuole, as well as in chloroplasts and functions as stabilizer of nucleic acid configuration [48]. Ions from the vacuole are potentially higher leachable as they are already dissolved. Remarkably, the amount of Ca and Mg ions removed stayed constant, while total concentration increased. The slope of the regression model predicting Al concentrations in the press cake indicated also a certain amount of fixed ions; however, the relative amount of soluble ions was highly proportional to the total concentrations. Similarly, the relative reduction of Na and P concentrations increased by up to 68 %, each, with increasing initial concentration. However, the relative reduction did not reach levels of K and Cl, but was in accordance with previous findings, which detected a relative reduction of Na concentration of 66 % and of P concentrations of 74 % [19] in grass from roadside verges applying the IFBB technique. Piepenschneider et al. [19] found a relative reduction of 29 % for N, which is higher than in this study with a maximum relative reduction of 15 %. Rather, small mass flows of N into the press fluid have also been reported by Hensgen et al. [20], who mentioned that in mature leaf tissue, most N is strongly bound in structural or insoluble proteins [49].
Noteworthy, all samples, including pure grass and leaf litter, as well as mixtures, were the basis for the highly valid linear regression models explaining the element concentration in the press cake by the element concentration in the silage. Thus, the material itself played a minor role in determining the quality of the press cake. This supports the target of the IFBB system to flexibly use different urban biomass types for energy recovery.
Energy Flux
The main share of energy contained in the silage (calculated from the heating value based on C, H, and N concentrations) was transferred into the press cake with a range from 67 to 89 % of energy contained in silage from pure grass and pure leaf litter samples, respectively (Fig. 3). With increasing energy flux into the press cake, the energy originating from biogas decreases continuously from pure grass to pure leaf litter samples. This is probably due to the decreasing mass flow of dry matter into the press fluid and the lower methane potential of leaf litter-rich samples. High energy fluxes into the press cake have been calculated by Bühle et al. [50], who detected a net energy transfer into the press cake of about 80 %. Losses of energy are most likely caused by undegraded substrate leaving the process in digestates, leading to values of less than 100 % for the accumulated energy derived from press cake and biogas. Standard deviations between tree genera were very low, indicating that effects of genera are of minor importance.
Conclusion
Grass and leaf litter biomass occurs in urban environments. A main challenge for utilizing these biomasses is their temporal irregular occurrence. However, ensiling for storage of pure grass and leaf litter, as well as of mixtures, is possible with a higher quality in grass samples or mixtures than in pure leaf litter samples. For energy recovery by combustion, the concentration of minerals in the fuel is crucial. Mineral (for example, K and Cl) concentration usually decreased with an increasing share of leaf litter, though concentrations of Ca and Mg, which have structure functions in plants, increased with increasing share of leaf litter. The IFBB procedure reduced the concentration of elements (Al, Ca, Cl, K, Mg, N, Na, P, S, Si) in the press cake, and concentrations in the press cake were highly depending on their concentration in the silage, except for S. Thus, no interactions between fractions concerning the element concentration in the press cake were detected, which underpins the high potential of the IFBB technique to treat different biomasses. However, highest methane potential, as well as COD, was measured in mixtures of 66 % grass and 33 % leaf litter. Possibly, there are some interactions between substrates, which concern the mass flow of carbohydrates and might issue from the combination of structure (from leaf litter) and sugars (from grass). The press cake as main product comprised major parts of the energy originally contained in the silage, and the lower heating value of ash-free biomass was not influenced by mixing of grass and leaf litter, but was constantly close to the lower heating value of wood. Therefore, bioenergy recovery from municipal biomasses with the IFBB technique is not only possible for pure materials but also for mixtures, as frequently occurring in urban maintenance practices. To evaluate the economic and ecological potential of the technique in cities, quantitative models of temporal and spatial biomass occurrence need to be developed.
