Abstract
The present study deals with the first systematic study on the isolation, characterization, and utilization of marine yeast for bioethanol production using seaweed biomass. The ability and efficiency of isolated marine yeast to grow and ferment sugar to ethanol in the presence of 2.5 % to 15 % salt concentration was validated by fermenting galactose in the presence of different salts at varied concentrations. Successively, this yeast was employed for fermentation of seaweed hydrolysate, containing high salt concentration, to ethanol. The hydrolysate having varying sugar as well as salt contents, from 2.7 % to 5.5 % and from 6.25 to 11.25 %, respectively, yielded 1.23–1.76 % ethanol. Through biochemical, fatty acid methyl ester analysis, and BioLog, the yeast was identified as Candida sp. The ability of this yeast to function at high salinity can be commercialized for its use to convert seaweed polysaccharide based hydrolysate, rich in salt, to ethanol without desalting process, ultimately making the process more efficient and economically viable. This is the first organized study for the utilization of marine yeast for converting Kappaphycus alvarezii, a red algal biomass, into ethanol as a byproduct, under highly saline condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Yeasts are ubiquitous in their distribution, and their population mainly depends on the type and concentration of organic matter present in the environment. Normally, yeast from terrestrial environment has been used for baking and brewing industries. Currently, biofuels are produced primarily from crops such as corn and sugarcane; however, using food crops for the production of biofuels has caused a controversy of “fuel versus food.” A lot of efforts are placed on commercializing the production of biofuels from terrestrial non-food crops due to their abundance and sustainability. Conversely, this too adds pressure on agricultural land. To resolve this issue, one has to look for marine resources, especially marine macroalgae. Higher growth rate, absence of lignin, and high carbohydrate content make it an attractive renewable source for the production of biofuels [7,10]. One of the challenges in using marine biomass is high salt content in the saccharified hydrolysate which hinders fermentation process by terrestrial yeast [4]. To overcome this problem, attempts were made in the present study to utilize a halophilic yeast for conversion of sugar-rich hydrolysate generated from marine biomass to ethanol.
Diverse yeast genera are reported from Indian waters, viz. Candida, Cryptococcus, Debaryomyces, Kluyveromyces, Metchnikowia, Pichia, Hansenula, Rhodotorula, Torulopsis, Trichosporon, Saccharomyces, Sporobolomyces, and black yeasts. However, the most frequently observed genera are Candida, Cryptococcus, Debaryomyces, and Rhodotorula [6]. Kuiran et al. [5] studied distribution and diversity of marine Candida tropicalis from tropical and subtropical marine environments and obtained 1,089 isolates; routine and molecular identification of those indicated that 44 strains belonged to C. tropicalis, indicating its wide distribution.
Various types of ethanol-producing marine yeasts from coastal waters were isolated and characterized by Urano et al. [12] who found that most of them belonged to the genera Candida and Debaryomyces, which could grow in varying salt concentrations, hence are categorized as euryhaline. They conducted a preliminary study on their fermentative ability by observing gas production in the presence of sodium chloride. However, they did not utilize these yeasts for the production of ethanol. According to Lee et al. [7], Saccharomyces cerevisiae was able to ferment galactose into ethanol, with less efficiency as compared to glucose. To address this problem, an inverse metabolic engineering approach was used to improve ethanol yield and productivity from galactose.
Usually, production of ethanol from biomass involves two steps; saccharification and fermentation. Chemical saccharification of marine biomass generates acidic hydrolysate, rich in monosaccharides as well as salt, which require neutralization as well as desalting prior to fermentation if terrestrially sourced yeast is to be used [8].
In the present study, isolation and characterization of marine yeast together with exploring its potential to ferment galactose under highly saline conditions was undertaken. Based on its potential to utilize galactose efficiently in the presence of 0–15 % salt concentration, it was used to ferment conventionally used sugarcane bagasse as well as Kappaphycus alvarezii, a red algal biomass, rich in carrageenan composed of galactose. Thus, by utilizing an indigenous halotolerant yeast, one can eliminate the energy intensive step of desalting, i.e., electrodialysis, making the whole process economically feasible. Structural features of native and pretreated K. alvarezii biomass were also investigated by scanning electron microscopy (SEM) and Fourier-transform infrared (FTIR) analysis.
