Abstract
Sleep disorders and changes in the profile of the cortisol awakening response (CAR) are potential predictive factors for the incidence of major depressive disorder (MDD). However, these parameters usually are evaluated separately, lacking information regarding the simultaneous association of sleep disorders and CAR, mainly throughout the MDD severity. This study addressed the relationship between sleep quality and CAR in patients with initial/mild depression (MD, n = 30) versus advanced/treatment-resistant (TRD, n = 28), compared with a group of healthy controls (CG, n = 49), aiming to point out in a clinical perspective which alterations in sleep and CAR have been observed along major depression severity stages. TRD patients presented a blunted CAR and poorer sleep quality comparing MD and CG groups. Additionally, MD patients showed worse sleep quality and larger CAR than CG. Taken together, both sleep quality and CAR were correlated with MDD symptoms and predictors of MDD severity, with a greater classification power for sleep quality. From sleep quality, specifically, the use of sleep medication and sleep efficiency predicted depression severity, discriminating mild and treatment-resistant depression. These results show the importance of assessing sleep quality and CAR in patients with major depression when there is a need for evaluation of the disorder’s severity in a clinical context. CAR and sleep quality can be useful complementary tools to help in the clinical identification of major depression severity and the understanding of their impact on MDD may support further studies that aim to improve intervention strategies to increase the effectiveness of treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep disorders may lead to significant distress or impairment in a variety of critical aspects in life, such as social interactions, work performance, financial incomes, among others (Bernardi et al., 2015; Corsi-Cabrera et al., 2015; Verleger et al., 2013). In recent years, sleep deprivation has been increasingly related to diverse mood swings, ranging from apathy and sadness to anxiety (Waters et al., 2018). Therefore, several studies have been carried out aiming to understand the relationship between sleep and mood disorders, raising several hypotheses about the influence that such conditions have on each other (Santiago et al., 2020; Wang et al., 2019; Zhai et al., 2015). Currently, it is suggested that sleep disorders can contribute to the progression of diseases in the depressive spectrum and are also considered a side effect of depression, thus exhibiting a bidirectional modulation of depression (Alvaro et al., 2013; Jansson-Fröjmark & Lindblom, 2008; Kaneita et al., 2009).
Insomnia is characterized as a dissatisfaction with the quantity or quality of sleep such as difficulty starting or maintaining sleep (Diagnostic and Statistical Manual of Mental Disorders 5; DSM-5) (American Psychiatric Association, 2013). If on the one hand insomnia has been associated with an increased risk of developing depression (Fang et al., 2019; Li et al., 2016), on the other hand, among sleep disorders, insomnia is the main complain of individuals already diagnosed with major depressive disorder (MDD) (Chellappa & Araujo, 2007). In addition, it is considered a predictor of the disorder relapse and the risk of developing suicidal behavior (Wang et al., 2019), and related to more clinically severe outcomes (de Menezes Galvão et al., 2021; Fang et al., 2019). The concomitant presence of sleep disturbances and MDD is also considered one of the risk factors for the development of drug treatment-resistant depression (Cepeda et al., 2018; de Menezes Galvão et al., 2021).
Changes in the Hypothalamus-Pituitary-Adrenal (HPA) axis, for instance, have been related to sleep disorders (Dedovic & Ngiam, 2015; Nicolaides et al., 2000). Some studies described increased serum cortisol levels in young people with primary insomnia (Eek et al., 2012). The pulsatile administration of corticotropin-releasing hormone (CRH) in healthy individuals can reduce slow-wave sleep, while the administration of a CRH antagonist normalizes sleep in depressed patients, increasing slow-wave sleep and improving sleep efficiency (Sculthorpe & Douglass, 2010). Furthermore, some of the antidepressants used as treatment for MDD target physiological pathways involved in sleep control, which points out possible evidence of the relationship between MDD and sleep disorders at both the clinical, behavioral, and neurochemical levels (Lee & Douglass, 2010).
It is important to mention that changes in the HPA axis have also been related to MDD (Chida & Steptoe, 2009; Dedovic & Ngiam, 2015; Vreeburg et al., 2013). The majority of these studies evaluate the cortisol response to the dexamethasone suppression test in MDD patients (Hubain et al., 1998; Staner et al., n.d.), and changes in this response as predictors of MDD incidence (Dedovic & Ngiam, 2015; Vreeburg et al., 2013; Zhai et al., 2015). Hyperactivation of the HPA axis and, consequently, high cortisol levels, has also been one of the most consistent biological findings in individuals with mild depression, while a hypocortisolemia is related with treatment-resistant MDD (Galvão et al., 2018, 2021; Varela et al., 2021). Thus, cortisol change is a factor associated with resistance to treatment (Cepeda et al., 2018; Galvão et al., 2018; Santiago et al., 2020) and disease recurrence (Fang et al., 2019; Riemann et al., 2001).