Abbreviations
- DM:
-
Dry matter
- FM:
-
Fresh matter
- GC-FID:
-
Gas chromatography with flame ionization detector
- ICP-OES:
-
Inductively coupled plasma optical emission spectrometry
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- IFBB:
-
Integrated generation of solid fuel and biogas from biomass
- LHV:
-
Lower heating value
- MF:
-
Mass flow
- NDF:
-
Neutral detergent fiber
- oDM:
-
Organic dry matter
- PF:
-
Press fluid
- PC:
-
Press cake
- RSC:
-
Ratio of standard deviation and standard error of cross-validation
- sd:
-
Standard deviation
References
Chiesura A (2004) The role of urban parks for the sustainable city. Landsc and Urban Plan 68(1):129–138. doi:10.1016/j.landurbplan.2003.08.003
Bolund P, Hunhammar S (1999) Ecosystem services in urban areas. Ecol Econ 29(2):293–301. doi:10.1016/S0921-8009(99)00013-0
Larondelle N, Haase D (2013) Urban ecosystem services assessment along a rural–urban gradient: a cross-analysis of European cities. Ecol Indic 29:179–190. doi:10.1016/j.ecolind.2012.12.022
Springer TL (2012) Biomass yield from an urban landscape. Biomass and Bioenergy 37:82–87. doi:10.1016/j.biombioe.2011.12.029
Kern M, Raussen T, Graven T et al (2012) Ökologisch sinnvolle Verwertung von Bioabfällen: Anregungen für kommunale Entscheidungsträger. BMU, Referat Öffentlichkeitsarbeit, Berlin
Prochnow A, Heiermann M, Plöchl M et al (2009) Bioenergy from permanent grassland – a review: 1. Biogas Bioresource Technol 100(21):4931–4944. doi:10.1016/j.biortech.2009.05.070
Jenkins BM, Baxter LL, Miles TR et al (1998) Combustion properties of biomass. Fuel Processing Technol 54(1–3):17–46. doi:10.1016/S0378-3820(97)00059-3
Liew LN, Shi J, Li Y (2012) Methane production from solid-state anaerobic digestion of lignocellulosic biomass. Biomass and Bioenergy 46:125–132. doi:10.1016/j.biombioe.2012.09.014
Wachendorf M, Richter F, Fricke T et al (2009) Utilization of semi-natural grassland through integrated generation of solid fuel and biogas from biomass. I. Effects of hydrothermal conditioning and mechanical dehydration on mass flows of organic and mineral plant compounds, and nutrient balances. Grass Forage Sci 64(2):132–143
Gamble JD, Jungers JM, Wyse DL et al. (2014) Harvest Date Effects on Biomass Yield, Moisture Content, Mineral Concentration, and Mineral Export in Switchgrass and Native Polycultures Managed for Bioenergy. Bioenerg. Res. doi:10.1007/s12155-014-9555-0
Tonn B, Dengler V, Thumm U et al (2011) Influence of leaching on the chemical composition of grassland biomass for combustion. Grass Forage Sci 66(4):464–473. doi:10.1111/j.1365-2494.2011.00804.x
Buxton DR, Muck RE, Harrison JH (eds) (2003) Silage Science and Technology, vol 42. American Society of Agronomy; Crop Science Society of America; Soil Science Society of America, Madison, Wis
McDonald P, Henderson N, Heron S (1991) The Biochemistry of Silage, 2nd edn. Chalcombe, Marlow, England
McEniry J, King C, O’Kiely P (2014) Silage fermentation characteristics of three common grassland species in response to advancing stage of maturity and additive application. Grass Forage Sci 69(3):393–404. doi:10.1111/gfs.12038
Khan N, Barman K, Rastogi A et al (2012) Chemical composition and digestion kinetics of mixed silages of maize fodder-tree leaves. Anim Nutr Feed Technol 12(2):271–278
Tjandraatmadja M, MacRae IC, Norton BW (1993) Effect of the inclusion of tropical tree legumes, Gliricidia sepium and Leucaena leucocephala, on the nutritive value of silages prepared from tropical grasses. J Agricultural Sci 120:397–406
