Abstract
Bio-energy is now emerging as a future source of energy. Pyrolysis is one of the main thermo-chemical processes that can provide a useful and valuable bio-fuel such as bio-oil and bio-char. Bio-oil was produced from an undesired bio-weed named Ipomoea carnea by thermal pyrolysis at a terminal temperature range from 350 to 600 °C with a heating rate of 10 °C/min. Maximum bio-oil yield (41.17 % of which 11.45 % is the oil phase) was obtained at a pyrolysis temperature of 550 °C. The oil obtained was characterized by Fourier transform infrared (FTIR), nuclear magnetic resonance (NMR; 1H and 13C), and gas chromatography–mass spectroscopy (GC-MS) which revealed the presence of various hydrocarbons and alcohols. The H/C molar ratio (1.49) of the bio-oil was found to be comparable with petroleum-derived diesel. However, the presence of oxygen (35.86 %) in the form of oxygenates especially organic acids make the bio-oil acidic, which needs to be upgraded to use in the current IC engine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energy is the backbone of any country for its sustainable economic growth. There is a sharp increase in the energy consumption in developing countries like India. The primary energy consumption of India during 2008 had been reported as 53.4 % coal, 31.2 % oil, 8.6 % natural gas, 6.0 % hydroelectricity, and 0.8 % nuclear energy [1, 2]. The consumption of oil in India is dependent on imports, which had been found increasing as 72, 76.5, 78, and 79.3 % in 2004–2005, 2006–2007, 2007–2008, and 2008–2009, respectively [2]. According to the World Bank, the energy import in India in terms of net percentage of energy use was 28 % in 2011. These imports are increasing significantly year after year with the growing economy of the country and contribute to continuous rise in the import bills [3]. Therefore, there is a dire need of alternate and renewable sources of energy for a sustainable growth of the country.
The renewable energy source, particularly biomass has received increased attention to meet the energy demand and costs [4, 5]. Biomass sources are considered a source of clean energy, as it contains negligible amounts of nitrogen, sulfur, and ash as compared with conventional fossil fuels resulting in lower emissions of SO2 and NO x than the conventional fossil fuels during combustion [6, 7]. Moreover, CO2 released from biomass is resolved into plants during photosynthesis.
Several processes exist for conversion of biomass into bio-fuels, among which pyrolysis (a thermo-chemical conversion process) is found to be a most promising one. It is a scalable thermo-chemical technology through which lignocellulosic biomass can be depolymerised in a single oxygen-starved reactor with a moderate temperature (400–600 °C) for a very short retention time [8]. The liquid product thus formed from the pyrolysis is known as bio-oil, which is a storable and transportable oxygenated bio-fuel. It can be directly used for heat and electricity generation in static burners. Recently, more attention has been given in upgrading bio-oil into transportation fuels and value-added chemicals [9, 10].
Ipomoea carnea is a bio-weed mostly found in the north-eastern part of India, particularly in Assam and West Bengal [11]. Although not a native plant of India, it is found in many parts of the country. The weed has high regenerative capacity and propagates through seeds and wood cuttings [11]. It is very difficult to eradicate this bio-weed by physical and chemical methods. This weed has spread very rapidly in many areas in northeast India and has been creating problems by covering large patches of agricultural fields and also by choking the passage of rivers, irrigation canals, lakes (both natural and man-made), thereby causing artificial floods in the rainy season. From a rough estimate of woody biomass productivity of I. carnea in northeast India, 100 t of woody stems could be harvested from an area of 1 ha of land covered by it [12]. The yield of green Ipomoea feedstock in Indian natural conditions is 160 tonne/ha [4]. The stems are unsuitable to be used as firewood due to its low heating value and emission of noxious gases during combustion process. In some rural areas, this wood is often used for fencing both home and agricultural fields [12].
Work on this feedstock has been very scanty. Konwer et al. has attempted slow pyrolysis of I. carnea with a heating rate of 5°/min [12]. In the present work, an attempt has been made to prepare bio-oil from I. carnea weed and characterization of liquid product. We have mostly emphasized our work on the characterization of the oil phase. Moreover, physico-chemical analysis and characterization of the aqueous oil phase is also reported.
