Abstract
Objectives
MotionFree® (AMF) is a data-driven respiratory gating (DDG) algorithm for image processing that has recently been introduced into clinical practice. The present study aimed to verify the accuracy of respiratory waveform and the effects of normal and irregular respiratory motions using AMF with the DDG algorithm.
Methods
We used a NEMA IEC body phantom comprising six spheres (37-, 28-, 22-, 17-, 13-, and 10 mm diameter) containing 18F. The sphere-to-background ratio was 4:1 (21.2 and 5.3 kBq/mL). We acquired PET/CT images from a stationary or moving phantom placed on a custom-designed motion platform. Respiratory motions were reproduced based on normal (sinusoidal or expiratory-paused waveforms) and irregular (changed amplitude or shifted baseline waveforms) movements. The “width” parameters in AMF were set at 10–60% and extracted data during the expiratory phases of each waveform. We verified the accuracy of the derived waveforms by comparing those input from the motion platform and output determined using AMF. Quantitative accuracy was evaluated as recovery coefficients (RCs), improvement rate, and %change that were calculated based on sphere diameter or width. We evaluated statistical differences in activity concentrations of each sphere between normal and irregular waveforms.
Results
Respiratory waveforms derived from AMF were almost identical to the input waveforms on the motion platform. Although the RCs in each sphere for expiratory-paused and ideal stationary waveforms were almost identical, RCs except the expiratory-paused waveform were lower than those for the stationary waveform. The improvement rate decreased more for the irregular, than the normal waveforms with AMF in smaller spheres. The %change was improved by decreasing the width of waveforms with a shifted baseline. Activity concentrations significantly differed between normal waveforms and those with a shifted baseline in spheres < 28 mm.
Conclusions
The PET images using AMF with the DDG algorithm provided the precise waveform of respiratory motions and the improvement of quantitative accuracy in the four types of respiratory waveforms. The improvement rate was the most obvious in expiratory-paused waveforms, and the most subtle in those with a shifted baseline. Optimizing the width parameter in irregular waveform will benefit patients who breathe like the waveform with the shifted baseline.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Whole-body 18F-fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) images that are acquired over several minutes contain many respiratory motion-related artifacts. Respiratory gating is a standard method to correct image blurring caused by respiratory motion, and enable the detection of small lesions [1]. A device gating with external equipment such as belts and infrared markers are still applied as respiratory gating [2]. Recently, device-less data-driven respiratory gating (DDG) has been introduced a commercial PET scanner for more robust operation and acquisition [3, 4].
A DDG is the means of respiratory gating that analyzes temporal sinogram data for PET. DDG algorithm provides automatically respiratory waveforms using various means, such as principal component analysis (PCA), spectral analysis and the center-of-mass method [5,6,7,8,9,10]. The motion correction based on derived respiratory waveform enhances spatial resolution and image quality and augments the standardized uptake value (SUV) of small lesions [11,12,13]. This can generate useful clinical information and has been applied to the staging and lesion classification of cancer in the vicinity of the diaphragm [14, 15]. The performance of DDG is at least equal to that of device gating [16,17,18,19] and it has also the less invasive and laborious procedures for patients and operators thanks to device-less [4]. MotionFree® (AMF; GE Healthcare, Milwaukee, WI, USA) with PCA algorithm has recently been introduced into commercial PET device [11].
The DDG algorithm that handle internal sinogram data more precisely detect respiratory signals than device gating [20] and can be estimated respiratory waveform even in a short acquisition time [21]. Derived respiratory waveforms reflect directly on the respiratory status of patients [22], which is classified some types. AMF methods improve the quality of PET images with respiratory motion, but the improved effect has not yet been clarified under various respiratory conditions. The present study aimed to verify the analysis accuracy of normal and irregular respiratory waveform and quantify improvements in these blurring by AMF.
Materials and methods
PET/CT systems
All PET data were acquired using a Discovery IQ PET/CT system (GE Healthcare) with a PET scanner comprising five rings of detector blocks with bismuth germanate (Bi4Ge3O12; BGO) crystals. The scintillator unit includes 21,888 BGO crystals 6.3 × 6.3 × 30 mm. The axial and transaxial fields of view (FOV) of the PET detector are 260 and 700 mm, respectively. The 16-slice CT detector has a transaxial FOV of 500 mm [23]. We reconstructed all PET images used the Bayesian penalized likelihood method, Q.Clear® (GE Healthcare). It includes point spread function modeling and controls image noise through a penalization factor (β value). The β value applied to control the global strength of smoothing was 300 same as clinical condition. The matrix was 192 × 192, the number and thickness of slices was 79 images and 3.27 mm, respectively.