Material and Methods
Isolation of Marine Yeasts
Seawater and sediment samples were collected into sterilized polyethylene bottles from Veraval N20º54′663″ E70º21′060″, the West coast of India during June 2010. A portion of the water (400 mL) was passed through a membrane filter (pore size, 0.45 μm, Millipore Ltd.) and the filter paper was soaked in 10 mL of GYP broth tubes consisting of g100 mL−1: glucose 1.0; peptone 0.5; yeast extract 0.5; sodium chloride 2.5, and incubated on a shaker at 30 ± 2 °C for 24 h, for enrichment. Similarly, sediment samples were suspended in GYP broth for enrichment. For the isolation of yeast, a loopful of the suspension was spread on GYP plates (consisting of g100 mL−1: glucose 1.0; peptone 0.5; yeast extract 0.5; sodium chloride 2.5, agar 2.5) and incubated at 30 ± 2 °C for 2–3 days. The individual colonies developed on the plates were picked up and observed under microscope (Nikon Labophot 2), and morphologically different yeast strains were preserved and used for further studies.
Identification of Promising Yeast
The identification of the promising yeast was carried out using biochemical as well as FAME analysis.
Biochemical Analysis
The identification of isolated yeast using biochemical test was done by procuring the HiCandida TM Identification Kit from Himedia Laboratories Pvt. Ltd, Mumbai. The kit consisted of sterile media for urease production and 11 different carbohydrate utilization tests.
FAME Analysis
For identification by FAME analysis, the yeast was streaked on Sabarouds agar plate and incubated at 30 °C for 24 h. Approximately, 20–30 mg of cells were harvested from the plate. The fatty acid methyl esters were extracted according to the standardized protocol of the Microbial Identification System (MIDI). The extracted samples were analyzed with Agilent GC6850, and the profiles were compared with the Sherlock YST28 library 6.0 version (Microbial ID, MIDI Inc.).
Analysis Using BIOLOG MicroStation™ System/MicroLog, Version 5.2.01
Biolog's technology uses each microbe's ability to use particular carbon sources or chemical sensitivity assays to produce a unique pattern or “Phenotypic Fingerprint” for that microbe. The isolate to be identified was grown on an agar medium and then suspended in a special “gelling” inoculating fluid (IF) at the recommended cell density. Then, the cell suspension was inoculated into the Gen-III Microplate, 100 μl per well, and the Microplate was incubated to allow the phenotypic fingerprint to form. The Biolog Gen-III Microplate provides 94 phenotypic tests, 71 carbon utilization assays, and 23 chemical sensitivity assays. For fungi, respiration and assimilation are detected. The color change in the wells is reddish orange due to the respiration of fungi, which reduces the dye. Assimilation or growth is detected by the turbidity of the well.
Preliminary Fermentation Test of Isolated Yeasts
To check the ability of isolated yeast strains to ferment glucose to ethanol, a preliminary test was undertaken by inoculating each yeast strain into 10 mL of GYP broth in a 25 mL volume test tube consisting of a Durham tube and incubated at 30 ± 2 °C for 2–3 days. The fermentation activity of yeast was confirmed by observing the Durham tube filled with CO2 gas [12]. Based on the CO2 positive test, yeast strains were selected for further studies.
Salt and pH Tolerance Study
The halophilic nature of the selected yeast was confirmed by cultivating it in GYP broth containing 2–15 % NaCl. Similarly, a pH tolerance study of isolated yeast was conducted with GYP broth having pH in the range of 2.0–10.0. Growth of the yeast was measured in terms of turbidity, spectrophotometrically at 578 nm (UV–VIS Shimadzu-1800).