Methods
Materials
The materials used in the study were I. carnea collected from a tropical (longitude 94° 09′ 31″ East and Latitude 26° 44′ 19″ North) region of Jorhat, India. The sample was cut into 5–7 cm in length and was then sun dried in ambient atmosphere for about 1–2 s. It was further ground to pass through 36 B.S. (0.422 mm) screen. The sample was then kept in a closed air-tight container to escape from moisture.
Raw Material Analysis
Proximate analysis (moisture content, volatile matter content, and ash content) of the sample was determined by TAPPI method T258 om-89, ASTM D 3175, and TAPPI method T 211 om-85, respectively. Fixed carbon content was determined by the difference. C, H, and N contents of the sample was determined using CHN analyzer (Perkin Elmer series 2, 2400 model). Calorific value was determined using Bomb Calorimeter (make: LECO, AC 350 LECO model). Cellulose, hemicelluloses, and lignin content of the sample were determined by using Fibertech I & M systems.
To study the thermal behavior of the I. carnea wood thermo-gravimetric analysis (TGA) was performed using 701 LECO instrument with a heating rate of 10 °C/min for terminal temperature of 800 °C in N2 atm and with a flow rate of 10 ml/min. The purity of nitrogen used was 99.9995 % with 2 ppm oxygen, 0.3 ppm CO2, 0.05 ppm CO, 2 ppm water, 0.1 ppm THC, and 0.01 ppm total VOC as impurities. The thermo-gravimetric weight loss curve (TG) and differential thermograph, i.e., rate of weight loss curve (DTG) were recorded as a function of temperature.
Pyrolysis Experiments and Bio-Oil Production
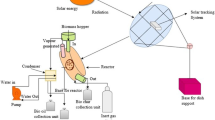
The pyrolysis of I. carnea was carried out in a laboratory-scale batch-type reactor. Figure 1 shows the schematic diagram of the pyrolysis apparatus used. The unit consists of pyrolysis reactor, furnace, PID controller, condenser, and measuring cylinder. The reactor had a length of 300, 6 mm thickness, and 52–53 mm internal diameter with electrical heating facility. The temperature of the furnace was maintained by highly sensitive PID controller using Al/Rh thermocouple. The average reaction temperature errors were within ±5 °C. The condensable phase (bio-oil) of the vapor was collected using a glass condenser maintained at ice cooling environment.
At the beginning of the pyrolysis experiment, a silica retort was filled with 7 g of the sample and placed in the reactor. The retort was then connected with the condensation assembly which was further connected with the non-condensable gas measuring unit (based on water displacement method) and finally released to the environment. During pyrolysis, the retort was flushed with nitrogen to create an inert atmosphere and then sealed using asbestos plug. The power switch of the reactor was then switched on and allowed to raise the temperature up to the desired terminal temperature. Experiments were conducted for six terminal temperatures viz., 350, 400, 450, 500, 550, and 600 °C with a heating rate of 10 °C/min. After reaching the terminal temperature, the sample was kept inside the reactor for about 30 min keeping the terminal temperature constant. The condensates collected during the experiment were found to be in a heterogeneous state with two distinct layers, aqueous and heavy oil fractions. The oil fraction was viscous black tarry component floating in a fairly clear brownish solution, but on standing, this tarry component eventually precipitated at the base and the sticks on the wall of the container. The two fractions were then separated by gravity separation method using separating funnel and collected in a glass container for further analyze. Moreover, the terminal temperature at which the maximum yield of bio-oil was obtained was taken into consideration for characterization and analysis. Adequate care was taken, so that the oil could not be exposed to the moisture of the surrounding environment. The product yield was calculated by using the following formula:
Analysis of the Pyrolytic Product
The bio-oil fractions obtained under optimum liquid yield conditions were well mixed and homogenized prior to their analysis. Physical properties of pyrolytic liquids, including density, viscosity, flash point, pour point, and ash content were determined by using the standard methods ASTM D1298, ASTM D445, ASTM D93, ASTM D97, and ASTM D482, respectively. The calorific value of the bio-oil fractions were determined by using bomb calorimeter, Leco AC-350. pH of the samples were determined using Digital pH meter EUTECH pH 510. Fuel properties for the aqueous phase were not determined as these fractions are generally used for extraction of valuable chemicals [13] such as alcohols, phenolic products, levulinic acid, xylitol, lactic acid, etc.