Phantom design
Respiratory motions were replicated using a National Electrical Manufactures Association (NEMA) International Electrotechnical Commission (IEC) body phantom (ITEC Co., Ltd., Tokyo, Japan) with a custom-designed motion platform. This phantom mimic the shape of the upper body of an average Japanese person weighting 60 kg. It contains six spheres (37-, 28-, 22-, 17-, 13-, and 10 mm diameter) and a simulated cylindrical (50 mm diameter) lung placed in the center. The measured content was 9,907 mL. We used this custom-designed platform that could move periodically in craniocaudal directions according to input waveform created by a personal computer [24]. We reproduced four respiratory motions with the phantom placed on the platform driven by a motor, two normal waveforms (sinusoidal or expiratory-paused ones) and two irregular waveforms (changed amplitude or shifted baseline ones) (Fig. 1a). The input information of the motion platform was 12 mm and 3.6 s/cycle as a standard based on mean lung tumor motions [25, 26]. The irregular respiratory waveforms were set to have the phantom moved by 12- and 18 mm displacement. Actual respiratory motion tends to dwell breathing for a few seconds around the maximum expiratory points. Therefore, the expiratory-paused waveform was set with 5.6 s/cycle, including 2-s pause.
The CT image was scanned for attenuation correction after the phantom stopped moving at the middle of the inspiratory and expiratory phases. Attenuation correction errors caused by misalignment between CT and PET images eliminated, because the density of 18F-FDG in the phantom was the same in the spheres and background (BG). The 18F-FDG concentrations in the spheres and background were 21.2 and 5.3 kBq/mL, respectively (sphere-to-background ratio = 4).
MotionFree
To describe image processing shows flow chart for AMF algorithm in Fig. 2. This algorithm has adopted PCA, dimensionality reduction processing, and can calculate three principal components and weight factors from sinogram data per 0.5 s (time index) [5]. The temporal resolution of 0.5 s is designed to reduce the sensitivity to the cardiac motion, and weight factors are determined by the following formula:
where r is the radial position of the line of response (LOR) in the transaxial plane, z is the axial coordinate, φ is the azimuthal angle between the LOR and the y-axis of the system. D (r, z, φ) is the three-dimensional coordinate per 0.5 s, and Ad (r, z, φ) is the mean coordinate on the total acquisition time. Thus, difference of between D (r, z, φ) and Ad (r, z, φ) reflects the degree of misalignments from mean coordinates. The P (r, z, φ) are the principal component vectors that are the coordinates of the sinogram data. The waveform corresponding to each principal component are created based on the weight factor, and one of three components contain motion-related the most reliable respiratory information. The determination of waveform including respiratory information is identified by the following formula:
A fast Fourier transformation is applied to each derived waveform and divide the processed data into respiratory frequency (0.1‒0.4 Hz) and other (> 0.4 Hz) bands. The ratio of the maximum power spectrum in 0.1‒0.4 Hz to the mean power spectrum above > 0.4 Hz is defined R1, R2, and R3 values as the intensity of respiratory motion. That is, the higher R value has a probability of respiratory waveform. Thereafter, the principal component with the highest R is re-transformed to produce final respiratory waveforms. The AMF consider the impact of respiratory motion on PET images when the selected R exceeds a threshold value, and applying respiratory gating with Q.Static® (GE Healthcare). The R is > 15 and ≤ 15 between two groups differed the mean increase rate of maximum SUV [19]. The SUV of small lesions are more improved in PET images with Q.Static® compared with non-gated images [14, 19, 27]. A maximum inspiration point every a cycle is marked as a trigger point by Q.Static®. AMF using Q.Static® divide each respiratory cycle between each trigger-to-trigger by 100 and arbitrary data extract by the parameters called “offset" and “width” (Fig. 3) [11]. The default settings on AMF extract 50% of the data from points at 30% away from the maximum inspiration [28].