Bazot S, Barthes L, Blanot D et al (2013) Distribution of non-structural nitrogen and carbohydrate compounds in mature oak trees in a temperate forest at four key phenological stages. Trees 27(4):1023–1034. doi:10.1007/s00468-013-0853-5
Tyler G (2005) Changes in the concentrations of major, minor and rare-earth elements during leaf senescence and decomposition in a Fagus sylvatica forest. For Ecology and Manag 206(1–3):167–177. doi:10.1016/j.foreco.2004.10.065
Piepenschneider M, de Moor S, Hensgen F et al (2015) Element concentrations in urban grass cuttings from roadside verges in the face of energy recovery. Environmental Sci Pollut Res 22(10):7808–7820. doi:10.1007/s11356-014-3881-9
Hensgen F, Richter F, Wachendorf M (2011) Integrated generation of solid fuel and biogas from green cut material from landscape conservation and private households. Bioresource Technol 102(22):10441–10450. doi:10.1016/j.biortech.2011.08.119
Jolliffe PA (2000) The replacement series. J Ecology 88(3):371–385. doi:10.1046/j.1365-2745.2000.00470.x
Hensgen F, Bühle L, Donnison I et al (2012) Mineral concentrations in solid fuels from European semi-natural grasslands after hydrothermal conditioning and subsequent mechanical dehydration. Bioresource Technol 118:332–342
Van Soest P, Robertson J, Lewis B (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74(10):3583–3597. doi:10.3168/jds.S0022-0302(91)78551-2
Friedl A, Padouvas E, Rotter H et al (2005) Prediction of heating values of biomass fuel from elemental composition. Analytica Chimica Acta 544(1–2):191–198. doi:10.1016/j.aca.2005.01.041
Bühle L, Reulein J, Stülpnagel R et al (2012) Methane yields and digestion dynamics of press fluids from mechanically dehydrated maize silages using different types of digesters. Bioenergy Res 5(2):294–305. doi:10.1007/s12155-011-9127-5
Zerr W (2006) Versuchsanlage zur energetischen Beurteilung von Substraten und Kofermentaten für Biogasanlagen. Umweltwissenschaften und Schadstoff-Forschung 18(4):219–227
Verein Deutscher Ingenieure (2006) Guideline VDI 4630 Fermentation of organic materials; Characterisation of the substrate, sampling, collection of material data, fermentation tests. Beuth, Berlin
R Core Team (2013) R: A Language and Environment. R Foundation for Statistical Computing, Vienna, Austria
Casler MD, Vermerris W, Dixon RA (2015) Replication Concepts for Bioenergy Research Experiments. Bioenergy Res 8(1):1–16. doi:10.1007/s12155-015-9580-7
Fox J, Weisberg S (2011) An {R} Companion to Applied Regression, 2nd edn. Sage, Thousand Oaks CA
Cherney J, Cherney DJR (2003) Assessing Silage Quality. In: Buxton DR, Muck RE, Harrison JH (eds) Silage Science and Technology. American Society of Agronomy; Crop Science Society of America; Soil Science Society of America, Madison, Wis
Pahlow G, Muck RE, Driehuis F et al (2003) Microbiology of Ensiling. In: Buxton DR, Muck RE, Harrison JH (eds) Silage Science and Technology. American Society of Agronomy; Crop Science Society of America; Soil Science Society of America, Madison, Wis
Hopkins A, Adamson AH, Bowling PJ (1994) Response of permanent and reseeded grassland to fertilizer nitrogen. 2. Effects on concentrations of Ca, Mg, K, Na, S, P, Mn, Zn, Cu, Co and Mo in herbage at a range of sites. Grass Forage Sci 49(1):9–20. doi:10.1111/j.1365-2494.1994.tb01971.x
McGechan MB (1990) A review of losses arising during conservation of grass forage: Part 2, storage losses. J Agricultural Eng Res 45(0):1–30. doi:10.1016/S0021-8634(05)80135-0
McEniry J, Finnan J, King C et al (2012) The effect of ensiling and fractionation on the suitability for combustion of three common grassland species at sequential harvest dates. Grass Forage Sci 67(4):559–568. doi:10.1111/j.1365-2494.2012.00902.x