The functional groups composition of the liquid products were analyzed by Fourier transform infrared (FTIR) spectroscopy using FTIR-system-2000, Perkin Elmer, USA with a resolution of 4 cm−1 and in the range of 400–4000 cm−1. NMR spectroscopy is one of the important analytical tools to characterize pyrolysis oil from numerous biomass feedstocks [14]. The 1H and 13C nuclear magnetic resonance (NMR) analyses of both the fractions were obtained by Advanced DPX-300 MHz, FT-NMR instrument. For NMR analysis, both the oil fractions were dissolved in CDCl3 and tetramethylsilane (TMS) was used as internal standard.
Gas chromatography–mass spectroscopy (GC-MS) study was carried out for both quantitative and qualitative analyses of the bio-oil fractions using Perkin Elmer Clarus 80 GC/600C MS Gas Chromatography (GC), and the compounds were determined using mass selectivity (MS) detector equipped with HP-5MS column (30.0 m × 0.25 mm × 0.25 μm). The initial oven temperature was maintained at 70 °C for 2 min and then ramped to 290 °C with a heating rate of 10 °C. At this temperature, the column was kept isothermal for 7 min. The injection and detector temperature was maintained at 200 and 250 °C, respectively. Prior to GC-MS analysis, 100 μL of crude sample was dissolved in 1 mL of methanol; 1 μL of the samples were injected with split ratio of 10:1 and He as a carrier gas. All the data were obtained by collecting the full-scan mass spectra within the scan range of 50–600 Da. The area percentage of the GC-MS chromatogram was considered as a good approximation indicating the approximate quantity of the various chemical compounds present in the oil fractions [5].
The identification and characterization of chemical compounds in various extracts was based on GC retention time. The mass spectra were computer matched with those of standards available in mass spectrum libraries.
Results and Discussions
Feedstock Characterization
The physical and bio-chemical characteristics of raw materials are essential for optimizing the efficiency of any thermo-chemical conversion process [15]. Their chemical compositions were not much different from other wood species. The cellulose, hemicellulose, and lignin contents of biomass form a complex bio-oil mixture; thus, structural composition is vital for analysis which provides information about the product transformation during the pyrolysis process [6]. The moisture content of biomass has a significant effect on its conversion efficiency and heating value. The proximate analysis, elemental composition, bio-chemical analysis, and calorific value of the ground I. carnea sample are shown in Table 1. The proximate analysis shows that the raw I. carnea should have minimum inherent moisture contents so that they can be pyrolysed easily. The biomass with high moisture content has high tendency to decompose resulting in energy loss during storage [7]. Raw I. carnea have a rather high volatile content which evolves in the form of gas, light hydrocarbons, and tars during pyrolysis. The ash content was medium in the I. carnea, which is an integral part of plant structure that consists of a wide range of mineral matters such as salt of calcium, potassium, silica, magnesium, etc. Liberating less fixed carbon from the sample makes them more useful for pyrolysis and gasification.
Thermo-gravimetric Analysis of I. carnea
The weight losses observed in TGA and DTG curves are found to be relevant to the composition of cellulose, hemicellulose, and lignin fractions in biomass. Generally, three different regions can be distinguished from a particular TGA curve. Weight change of a sample is recorded as a function of time or temperature and portrayed by a TGA curve; on the other hand, DTG emphasizes the zones of reaction where various reaction steps are taking place over the entire temperature range.