Fermentation Efficiency of Selected Yeast in GYP Medium Containing Galactose with Different Salts at Varying Concentrations
Effect of type of salt on the fermentation efficiency of the selected isolate was studied by inoculating isolated yeast (5 % having 1.5 × 107 CFU mL−1) in GYP broth containing 5.0 % galactose and varying concentrations of NaCl, KCl, and CaCl2 ranging from 0 % to 15 %. The flasks were incubated at 30 ± 2 °C on a shaker. Reduction in reducing sugar concentration [9] along with ethanol production were measured (GC-MS) at various time intervals. Here, galactose is used instead of glucose as K. alvarezii polysaccharide is mainly composed of galactose.
Preparation of Sugarcane Bagasse Hydrolysate
Sugar cane bagasse was obtained from a local market, air-dried, powdered, and stored in dry condition until used. Acid hydrolysis was carried out by mixing 5 g biomass with 100 mL of different acids (2.5 % v/v of H2SO4 and HCl) and cooked at 100 °C for 1 h. The hydrolysate was filtered and same amount of new, dry biomass was added to the filtrate and hydrolyzed under similar conditions. This process was repeated 3–5 times to achieve desired reducing sugar concentration which was monitored spectrophotometrically [9]. The pH of the resultant filtrates was highly acidic (around 0.5–1.0), which was neutralized to pH 6.5–6.7 using Ca(OH)2. In the case of hydrolysate prepared using H2SO4, during neutralization, insoluble CaSO4 precipitates were generated which were removed by filtration/centrifugation; however, when HCl was used, the neutralization process yielded soluble salt of CaCl2 which resulted in an increase in the Total Dissolved Solids (TDS) content of the hydrolysate. Conductivity as well as TDS of all the samples were measured by conductivity meter (Century Instruments Pvt. Ltd., Chandigarh; CC601) and TDS meter (Oakton, Waterproof TDS Testr, Eutech Instruments), respectively.
Fermentation of Sugarcane Bagasse Hydrolysate Using Marine Yeast
The fermentative capacity of the isolated marine yeast to ferment sugarcane bagasse hydrolysates having varying TDS was evaluated by supplementing them with 0.5 % of each peptone and yeast extract, autoclaved at 100 °C for 20 min, and inoculated with marine yeast isolate (5 % of ~1.5 × 107 CFU mL−1). Samples were collected at regular intervals and analyzed for reduction in reducing sugar content [9], and ethanol production was monitored using a Shimadzu GC-MS (Shimadzu GC-2010 coupled with Shimadzu mass spectrometer GC-MS-QP2010) with AOC-5000 auto sampler headspace injection system, on a Rtx-5 column (length 30 m, ID 0.25 mm, and film thickness 0.25 μm) with isothermal column oven temperature of 40 °C and injector temperature of 230 °C. Fermented broth (1.0 mL) was incubated for 5 min at 80 °C. Gas sample (250 μl) was injected using auto sampler and ethanol produced was quantified by comparing the peak area of sample with that of standard ethanol obtained through calibration curve.
Collection of Seaweed and Preparation of Hydrolysate
Processing of K. alvarezii was performed as reported by Khambhaty et al. [4], where the fresh algal biomass was crushed to remove sap, leaving behind carrageenan-rich granular biomass [1, 8], which was subsequently dried and used for bioethanol production. The κ-carrageenan rich granules were quick-washed with water to remove adhering salt and were dried. Saccharification was done using H2SO4 in the same manner as described for sugarcane bagasse. Inspite of removal of insoluble salts from the hydrolysate, it contained large amount of soluble salts, contributed by biomass of marine origin. This hydrolysate was used for fermentation after required dilutions.
Fermentation of K. alvarezii Hydrolysate Using Marine Yeast
To check the efficiency of the isolated yeast to ferment K. alvarezii hydrolysate having different salt and sugar contents, different dilutions of neutralized hydrolysate {undiluted, seaweed hydrolysate: distilled water, v/v in a ratio of 3:1, 2:1, and 1: 1} were prepared, and their TDS, conductivity, and reducing sugar contents were measured before inoculation. All the sets were supplemented with 0.5 % of each peptone and yeast extract, autoclaved at 100 °C for 20 min, and inoculated with marine yeast (5 % of ~1.5 × 107 CFU mL−1) as mentioned above. Samples were collected at regular intervals and analyzed for reduction in reducing sugar and ethanol production as mentioned above.