Scheffer F, Schachtschabel P, Blume H (2010) Lehrbuch der Bodenkunde, 16th edn. Spektrum Lehrbuch. Spektrum, Akad. Verl, Heidelberg, Berlin
Bühle L, Dürl G, Hensgen F et al (2014) Effects of hydrothermal conditioning and mechanical dewatering on ash melting behaviour of solid fuel produced from European semi-natural grasslands. Fuel 118:123–129. doi:10.1016/j.fuel.2013.10.063
Lin P, Wang W (2001) Changes in the leaf composition, leaf mass and leaf area during leaf senescence in three species of mangroves. Ecological Eng 16(3):415–424. doi:10.1016/S0925-8574(00)00126-9
Hagen-Thorn A, Varnagiryte I, Nihlgård B et al (2006) Autumn nutrient resorption and losses in four deciduous forest tree species. For Ecology Manag 228(1–3):33–39. doi:10.1016/j.foreco.2006.02.021
Bonsi M, Osuji P, Tuah A (1995) Effect of supplementing teff straw with different levels of leucaena or sesbania leaves on the degradabilities of teff straw, sesbania, leucaena, tagasaste and vernonia and on certain rumen and blood metabolites in Ethiopian Menz sheep. Anim Feed Sci Technol 52(1–2):101–129. doi:10.1016/0377-8401(94)00702-B
Salem AZM, Zhou C, Tan Z et al (2013) In vitro Ruminal Gas Production Kinetics of Four Fodder Trees Ensiled With or Without Molasses and Urea. J Integr Agriculture 12(7):1234–1242. doi:10.1016/S2095-3119(13)60438-4
Molina-Alcaide E, Yáñez-Ruiz D (2008) Potential use of olive by-products in ruminant feeding: a review. Anim Feed Sci Technol 147(1–3):247–264. doi:10.1016/j.anifeedsci.2007.09.021
Sharma R, Singh B, Sahoo A (2008) Exploring feeding value of oak (Quercus incana) leaves: Nutrient intake and utilization in calves. Livest Sci 118(1–2):157–165. doi:10.1016/j.livsci.2008.01.022
Lewandowski I, Kicherer A (1997) Combustion quality of biomass: practical relevance and experiments to modify the biomass quality of Miscanthus x giganteus. Eur J Agronomy 6(3–4):163–177. doi:10.1016/S1161-0301(96)02044-8
Heckman J, Kluchinski D (1996) Chemical composition of municipal leaf waste and hand-collected urban leaf litter. J Environ Qual 25(4):930. doi:10.2134/jeq1996.00472425002500040048x
Joergensen RG, Scholle GA, Wolters V (2009) Dynamics of mineral components in the forest floor of an acidic beech (Fagus sylvatica L.) forest. Eur J Soil Biol 45(4):285–289. doi:10.1016/j.ejsobi.2009.04.006
Steenari B, Lundberg A, Pettersson H et al (2009) Investigation of ash sintering during combustion of agricultural residues and the effect of additives. Energy Fuels 23(11):5655–5662
Maathuis FJM (2009) Physiological functions of mineral macronutrients. Curr Opin in Plant Biol 12(3):250–258. doi:10.1016/j.pbi.2009.04.003
Mattson W (1980) Herbivory in relation to plant nitrogen content. Annu Reviews Ecology Systematics 11:119–161
Bühle L, Hensgen F, Donnison I et al (2012) Life cycle assessment of the integrated generation of solid fuel and biogas from biomass (IFBB) in comparison to different energy recovery, animal-based and non-refining management systems. Bioresource Technol 111:230–239
Acknowledgments
The authors are grateful for receiving funding from the European Union through the Interreg IV B regional development fund for the COMBINE Project (Nr. 299J)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piepenschneider, M., Bühle, L. & Wachendorf, M. Solid Fuel Generation from Urban Leaf Litter in Mixture with Grass Cuttings: Chemical Composition, Energetic Characteristics, and Impact of Preprocessing. Bioenerg. Res. 9, 57–66 (2016). https://doi.org/10.1007/s12155-015-9661-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-015-9661-7