In the current investigation, a temperature below 200 °C in the TG curve (Fig. 2) was observed as a first stage of weight loss, which corresponds to the drying of the sample (I. carnea) where light volatiles, mainly water were liberated. Slight decay of sample weight around 14 % was observed signifying the DTG peak at 103 °C. The second stage of weight loss observed corresponds to the de-volatilization of the biomass material with high decomposition rate known as an active pyrolysis zone. During this stage, the intermolecular associations and weaker chemical bonds are destroyed. The side aliphatic chains may be broken and some small gaseous molecules are produced because of the lower temperature. A rapid decomposition of the sample was observed between 200 and 500 °C, representing around 64 % weight loss, and DTG curve shows maximum degradation peak occurring at 312.8 °C for this region. Decomposition in this region indicates the formation of volatiles mainly due to decomposition of hemicellulose followed by the cellulose. At this stage, weight of the sample material is reduced to below 50 %. This is primarily due to the fact that around 70–80 wt.% of biomass is formed by volatile fraction, and only around 20–30 wt.% is in the form of solid carbonaceous residue [16]. This shows that volatile products play a very important part in pyrolysis of biomass materials and product gas composition. By around 500 °C, the degradation rate of biomass becomes relatively constant.
During the 3rd stage, the lignin residue slowly decomposed, with the weight loss velocity becoming smaller and smaller and the residue ratio tends to be constant at the end the decomposition of hydrocarbon (Table 1). Higher temperatures lead to further pyrolysis of lignin which slowly decomposes over a much wider temperature range (160–900 °C), mostly yielding char [17, 18]. At higher temperature, chemical bonds are broken and the parent molecular skeletons are destroyed. As a result, the larger molecule decomposes to smaller molecules and additional release of volatiles from decomposition of lignin at above 600 °C. Vellojos et al. also observed a similar profile when TGA of lignin was performed in N2 atm [19]. Based on the TG and DTG results, we chose a reaction temperature range between 350 and 600 °C for estimation of optimum yield of bio-oil.
Effects of Pyrolysis Temperature on Pyrolytic Oil Yields
Table 2 shows the mass balance for slow pyrolysis of I. carnea with a heating rate of 10 °C/min. Since the pyrolytic oil was viscous and very difficult to fully separate, the oil yield was calculated by difference, using the char and gas yields. As the terminal temperature increases, the char yield decreases from 34.72 % at 350 °C to 28.31 % at 600 °C by releasing more vapors. The pyrolytic oil yield from 40.15 % (of which oil phase consists of 8.53 %) at 350 °C to 44.17 % (of which oil phase consists of 11.45 %) at 550 °C by weight. No further increase in oil content was observed when pyrolysis temperature was raised above 550 °C. This was due to the secondary reactions of the heavy molecular weight compounds in the pyrolysis vapors, which is known to become active at temperatures higher than 500 °C [20]. This eventually leads to formation of more amounts of non-condensable gases/volatiles. Thus, yield of liquid and completion time of pyrolysis is a function of temperature. Hence, 550 °C was considered as the optimum temperature for pyrolysis of I. carnea to get maximum yield of oil with less completion time [21, 22].
Analysis of Product Liquids
Fuel Properties of the Bio-Oil
The properties of the bio-oil vary mainly due to feedstock variation, production processes, reaction conditions, and collecting efficiency [23]. Elemental analysis and properties of oil phase from I. carnea pyrolysis at optimum temperature was compared with commercial diesel (Table 3). The oil phase fraction was oxygenated hydrocarbons containing 35.86 % oxygen, which falls within the range (35–40 % oxygen in bio-oil) reported in other studies [24–26]. Oxygen is distributed among various compounds, depending on the resource of biomass and conditions of pyrolysis. Presence of oxygen results in lower energy density, high acidity, and immiscibility with hydrocarbon fuels [26]. The density of oil fraction was higher than that of commercial diesel because of the presence of a large amount of water and oxygen containing macromolecule such as hydroxyaldehydes, hydroxyketones, sugars, carboxylic acids, and oligomeric phenolic compounds which reduces the heating value of bio-oil as compared with the hydrocarbon fuels [27]. Viscosity of the bio-oil fractions was found higher than that of diesel, which generally varies over a wide range depending on the feedstock and its process conditions [27, 28]. Viscosity of a fuel has a significant importance in design and operation of a fuel injection system, automization quality, and subsequent combustion properties [29]. The flash point of the oil was found to be 82 °C, which was not too high in comparison with the petroleum-refined fuels like diesel fuel (∼55 °C) and light fuel oil (∼60 °C). Flash point of the bio-oil was high due to its complex mixture of components having a wide distillation range and oxygenated hydrocarbons [27, 30]. Flash points of bio-oil usually vary in the range of 40–70 °C or above 100 °C, depending on the contents of light organic volatile. Bio-oils usually contain about 7–12 wt.% acids and have a pH of in the range 2–4 [27]. Thus, storage and handling of the liquids can be problematic in industrial usage due to corrosiveness of acids.