Principle of data extraction with AMF. Grey crosses are trigger landmarks at maximum inspiration. Blue line divides each cycle between two triggers into hundredths. Red and green lines are default 30% offset and 50% width, respectively. Offset is range from trigger to a specific point (red and green circles), and width is distance between points in the same data
Data acquisition and image reconstruction
All emission data were acquired in 3D list mode. A phantom was placed on the platform in the PET/CT system and defined a “stationary” as no movement. The stationary images and normal waveforms with the platform moved were acquired emission data for 20 min. The irregular waveforms with a changed amplitude and a shifted baseline were acquired from emission data for 23 and 27 min to correct 18F decay. We resampled the list-mode data of these waveforms and reconstructed them with the following three ways. First, we reconstructed the list-mode data with default AMF and created gated images for 10 min (AMFon) and non-gated images for 10 min (AMFoff). Second, we reconstructed 2-min images with the default AMF parameter and evaluated significant differences among waveforms. These reconstructed images were, respectively, created nine ones with staggering time phase to PET data of waveforms. Finally, we reconstructed 2-min images with the variable AMF parameters (width: from 10% to 60%, offset: from 50% to 25%). To activate AMF, the threshold of R value was set 0 at all emission data.
Data analysis
The reconstructed PET images were analyzed using Osirix Lite software v.12.0.1 (Pixmeo Sàrl, Bernex, Switzerland). We measured maximum activity concentrations by placing regions of interest (ROI) of the same size on each sphere of the stationary, AMFoff and AMFon images. Recovery coefficients (RCs) were calculated as
\(RC=\frac{{AC}_{max,i}}{{AC}_{max,37}}\),
where i is the diameter of hot spheres. where ACmax,i is the maximum measured activity concentration on the center of the stationary, AMFoff and AMFon images, and ACmax,37 is the maximum activity concentration on the center of a 37 mm hot sphere in the stationary images. The Improvement rate (%) between AMFoff and AMFon images was calculated as
\(Improvement rate \left(\mathrm{\%}\right)= \left(\frac{{RC}_{AMF,i}-{RC}_{nonAMF,i}}{{RC}_{nonAMF, i}}\right)\times 100\),
where RCAMF,i and RCnonAMF,i represent RC with and without AMF, respectively, on the four respiratory waveforms, and i represents hot sphere diameter (37, 28, 22, 17, 13, and 10 mm). The rate of improvement (%change) in images of variable parameters was calculated as
\(\mathrm{\%}change =\frac{1}{6}{\sum }_{i=\mathrm{10,13,17,22,28,37}mm}\left(\frac{\left({RC}_{width,i}-{RC}_{50\mathrm{\%},i}\right)}{{RC}_{50\mathrm{\%},i}}\right)\times 100\),
where RCwidth,i represents the RC of each hot sphere on AMFon images with variable width, and thus RC50%,37 represents the RC of a 37-mm hot sphere on AMFon images with width 50%.
Statistical analysis
We compared activity concentrations of each spheres using Dunn tests for various waveforms. Calculated values were considered statistically significant at P < 0.05. All data were analyzed using Prism 9 (GraphPad Software LLC., San Diego, CA, USA).
Results
Figure 4 shows the four respiratory waveforms derived from sinogram data using AMF algorithm. Table 1 shows the cycle, R, the number of triggers and the acquisition time of the analyzed waveform information. All respiratory waveforms derived with AMF almost matched the shape of the input waveform. The maximum R values were < 5 for the stationary, 170 for the expiratory-paused waveform and > 240 for others.
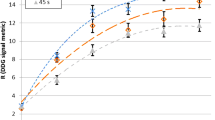
Figure 5 shows the PET images and the concentration profiles of the phantom on the four waveforms, and Fig. 6 shows the RCs of the stationary and the respiratory waveforms. Image blurring by respiratory motion were visually improved in all respiratory waveforms using AMF. The RCs of all respiratory waveforms decreased as the sphere diameter were smaller. The RC of these waveforms with AMF was the lowest of the shifted baseline waveform, followed by those with a changed amplitude, and the sinusoidal, expiratory-paused types. The RCs were, respectively, 0.95, 0.96 and 0.92 for the 28, 22 and 17 mm spheres in the stationary images, and 0.62 and 0.35 for the 13- and 10-mm spheres, respectively. The RCs of the expiratory-paused waveform (37–10 mm spheres) were 0.98, 0.97, 0.96, 0.91, 0.65 and 0.40, which were similar to those of the stationary images.