SEM Analysis
Physical changes in native and sulfuric acid-treated K. alvarezii granules were observed using a LEO 1430 VP, scanning electron microscope. Images of surfaces of native and treated biomass were taken at magnification of 15KX. Specimens were mounted on a conductive tape and coated with gold palladium using a LEO 1430 VP fine coater and observed using a voltage of 18 kV.
FTIR Spectroscopic Analysis
FTIR analysis was carried out to detect modifications in the structural changes in κ-carrageenan present in K. alvarezii granules, due to acid hydrolysis. Native as well as treated biomass (3 mg each) was dispersed in spectroscopic grade KBr (300 mg) and subsequently pressed into disks at 10 Mpa for 3 min. A total of 25 scans with a 4 cm−1 resolution were signal-averaged and stored. Wave number range recorded was between 4,000 and 400 cm−1.
Results and Discussion
Isolation of Yeasts
Thirteen, morphologically different strains of yeast were isolated from different samples collected from West coast of India. These were purified on GYP agar medium and tested for their ability to ferment sugar. Based on CO2 gas generated in Durhams tubes, one isolate was identified as the potential fermentative marine yeast.
Identification of Yeast Used in the Present Study
The identification of selected yeast was initially done using Himedia test kit. The ability of the yeast to ferment various sugars was ensured. It was observed that this yeast could ferment sugars viz. melibiose, lactose, mallose, galactose, cellulobiose, xylose, and raffinose. However, it was not able to utilize sucrose, inositol, dulcitol, trehalose, and urease. Successively, the yeast was identified to be Candida sp. The identification was further confirmed by FAME analysis, conducted using MIDI Sherlock® Microbial Identification System. This yeast possessed 18:1 CIS 9 (46.92 %), summed feature 8 as the major cellular fatty acid. The YST28 3.80 library matches from Sherlock® software showed Similarity Index (SI) value of 0.330 with Candida albicans. However, when the identification was performed using BIOLOG MicroStation™ System, it was observed that after 30 h of incubation the yeast exhibited 0.218 similarity to C. albicans, which after a further incubation at 48 and 72 h revealed a 0.709 and 0.907 similarity index to C. tropicalis B. It was also observed that all of the wells in the MicroPlate start out colorless when inoculated. During incubation process there is increased respiration in the wells where cells utilize a carbon source or grow in the presence of inhibitory chemicals. Increased respiration causes reduction of the tetrazolium redox dye, forming a purple color. In addition to this, an increase in substrate utilization was also observed with the progress in incubation time.
Study on Salt Tolerance and pH Tolerance of Marine Yeast
In the presence of 2–13 % of salt, luxuriant growth of yeast was observed, which subsequently decreased at 14 % and 15 % NaCl concentrations. Thus, the yeast isolated in the present study was found to be euryhaline in nature, which made it suitable for fermentation of sugar to ethanol in the presence of high salt concentration and helped in eliminating the step of desalting of the hydrolysate while using marine biomass as substrate. Growth of yeast isolates in different concentrations of NaCl was studied by Gupta [2], where it was reported that various species of yeasts like Debaryomyces, Rhodotorula, Candida, and Saccharomyces could tolerate NaCl concentration ranging from 0 % to 16 %. Apart from these, yeasts that could tolerate sodium chloride concentration ranging from 0 to 3.5 M have also been documented [6].
In the present study, the isolated yeast was inoculated into GYP broth containing pH ranging from 2.0 to 11.0. Growth of the yeast in terms of turbidity was measured spectrophotometrically. It was observed that after 48 h, maximum growth was observed at pH ranging from 4 to 9, which was decreased with a further increase in pH. This property indicated that the isolate had a wide range of pH tolerance, from acidic to alkaline. According to a report [11], the optimum pH of yeasts M15, M23, and M28 was 5. However, M10, identified as Candida, exhibited a wide pH tolerance from 4 to 10 with very little difference in growth. Low growth was noticed in the alkaline range for the other strains. The ability of the yeast to tolerate a wide range of pH might be due to the fact that pH of the cell interior of the yeast remains quite constant at about pH 5.8, regardless of pH variations in the fermenting medium. The enzymes involved in fermentation thus operate in an optimum pH environment within the yeast cell that is largely unaffected by external changes in pH [3].