Complete chemical characterization of bio-oils is difficult mainly due to the presence of pyrolytic lignin. However, elemental analysis, H/C and C/O molar ratios, empirical formula, and gross calorific values (GCV) of the bio-oil were determined. The average chemical composition of the oil phase obtained from I. carnea was obtained to be CH1.49O0.5N0.06. The H/C molar ratio 1.49 of the oil phase indicates that such oils are a mixture of aliphatic and aromatic compounds and is comparable (H/C molar ratio = 1.86) with the petroleum-derived sulfur-free diesel [31]. The nitrogen content was also found higher than that of commercial diesel. Based on its fuel properties, the oil fraction derived from I. carnea may be considered as a valuable component for use with automotive diesel fuels. Moreover, the liquids may be directly used as fuels for industrial furnaces, power plants, and boilers [32]. To get more into the bio-oil derived from I. carnea, further analysis such as FTIR, NMR, and GC-MS were carried out to understand the functional characteristics and composition.
Chemical Composition of the Product Liquids
FTIR analysis for pyrolytic oil is shown in Fig. 3. Similar results were also reported by other researchers [33–35] for pyrolytic liquids derived from I. carnea. The O–H stretching vibrations between 3200 and 3600 cm−1 of the bio-oil indicate the presence of phenols and alcohols. Since the peak is broad, it represents the intramolecular hydrogen bonds. Absorption arising from C–H stretching vibration in the alkanes occurs in the general region of 3000–2840 cm−1. The FTIR shows absorption between 2843 and 2950 cm−1 which indicates the presence of alkanes and C–H deformation vibrations between 1300 and 1468 cm−1, indicating the presence of a terminal methyl group in the pyrolytic oil derived from I. carnea [36]. The C=O stretching vibrations between 1600 and 1718 cm−1 indicate the presence of conjugated systems such as ketone, quinone, aldehyde groups, etc. The absorbance peaks between 1547 and 1675 cm−1 represent C=C stretching vibrations which indicate the presence of alkenes and aromatics. The absorbance peak between 1300 and 950 cm−1 indicates the presence of primary, secondary, and tertiary alcohols, phenols, esters, and ethers. Furthermore, mono- and polycyclic and substituted aromatic groups are indicated by the absorption peaks between 700 and 900 cm−1. The presence of the oxygenated functional groups such as O–H, C=O, C–O, and aromatic compounds makes the oil highly oxygenated, acidic, and low in calorific value. Moreover, the oil has a potential to be used as fuel in industrial burners due to the presence of hydrocarbons (such as C–H, C=C) and oxygenates (such as alcohols).
The 1H NMR analysis for the proton content of pyrolytic oil was carried out within a chemical shift range through which the types of chemical functional groups are known is shown in Table 4 [36]. Figure 4a, b shows the 1H NMR spectra for oil phase and aqueous phase of bio-oil, respectively. Both the fractions show the peak corresponding to the proton content in all ranges presented in Table 4. The pronounced presence of aliphatic adjacent to aromatic/alkene groups and aliphatic bonded to aliphatic only and relatively lower phenolic/olefinic protons (4.5–6.5 ppm) and aromatics (6.5–9 ppm) in both the fractions was found. The long alkyl and alkanes spectra mainly found are mostly derived from the I. carnea.