Recovery coefficients (RC) of four respiratory waveforms. Dotted line is RC of stationary images. Filled and unfilled symbols, AMFon and AMFoff images, respectively. Sinusoidal (a) (Filled Circle, Circle), expiratory pause (b) (Filled Triangle, Triangle), changed amplitude (c) (Filled Square, Square), and shifted baseline (d) (Filled Diamond, Diamond)
Table 2 shows the improvement rate between the images with AMFoff and AMFon in each sphere. On all respiratory waveforms, the improvement rate in the 37 mm sphere was the lowest. Although these increased as the sphere diameter is smaller, the improvement rate in the 10 mm sphere was lower than that in the 13 mm sphere. The improvement rates of the sinusoidal and the expiratory-paused waveforms in the 13 mm sphere were maximal (35.9% and 52.9%, respectively). The 22- and 17-mm spheres in which waveforms with a changed amplitude and a shifted baseline improved the most (42.6% and 42.5%, respectively).
Table 3 shows statistical differences in activity concentrations of PET images between two waveforms with AMF. No significant differences were found among normal and among irregular waveforms regardless of sphere diameter. In contrast, normal and irregular waveforms significantly differed in spheres < 28 mm.
Figure 7 shows the result of the %change as the width changes. The %change of the expiratory-paused waveform was constant regardless of width. The %change increased in the sinusoidal and changed amplitude waveforms as width decreased, then gradually decreased when the width was < 20%. The %change increased as width decreased in the waveform with the shifted baseline.
Discussion
Previous phantom studies with AMF remain at visual and image evaluations with sinusoidal waveforms [26, 29, 30]. Phantom experiments are useful and essential to validate the features of the quantitative accuracy of respiratory gating that cannot be determine in clinical patients, because they can be constructed with known materials and activity concentrations. Here, we evaluated the accuracy of the derived waveform and the quantitative improvement effects using four respiratory waveforms and a NEMA IEC body phantom. We found that PET imaging with AMF provided precise waveforms and improvement of image blurring regardless of respiratory types. However, the improvement effect on the irregular waveforms decreased, and the waveform with a shifted baseline compared to normal ones significantly differed. Decreasing the width parameter can be effectively improved respiratory motion on the waveform with the shifted baseline.
The trigger points of maximum inspiration points are an important factor to improve the image blurring by AMF. The discrimination errors of trigger might occur depending on the type of respiratory motions, but the triggers were located at the maximum inspiration points regardless of respiratory waveforms. This is explained in terms of unity of acquisition time per cycle and the number of triggers. In addition, stationary waveform with no movement was also detected the R value. The AMF computationally determines respiratory motions from whole emission data in the body. Thus, it can stably detect trigger point and waveform even if imperceptible motion, such as stationary [16]. That is, the AMF algorithm can resolve various errors of device gating in clinical patients with shallow and irregular breathing [20].
Whether or not the motion correction by Q.Static® activates depends on a set R value. The maximum R value derived from 157 patients injected with 18F-FDG was around 50, and a recommended R value is a 15 [19]. However, our results considerably exceeded the default R = 15. Two factors can explain this. One is that we did not consider amplitude exceeding 0.4 Hz (such as motion derived from other organ), because we repeated experiments with the motion platform moving at a constant amplitude of 0.28 or 0.18 Hz (3.6 or 5.6 s/cycle). The other is that the acquisition time in this study was longer than clinical acquisition time. The R values increase in proportion to the acquisition time (the number of trigger), because the power spectrum of the respiratory frequency band at 0.1–0.4 Hz is higher than that of > 0.4 Hz in respiratory principal component [19]. The lower R value on the expiratory-paused waveform was due to longer respiratory cycles than the others. Each facility should be carefully set at R values considering that it varies according to respiratory cycles and acquisition time in clinical patient.