Although there is a wide literature on sleep and HPA axis alterations in depression, the studies have investigated them separately. Simultaneous analysis of the relationship between such alterations through the clinical severity of the disease is scarce (Hubain et al., 1998). Moreover, when sleep or HPA axis changes are investigated as comorbidity to MDD, it is often done as predictive investigations of MDD incidence or recurrence, or patients with a specific degree of severity, and not along multiple disease stages (Chellappa & Araujo, 2007; Lopez-Duran et al., 2009; Stetler & Miller, 2005; Vreeburg et al., 2013).
Therefore, this study aimed to evaluate how a measure of HPA axis, cortisol awakening response (CAR) and quality of sleep behaved in the different severity stages of MDD, evaluating initial/mild versus advanced/treatment-resistant patients and comparing them with a healthy control group. We also analyzed different components of the sleep questionnaire to check which one is better correlated with MDD severity.
We expect to find low sleep quality and changed CAR in patients compared with the control group. More specifically, we expect a negative correlation between cortisol levels and depression severity, while an impaired sleep quality would be positively correlated with MDD severity. We also believe that both cortisol changes and sleep impairments, mainly insomnia, would be predictors of MDD severity.
Methods
Ethical aspects
The study was approved by the Hospital Universitário Onofre Lopes (HUOL) Medical Research Ethics Committee (Registration number 579.479), and the Human Research Ethics Committee of the Federal University Rio Grande do Norte (UFRN) (Registration number 2.628.202). All volunteers signed a consent form in which they state that they are aware of the study methods, and that guarantees that they were free to interrupt their participation in the study, without any harm. Moreover, this study fulfills the ethical standards of the relevant national and institutional committees for human experimentation and with the Declaration of Helsinki (1975, revised in 2008).
Subjects
The study consisted of a sample of 107 adults volunteers, who underwent clinical screening with psychiatrists from the Department of Psychiatry at UFRN where the Structured Clinical Interview for the DSM-IV (SCID) (Guze, 1995) and the Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960) were used for MDD diagnosis. The DMS-IV was used because it was the instrument approved by the ethics committee at the time of the study protocol evaluation. After screening, volunteers were divided into the following groups (See Supplementary Fig. 1 for CONSORT flow diagram and Supplementary Table 1 for sociodemographic characteristics):
Control Group (CG: n = 49; Men = 19; Women = 30): This group was composed of healthy volunteers. The exclusion criteria for the CG were history, diagnosis, or clinical symptoms of psychological or physical diseases, use of psychotropics or medications with effects on cognition and neurovegetative functions, alcohol, or other illicit drugs dependency. For women, having given birth in the last 6 months or being pregnant also were an exclusion criterion.
Patient group (PG: n = 58; Men = 21; Women = 37): Volunteers diagnosed with major depression. According to the severity of the current depressive episode, which was measured by the HAM-D (mild: 7 ≤ scores ≤ 17 points; moderate: 18 ≤ scores ≤ 24 points; severe: scores ≥ 25 points) (Moreno & Moreno 1998), these volunteers were distributed in two subgroups:
-
a)
Mild depression group (MD: n = 30; Men = 14; Women = 16): Patients in a current mild depressive episode, who never used antidepressants and without comorbidities. Therefore, the exclusion criteria for MD group were previous use of antidepressants and psychiatric comorbidities and, for women, having given birth in the last 6 months or being pregnant, to prevent the inclusion of people with a disorder other than major depression, such as postpartum depression or other pregnancy-related disorder.
-
b)
Treatment-resistant depression group (TRD: n = 28; Men = 7; Women = 21): Patients who were under a moderate to severe depressive episode, who failed to respond to at least two previous treatments with commercial antidepressants. During the study, these subjects went through a 15-day wash-out period, that is, they were not taking any antidepressants. This procedure was necessary in order to change antidepressant medication. Some patients presented personality disorder (histrionic: n = 10/50%; borderline: n = 9/45%; schizoid: n = 1/5%) and anxiety disorders (generalized anxiety: n = 10/83.33%; panic disorder: n = 5 / 17.24%; Social phobia: n = 2/16.67%) as comorbidities. The diagnosis of comorbidities was also performed according to the DSM-4 (SCID) (Guze, 1995). The exclusion criteria for TRD women patients also included having given birth in the last 6 months or being pregnant, to prevent the inclusion of people with a disorder other than major depression, such as postpartum depression or other pregnancy-related disorder.