An overview of the 13C NMR spectra for the entire carbon content within a given chemical shift range and information on the types of chemical functional groups present in that range are shown in Table 4 [36]. Figure 5a, b shows the 13C NMR spectra of the oil phase and aqueous phase of bio-oils obtained. Both the fraction shows the presence of short, long, and branched aliphatic (1–54 ppm), alcohols, esters, phenolic-methoxy, carbohydrate sugars (55–95 ppm), aromatics, olefins (95–165 ppm), esters, and carboxylic acids (165–180 ppm).
Both oil and aqueous phase of the bio-oil contains all the chemical components as discussed above; however, their distribution in both the phase differs. This is mostly due the miscibility nature of the chemicals present in the bio-oil in either of the oil phases. Although quantitative explanation of the product seems to be difficult, qualitative assessment of the 1H NMR and 13C NMR spectra show differences in the product distribution. One of the peaks at 4.95 ppm in the 1H NMR spectra of oil phase is shifted to 5.16 ppm in aqueous phase. These two peaks correspond to phenolic (OH) or olefinic proton such as methoxy and carbohydrate. Intensity of one of the peaks at around 3.63 ppm present in oil phase is reduced in aqueous phase, which corresponds to aliphatic adjacent to oxygen/hydroxyl group such as alcohols and methylene dibenzene, whereas peak at around 1.16–1.27 ppm present in the oil phase shows significantly reduced intensity in the aqueous phase which corresponds to other aliphatic (bonded to aliphatic only) such as alkanes. Moreover, two peaks at around 2.1 and 4.25 ppm can be observed in aqueous phase and is substantially low in the oil phase. The first peak at around 2.1 ppm peaks represents aliphatic adjacent to aromatic/alkene group whereas the second peak at around 4.5 ppm represents phenolic (OH) or olefinic proton such as methoxy, carbohydrates. 13C NMR spectra of the bio-oil phases show two major differences. Oil phase shows one group of peaks at around 76.68–77.53 ppm apart from one at 50.32 ppm whereas aqueous phase shows 46.90–48.60 ppm apart from one at 19.60 ppm. The peaks at around 76.68–77.53 ppm corresponds to alcohols, esters, phenolic-methoxys, and carbohydrate sugars, whereas peak at around 46.90–48.60 ppm represents short, long, and branched aliphatics.
The compositions of bio-oil fractions were further analyzed with GC-MS (Fig. 6a, b). Although, the GC-MS analysis of raw un-fractionated bio-oil is not a confirmatory test for compounds. However, it gives us insight about the most probable compounds using standard library. In the present investigation, NIST library was used. The identified chemical compounds of oil fraction and aqueous fraction are shown in Tables 5 and 6, respectively. GC-MS analysis of the oil fraction revealed the presence of extremely complex mixture of numerous organic compounds. The components present in oil fraction of bio-oil produced mainly comprised the branched/long chains, phenol and its derivatives, mixed acids, esters, alcohols, and various aromatics (indene, methyl-indene, benzene, toluene, methyl-naphthalene, etc.), and the rest mostly are furans and ketones, which are similar with those obtained from the slow pyrolysis of wood. The oxygenates and aromatic compounds present in the oil fraction is derived mainly from its bio-polymer textures such as cellulose and hemicellulose. So, the highly oxygenated organic components or oxygenates are required to be separated first and then upgraded in order to raise their heating values and reduce the corrosiveness when they are used as a potential liquid fuel [37]. The aqueous phase comprised mainly of carboxylic acids, various branched ketones, small amounts of methyl and methoxy phenols, and minor aromatic hydrocarbons. The presence of abundant aldehydes and ketones make this aqueous phase hydrophilic [38] and highly hydrated due to its polar nature, which makes it difficult to eliminate water from the bio-oil via gravity separation. However, the aqueous phase of the pyrolytic product can be used as a useful source of chemicals to supplement the needs of chemicals industry [13].