The RCs with AMF improved more than without AMF regardless of respiratory types. However, the activity concentration was significantly lower for irregular, than normal waveforms because of differences in motion displacement (Table 3). The difference in activity concentration can be explained by the relationship between respiratory waveforms and sphere diameters. When respiratory motion occurs, the image blurring caused by spill-out of the partial volume effects (PVE) expand and underestimate activity concentrations in a ROI [31]. Quantitative values exponentially decrease in accordance with the motion displacement [32, 33], and PVE increases as a lesion size is smaller [34, 35]. Therefore, the RC without AMF was lower than the other waveforms, because the waveform with changed amplitude had the most repeats of motion displacement of 1.8 cm. Irregular waveforms with great motion displacement have a critical influence as a sphere size is small. Enhanced AMF performance has an important role in oncology patients with metastases 1 cm or less is achieved to optimal treatment strategy [3].
The Q.Static® using phase gating algorithm extracts emission data based on the premise of repeated movement at the same expiratory point. Thus, irregular waveforms cannot eliminate image misalignment of the craniocaudal directions [36, 37]. The waveform with the shifted baseline that has most residual misalignment resulted in a significantly lower the activity concentration than the normal waveforms (Table 3). Previous gating studies have indicated that the optimal width is < 50% [38, 39]. Because PET images with width at 50% could not suppress respiratory motions in some clinical images, setting the width at 25‒50% for phase gating have been recommended for data acquisition [20, 26]. Kesner et al. was also described that data are optimal at 35–40% [40]. Our results showed the %change in the irregular waveforms improved up to width 20% beyond the default setting (Fig. 7c, d). The waveform with a changed amplitude compared to sinusoidal one should theoretically improve even below width 20% due to the greater motion displacement. Nevertheless, the %change of that decreased below width 20%, because the sphere overlap separated by expiratory misalignment. In contrast, waveform with a shifted baseline had already separated the sphere from width 50%, and thus %change kept increasing as a function of smaller width. The count loss with smaller width can be overcome by extending acquisition time. We suggest setting the width at 30‒35% for irregular waveforms with expiratory misalignment. Although AMF can effectively demonstrate intrinsic performance, a balance between quantitative improvement and acquisition time should be considered.
Recent advanced developments in silicon photomultiplier-based PET imaging have achieved high spatial resolution of 2‒5 mm [41], but its relationship with the impact of respiratory motion is a trade-off [32]. Therefore, the clinical importance of PET imaging with AMF is likely to increase in the future. However, the present study has some limitations, because we used only an average-sized phantom, and did not consider the effects of physique. Therefore, the influence of factors, such as counts and noise, remain unclear. Future investigations of clinical PET images should focus on patient-associated parameters, such as habitus, weight, injected dose and acquisition time. Acquiring these data in various types of patients would provide further the clinical benefits of PET imaging with AMF.
Conclusions
Device-less respiratory gating using the AMF algorithm improved the stable discrimination and the quantitative accuracy of four types of respiratory waveforms. The improvement rate was the most obvious and the most subtle, respectively, with the expiratory-paused, and shifted baseline waveforms. Optimizing the width at 30‒35% will especially benefit the superior PET images for patients who irregularly breath like a shifted baseline.
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
van der Vos CS, Koopman D, Rijnsdorp S, Arends AJ, Boellaard R, van Dalen JA, et al. Quantification, improvement, and harmonization of small lesion detection with state-of-the-art PET. Eur J Nucl Med Mol Imaging. 2017;44:4–16.
Pepin A, Daouk J, Bailly P, Hapdey S, Meyer ME. Management of respiratory motion in PET/computed tomography: the state of the art. Nucl Med Commun. 2014;35:113–22.
Aide N, Lasnon C, Kesner A, Levin CS, Buvat I, Iagaru A, et al. New PET technologies - embracing progress and pushing the limits. Eur J Nucl Med Mol Imaging. 2021;48:2711–26.
Kesner AL, Schleyer PJ, Büther F, Walter MA, Schäfers KP, Koo PJ. On transcending the impasse of respiratory motion correction applications in routine clinical imaging—a consideration of a fully automated data driven motion control framework. EJNMMI Phys. 2014;1:8.
Thielemans K, Rathore S, Engbrant F, Razifar P. Device-less gating for PET/CT using PCA. In: 2011 IEEE Nuclear Science Symposium Conference Record; 2011. p. 3904–10.
Thielemans K, Schleyer P, Marsden PK, Manjeshwar RM, Wollenweber SD, Ganin A. Comparison of different methods for data-driven respiratory gating of PET data. In: 2013 IEEE Nuclear Science Symposium and Medical Imaging Conference (2013 NSS/MIC); 2013. p. 1–4.