Conversion of Galactose to Ethanol in the Presence of Different Salts at Varying Concentrations
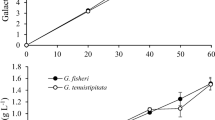
Usually, yeasts have preference towards glucose as compared to galactose, but since the seaweed biomass, used in the present study is rich in polysaccharide composed of galactose, all the experiments were conducted using galactose. To check the efficiency of isolated marine yeast to ferment galactose, the experiments were conducted using galactose in the fermenting media containing different salts at varying concentration. In the presence of 0–10 % of KCl, CaCl2, and NaCl, GYE broth containing 5 % galactose yielded approximately 2.1–2.4 % ethanol within 24 h, indicating 85–100 % conversion efficiency in the presence of low to high salt concentration, whereas in the presence of 15 % KCl and 15 % NaCl, fermentation process becomes slow with reduced efficiency as it produced approximately 1.86 % and 1.62 % ethanol, respectively, after 96 h, indicating the inhibitory effect of high salt concentration, while the presence of CaCl2 at 15 % concentration showed inhibitory effect on fermentation as it could produce only 0.81 % ethanol even after 120 h of incubation (Table 1). It is well known that K. alvarezii has propensity to accumulate KCl from the seawater and being seaweed growing in the seawater, it may have traces of NaCl. Thus, the possibility of presence of KCl or mixture of KCl and NaCl in the hydrolysate is expected, which could affect fermentation. As both the salts did not show any adverse effect on fermentation even at 10 % concentration, the isolated yeast can be the best choice for fermentation of K. alvarezii hydrolysate having high salt concentration.
Hydrolysis of Sugarcane Bagasse and Subsequent Fermentation to Ethanol by Isolated Yeast
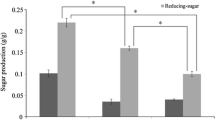
Keeping in mind the fermentative ability of the isolated yeast over a varying range of NaCl concentration, it was assessed for its ability to ferment hydrolysate obtained from conventionally used sugarcane bagasse where salt concentration is relatively less. At the end of three cycles of hydrolysis with H2SO4, 7.17 % of reducing sugar was obtained where TDS was 0.71 %, while conductivity was 22.52 mS as neutralization with Ca(OH)2 yielded insoluble CaSO4, which was removed by filtration/centrifugation. On the contrary, when the same biomass was subjected to hydrolysis by HCl, almost similar sugar concentration, i.e., 7.57 % was achieved with TDS and conductivity as high as 4.5 % and 86.95 mS, respectively (Table 2), due to generation of soluble CaCl2 during neutralization with calcium hydroxide. In the sugarcane hydrolysates, prepared using H2SO4 and HCl, ethanol production achieved was 2.28 % and 1.89 %, respectively, with conversion efficiency of 66 % and 55 % after 48–72 h (Fig. 1a, b). This proved the competence of the isolated yeast to ferment sugar to ethanol in the presence of low as well as high salt concentration.
Hydrolysis of Granules and Neutralization of Hydrolysate
The conditions used for saccharification of washed and dried K. alvarezii granules and sugar generated during saccharification in each cycle are detailed in Table 3.
Fermentation of Obtained Algal Hydrolysate
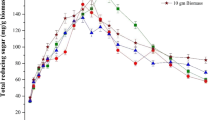
Reducing sugar content, TDS, and conductivity of different dilutions of hydrolysate used for the experiment are indicated in Table 4. It was observed that in the case of undiluted hydrolysate, where reducing sugar content was 5.5 % and salt concentration was as high as 11.25 %, ethanol production was 1.23 % after 72 h of incubation representing 50 % conversion efficiency. However, in case of 3:1 diluted hydrolysate having 3.77 % reducing sugar and salt content of 9.0 %, 1.76 % ethanol was obtained within 48 h, exhibiting 100 % conversion. Similarly, in the case of 2:1 as well as 1:1 dilutions, 100 % conversion was observed within 48 h. As indicated above, though the growth of yeast was luxuriant in presence of 13 % salt, fermentation efficiency was relatively low at 11.25 % salt concentration, which revealed inhibitory effect of high salt content on fermentation; however, in the presence of 9–6.25 % salt, conversion of sugar to ethanol was 100 % (Fig. 2).