Conclusion
The optimal pyrolysis temperature for the production of bio-oil from I. carnea was 550 °C. FTIR, NMR (1H and 13C), and GC-MS analysis revealed the presence of hydrocarbons along with other oxygenates. The oxygen content was found to be 35.86 % which reduces the heating value (22.304 MJ/kg) and increases the viscosity (19.32 cst) of the bio-oil. The presence of oxygenates especially organic acids makes the bio-oil acidic. However, the H/C molar ratio of the bio-oil was found to be 1.49, which is comparable with the petroleum-derived diesel and can be a potential fuel for industrial furnaces.
References
Sagi S, Patel A, Hornung A, Singh H, Apfelbacher A, Berry R (2012) Decentralised off-grid electricity generation in India using intermediate pyrolysis of residue straws. In: World Renewable Energy Forum (WREF)
Singh J, Gu S (2010) Biomass conversion to energy in India—a critique. Renew Sust Energ Rev 14:1367–1378
Ghosh S (2009) Import demand of crude oil and economic growth: evidence from India. Energ Policy 37(2):699–702
Bioenergy India (2010) A quarterly magazine on biomass energy, published under the UNDP-GEF biomass power project of MNRE, Govt. of India. Published by Winrock International India
Heo HS, Park HJ, Yim JH, Sohn JM, Park J, Kim SS, Ryu C, Jeon JK, Park YK (2010) Influence of operation variables on fast pyrolysis of Miscanthus sinensis var. purpurascens. Bioresour Technol 101:3672–3677
Yaman S (2004) Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers Manage 45:651–671
Jenkins BM, Ebeling JM (1985) Thermochemical properties of biomass fuels. California Agriculture. May–June, 14–16
Skoulou V, Zabaniotou A (2012) Fe catalysis for lignocellulosic biomass conversion to fuels and materials via thermochemical processes. Catal Today 196(1):56–66
Li Z, Savage PE (2013) Feedstocks for fuels and chemicals from algae: treatment of crude bio-oil over HZSM-5. Algal Res 2(2):154–163
Zhou M, Tian L, Niu L, Li C, Xiao G, Xiao R (2014) Upgrading of liquid fuel from fast pyrolysis of biomass over modified Ni/CNT catalysts. Fuel Process Technol 126:12–18
Sushil K (2011) Aquatic weeds problems and management in India. Indian J Weed Sci 43(3&4):118–138
Konwer D, Kataki R, Saikia M (2007) Production of solid fuel from Ipomoea carnea wood. Energy Sources Part A-Recovery Utilization Environ Effects 29(9):817–822. doi:10.1080/00908310500281189
Demirbas A (2009) Biorefineries: current activities and future developments. Energy Convers Manage 50:2782–2801
Mullen CA, Strahan DG, Boateng AA (2009) Characterization of various fast-pyrolysis bio-oils by NMR spectroscopy. Energy Fuels 23:2707–2718
Kanaujia PK, Sharma YK, Garg MO, Tripathi D, Singh R (2014) Effects of temperature on the physicochemical characteristics of fast pyrolysis bio-chars derived from Canadian waste biomass. Fuel 125:90–100
Demirbas A (2007) The influence of temperature on the yields of compounds existing in bio-oils obtained from biomass samples via pyrolysis. Fuel Process Technol 88:591–597
Yang H, Yan R, Chen H, Lee DH, Zheng H (2007) Characteristics of hemicelluloses, cellulose and lignin pyrolysis. Fuel 86:1781–1788
Gasparovic L, Korenova Z, Jelemensky L (2009) Kinetic study of wood chips decomposition by TGA. Proceedings of 36th International Conference of Slovak Society of Chemical Engineering, May 25–29, Tatranske Matliare, Slovakia, ISBN 978-80-227-3072-3, pp. (178) 1–14
Vallejos ME, Felissia FE, Curvelo AAS, Zambon MD, Ramos L, Area MC (2011) Analysis of lignin fractions. BioResources 6(2):1158–1171