Schleyer P, Hong I, Jones J, Hamill J, Panin V, Fuerst S. Data-driven respiratory gating whole body PET using continuous bed motion. In: 2018 IEEE nuclear science symposium and medical imaging conference proceedings (NSS/MIC); 2018. p. 1–5.
Schleyer PJ, O’Doherty MJ, Barrington SF, Marsden PK. Retrospective data-driven respiratory gating for PET/CT. Phys Med Biol. 2009;54:1935–50.
Bundschuh RA, Martinez-Moeller A, Essler M, Martinez MJ, Nekolla SG, Ziegler SI, et al. Postacquisition detection of tumor motion in the lung and upper abdomen using list-mode PET data: a feasibility study. J Nucl Med. 2007;48:758–63.
Feng T, Wang J, Sun Y, Zhu W, Dong Y, Li H. Self-gating: an adaptive center-of-mass approach for respiratory gating in PET. IEEE Trans Med Imaging. 2018;37:1140–8.
KhamisH WS. MotionFree:Device-less digital respiratory gating technique, seamlessly integrated in PET imaging routine. In: Co. GE, editor.
Sebastian Fuerst JH, Inki Hong, Judson Jones, Paul Schleyer. OncoFreeze: Deviceless motion management for PET imaging. In: Siemens Medical Solutions USA I, editor.
Feng T, Yang G, Liu H, Ding Y, Lv Y, Li H, et al. Data-driven phase-matched PET/CT: a solution for axial location-dependent respiratory phase in CT. J Nucl Med. 2021;62:1420.
Morley NC, McGowan DR, Gleeson FV, Bradley KM. Software respiratory gating of positron emission tomography-computed tomography improves pulmonary nodule detection. Am J Respir Crit Care Med. 2017;195:261–2.
Liberini V, Kotasidis F, Treyer V, Messerli M, Orita E, Engel-Bicik I, et al. Impact of PET data driven respiratory motion correction and BSREM reconstruction of (68)Ga-DOTATATE PET/CT for differentiating neuroendocrine tumors (NET) and intrapancreatic accessory spleens (IPAS). Sci Rep. 2021;11:2273.
Buther F, Jones J, Seifert R, Stegger L, Schleyer P, Schafers M. Clinical evaluation of a data-driven respiratory gating algorithm for whole-body PET with continuous bed motion. J Nucl Med. 2020;61:1520–7.
Kang SY, Moon BS, Kim HO, Yoon HJ, Kim BS. The impact of data-driven respiratory gating in clinical F-18 FDG PET/CT: comparison of free breathing and deep-expiration breath-hold CT protocol. Ann Nucl Med. 2021;35:328–37.
Kesner AL, Chung JH, Lind KE, Kwak JJ, Lynch D, Burckhardt D, et al. Validation of software gating: a practical technology for respiratory motion correction in PET. Radiology. 2016;281:239–48.
Walker MD, Morgan AJ, Bradley KM, McGowan DR. Evaluation of data-driven respiratory gating waveforms for clinical PET imaging. EJNMMI Res. 2019;9:1.
Walker MD, Morgan AJ, Bradley KM, McGowan DR. Data-driven respiratory gating outperforms device-based gating for clinical (18)F-FDG PET/CT. J Nucl Med. 2020;61:1678–83.
Walker MD, Bradley KM, McGowan DR. Evaluation of principal component analysis-based data-driven respiratory gating for positron emission tomography. Br J Radiol. 2018;91:20170793.
Buther F, Ernst I, Frohwein LJ, Pouw J, Schafers KP, Stegger L. Data-driven gating in PET: Influence of respiratory signal noise on motion resolution. Med Phys. 2018;45:3205–13.
Reynes-Llompart G, Gamez-Cenzano C, Romero-Zayas I, Rodriguez-Bel L, Vercher-Conejero JL, Marti-Climent JM. Performance characteristics of the whole-body discovery IQ PET/CT System. J Nucl Med. 2017;58:1155–61.
Tachibana H, Kitamura N, Ito Y, Kawai D, Nakajima M, Tsuda A, et al. Management of the baseline shift using a new and simple method for respiratory-gated radiation therapy: detectability and effectiveness of a flexible monitoring system. Med Phys. 2011;38:3971–80.
Seppenwoolde Y, Shirato H, Kitamura K, Shimizu S, van Herk M, Lebesque JV, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53:822–34.