Characterization of Native and Treated Biomass
SEM observations of native and treated K. alvarezii granules (Fig. 3) exhibited that acidic treatment caused drastic physical changes in the biomass. It hydrolyzed the cell wall structure of the biomass, which in turn released the fermentable sugar in the medium, accessible to yeast. This was further confirmed by performing FTIR spectra of native (Fig. 4a) and treated biomass (Fig. 4b), indicating that some of the distinct peaks of κ-carrageenan observed in native granules (at 1,260 cm−1 of S=O of ester sulfate, at 930 cm−1 of C–O of 3,6 anhydrogalactose and at 845 cm−1 of C–O–SO4 for C4 of galactose) disappeared in the treated biomass, indicating structural changes in the biomass.
Conclusion
The present study reveals utilization of marine yeast for converting K. alvarezii biomass into bioethanol as a byproduct. Tolerance of isolated marine yeast at wide ranges of salts and pH makes it a potential candidate for its use in fermentation under diverse environmental conditions. Utilization of marine yeast for converting algal sugar to ethanol would eliminate an energy intensive step of electrodialysis, thus making the whole fermentation process economically viable. To the best of our knowledge, this is the first report on utilization of marine yeast for the production of seaweed-based ethanol.
References
Eswaran K, Ghosh PK, Siddhanta AK, Patolia JS, Periasamy C, Mehta AS et al (2005) Integrated method for production of carrageenan and liquid fertilizer from fresh seaweeds. United States Patent No.6893479, May 17, 2005
Gupta R (1996) Growth of marine yeast on different strength of stress solutes. Proc Second Workshop on Scientific Results of FORV Sagar Sampada. Department of Ocean Development, New Delhi, pp. 91–95
http://home.earthlink.net/~ggda/The_Artisan_Yeast_Treatise_Section_Two.htm
Khambhaty Y, Mody K, Gandhi MR, Thampy SK, Maiti P, Brahmbhatt H et al (2012) Kappaphycus alvarezii as a source of Bioethanol. Bioresour Technol 103:180–185
Kuiran Y, Ying Z, Zhenming C (2010) Distribution and diversity of Candida tropicalis strains in different marine environments. J Ocean Univ China 9(2):139–144
Kutty NS, Philip R (2008) Marine yeasts—a review. Yeast 25:465–483
Lee KS, Hong ME, Jung SC, Ha SJ, Yu BJ, Koo HM et al (2011) Improved galactose fermentation of Saccharomyces cerevisiae through inverse metabolic engineering. Biotechnol Bioeng 108(3):621–631
Mody KH, Ghosh PK, Barindra S, Gnanasekaran G, Shukla AD, Eswaran K et al. A process for integrated production of ethanol and seaweed sap from Kappaphycus alvarezii WO2011/027360A1, March 10, 2011
Nelson M (1944) A photometric adaption of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Packer M (2009) Algal capture of carbon dioxide; biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energ Policy 37:3428–3437
Rhishipal R, Philip R (1998) Selection of marine yeasts for the generation of single cell protein from Prawn-shell waste. Bioresour Technol 65:255–256
Urano N, Yamazaki M, Ueno R (2001) Distribution of halotolerant and/or fermentative yeasts in aquatic environments. J Tokyo Univ Fish 87:23–29
Acknowledgments
Y Khambhaty acknowledges Council of Scientific and Industrial Research, New Delhi for award of Senior Research Associateship. The authors are also thankful to the Ministry of New and Renewable Energy (MNRE), New Delhi for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khambhaty, Y., Upadhyay, D., Kriplani, Y. et al. Bioethanol from Macroalgal Biomass: Utilization of Marine Yeast for Production of the Same. Bioenerg. Res. 6, 188–195 (2013). https://doi.org/10.1007/s12155-012-9249-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-012-9249-4