Park HJ, Heo HS, Park Y, Yim J, Jeon J, Park J, Ryu C, Kim S (2010) Clean bio-oil production from fast pyrolysis of sewage sludge: effects of reaction conditions and metal oxide catalysts. Bioresour Technol 101:S83–S85
Ucar S, Karagoz S (2009) The slow pyrolysis of pomegranate seeds: the effect of temperature on the product yields and bio-oil properties. J Anal Appl Pyrolysis 84:151–156
Sensoz S, Angin D (2008) Pyrolysis of safflower (Charthamus tinctorius L.) Seed press cake: part 1: the effects of pyrolysis parameters on the product yields. Bioresour Technol 99(13):5492–7
Farha S, Farha K, Farha P (2014) Method for the production of polyethylene terephthalate with low carbon footprint. World patent WO2014079572 A1, filed November 21, 2013 and issued May 30
Mullen CA, Boateng AA, Goldberg NM, Lima IM, Laird DA, Hicks KB (2010) Bio-oil and bio-char production from corn cobs and stover by fast pyrolysis. Biomass Bioenergy 34:67–74
Agblevor FA, Beis S, Kim SS, Mante NO (2010) Biocrude oils from the fast pyrolysis of poultry litter and hardwood. Waste Manage 30:298–307
Czernik S, Bridgwater AV (2004) Overview of applications of biomass fast pyrolysis oil. Energy Fuels 18:590–598
Oasmaa A, Czernik S (1999) Fuel oil quality of biomass pyrolysis oils state of the art for the end users. Energy Fuels 13(4):914–921
Zhang R, Zhong Z, Huang Y (2009) Combustion characteristics and kinetics of bio-oil. Front Chem Eng China 3(2):119–124
Lehto J, Oasmaa A, Solantausta Y, Kyto M, Chiaramonti D (2013) Fuel oil quality and combustion of fast pyrolysis bio-oils. Espoo. VTT Technology 79. 87
Radlein D, Wang J, Yuan Y, Quignard A (2013) Methods of upgrading bio-oil to transportation grade hydrocarbon fuels. E U patent EP2638129 A2, filed September 12, 2011 and issued September 18
Schaberg PW (2005) Crude oil derived and gas-to-liquids diesel fuel blends. World Patent WO2005105961 A1, filed April 22, 2005 and issued November 10
Islam MR, Tushar MSHK, Haniu H (2008) Production of liquid fuels and chemicals from pyrolysis of Bangladeshi bicycle/rickshaw tire wastes. J Anal Appl Pyrolysis 82:96–109
Ates F, Putun E, Putun AE (2004) Fast pyrolysis of sesame stalk: yields and structural analysis of bio-oil. J Anal Appl Pyrolysis 71(2):779–790
Putun AE, Apaydın E, Putun E (2004) Rice straw as a bio-oil source via pyrolysis and steam pyrolysis. Energy 29(12–15):2171–2180
Sensoz S, Demiral I, Gercuel HF (2006) Olive bagasse (Olea europea L.) pyrolysis. Bioresour Technol 97(3):429–436
Silverstein RM, Bassler GC, Morrill TC (1991) Spectrometric identification of organic compounds. 5th Ed, John Wiley & Sons, Inc
Xu Y, Hu X, Li W, Shi Y (2011) Preparation and characterization of bio-oil from biomass, progress in biomass and bioenergy production, Dr. Shahid Shaukat (Ed.), ISBN: 978-953-307-491-7
Jone M, Fleming SA (2010) Organic chemistry. Norton, 4th edn. pp14–16
Acknowledgments
The authors are thankful to the Council of Scientific and Industrial Research (CSIR) for funding this work and Director, CSIR-North East Institute of Science and Technology (NEIST), Jorhat, Assam for providing facilities to complete this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saikia, P., Gupta, U.N., Barman, R.S. et al. Production and Characterization of Bio-Oil Produced from Ipomoea carnea Bio-Weed. Bioenerg. Res. 8, 1212–1223 (2015). https://doi.org/10.1007/s12155-014-9561-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-014-9561-2