Liu C, Alessio A, Pierce L, Thielemans K, Wollenweber S, Ganin A, et al. Quiescent period respiratory gating for PET/CT. Med Phys. 2010;37:5037–43.
Shirato H, Seppenwoolde Y, Kitamura K, Onimura R, Shimizu S. Intrafractional tumor motion: lung and liver. Semin Radiat Oncol. 2004;14:10–8.
Sigfridsson J, Lindstrom E, Iyer V, Holstensson M, Velikyan I, Sundin A, et al. Prospective data-driven respiratory gating of [(68)Ga]Ga-DOTATOC PET/CT. EJNMMI Res. 2021;11:33.
Kim DH, Yoo EH, Hong US, Kim JH, Ko YH, Moon SC, et al. Image Registration of (18)F-FDG PET/CT Using the MotionFree Algorithm and CT Protocols through Phantom Study and Clinical Evaluation. Healthcare (Basel). 2021;9.
Yamashita K, Miyaji N, Motegi K, Ito S. Terauchi T [Effects of CT-based attenuation correction on pet images using data-driven respiratory gating]. Nihon Hoshasen Gijutsu Gakkai Zasshi. 2021;77:1317–24.
Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48(6):932–45.
Daou D. Respiratory motion handling is mandatory to accomplish the high-resolution PET destiny. Eur J Nucl Med Mol Imaging. 2008;35:1961–70.
Okubo M, Nishimura Y, Nakamatsu K, Okumura M, Shibata T, Kanamori S, et al. Static and moving phantom studies for radiation treatment planning in a positron emission tomography and computed tomography (PET/CT) system. Ann Nucl Med. 2008;22:579–86.
Teo BK, Saboury B, Munbodh R, Scheuermann J, Torigian DA, Zaidi H, et al. The effect of breathing irregularities on quantitative accuracy of respiratory gated PET/CT. Med Phys. 2012;39:7390–7.
Alessio AM, Kinahan PE. Improved quantitation for PET/CT image reconstruction with system modeling and anatomical priors. Med Phys. 2006;33:4095–103.
Frood R, McDermott G, Scarsbrook A. Respiratory-gated PET/CT for pulmonary lesion characterisation-promises and problems. Br J Radiol. 2018;91:20170640.
Tsutsui Y, Kidera D, Taniguchi T, Akamatsu G, Komiya I, Umezu Y, et al. Accuracy of amplitude-based respiratory gating for PET/CT in irregular respirations. Ann Nucl Med. 2014;28:770–9.
van Elmpt W, Hamill J, Jones J, De Ruysscher D, Lambin P, Ollers M. Optimal gating compared to 3D and 4D PET reconstruction for characterization of lung tumours. Eur J Nucl Med Mol Imaging. 2011;38:843–55.
Kim JS, Park CR, Yoon SH, Lee JA, Kim TY, Yang HJ. Improvement of image quality using amplitude-based respiratory gating in PET-computed tomography scanning. Nucl Med Commun. 2021;42:553–65.
Kesner AL, Meier JG, Burckhardt DD, Schwartz J, Lynch DA. Data-driven optimal binning for respiratory motion management in PET. Med Phys. 2018;45:277–86.
Chen S, Hu P, Gu Y, Yu H, Shi H. Performance characteristics of the digital uMI550 PET/CT system according to the NEMA NU2-2018 standard. EJNMMI Phys. 2020;7:43.
Acknowledgements
We thank the staff at the Diagnostic Imaging Center at Cancer Institute Hospital of JFCR which contributed to the study design and phantom data acquisition. We are also grateful to Mr. Miyachi (GE Healthcare Co., Ltd.) and Mr. Saito for technical support with the AMF. This study was supported in part by the National Cancer Center Research and Development Fund (2020-J-3) and by a KAKENHI Grant-in-Aid for Young Scientists (No. 22K18234) and from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), the Japanese Government.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethics approval and consent to participate
This article does not contain any studies with human participants performed by any of the authors.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miyaji, N., Miwa, K., Yamashita, K. et al. Impact of irregular waveforms on data-driven respiratory gated PET/CT images processed using MotionFree algorithm. Ann Nucl Med 37, 665–674 (2023). https://doi.org/10.1007/s12149-023-01870-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-023-01870-9