Study design
Volunteers of all groups stayed the night before the experiment individually in a room at a UFRN facility reserved exclusively for this study. Before sleep they filled out the Pittsburgh Sleep Quality Index (PSQI) to investigate different aspects of their sleep. On the following day, around 6 AM, 3 saliva samples were collected to measure the cortisol awakening response (CAR). During saliva collection the volunteers had spent 8 h fasting.
Psychometric instruments
Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960): It has been used worldwide to track major depression diagnosis. It is a semi-structured interview to identify depressive symptoms, evaluating their frequency and intensity, as well as remission of the condition during and after clinical treatments. Good internal consistency is often reported for this instrument, ranging from α = 0.74 to α = 0.93 (Lin et al., 2019), and reaching α = 0.74 for our sample of patients.
Pittsburgh Sleep Quality Index (PSQI): It was developed to record the key elements of main sleep disorders, as well as the clinical, behavioral, and cognitive aspects directly or indirectly involved in sleep, such as: sleep hygiene and habits, insomnia, daytime sleepiness, sleep movement and breathing disorders, parasomnias, narcolepsy, mood and cognition, consumption of alcohol, coffee consumption, and consumption of drugs of abuse and medications. The PSQI has an overall score ranging from 0 to 21 points - categorized into good sleep (0–4 points), poor sleep (5–10 points) and sleep disturbance (greater than 10 points) - and evaluates seven components of sleep, namely: subjective quality, sleep efficiency, sleep disorders, use of medications, daily dysfunction, sleep latency and sleep duration, the latter two being used to infer insomnia. The instrument was validated to the Brazilian population with a good internal consistency (α = 0.82) (Bertolazi et al., 2011), and reached α = 0.68 for our sample of patients.
Salivary cortisol
Saliva collections were performed with Salivette™ (a device consisting of a plastic tube with cotton, specific for saliva collection). Each participant carried out the collection under guidance and supervision of a researcher: (a) 1st collection was done immediately after awakening; (b) 2nd collection, 30 min after awakening; and (c) 3rd collection, 45 min after awakening. Before or during saliva collection, volunteers were instructed not to wash their mouths with water, to not to eat food or drinks, and to not to perform physical activities or sudden movements. The collected saliva was sent to the Laboratory of Hormone Measurements (LHM) of the Department of Physiology and Behavior at UFRN, and then dosed using a competitive ELISA technique with the DGR-SLV 4635 kit.
Statistical analysis
The groups (MD, TRD and CG) were used as independent categorical variables. From the three samples, the area under the curve (AUC) of salivary cortisol was calculated to use in statistical analysis (Pruessner et al., 2003). The sociodemographic characteristics were evaluated as potential covariates using a chi-squared test for categorical (sex, scholarity) and ANOVA for numerical (age) variables. Both CAR (AUC) and the total PSQI score were used as numerical dependent variables. Salivary CAR (AUC) was log-transformed to reach a normal distribution, allowing the use of parametric statistical tests. The rationale behind this statistical analysis involves two steps: (1) comparing patients with a control group of healthy volunteers, using the General Linear Model (GLM) and Fisher’s Least Significant Difference (LSD) post-hoc to assess possible differences across groups according to dependent variables, with age as a covariate. Outliers were identified as \(case<{Q}_{1}-1.5 \times IQR\) or \(case>{Q}_{3}+1.5 \times IQR\), where Q1 and Q3 stand for quartile 1 (25%) and 3 (75%) respectively. The inspection found 5 outliers for CAR (CG = 4, MD = 1) and 1 outlier for PSQI (CG = 1), which were excluded from GLM analysis of CAR and PSQI, respectively. (2) If we had significant results on the first step, we proceeded with the analysis of severity where Spearman’s correlation was performed between disease severity (HAMD-17) and: PSQI and CAR, only for the groups with depression (MD and TRD). In order to assess which components of the PSQI were relevant for discriminating groups of depressed patients (MD and TRD) the Boruta test was used. The variables that scored above the importance of a shuffled data (I > 0.35) were categorized as relevant (Kursa & Rudnicki, 2010), and then a t-test was performed to compare them in relation to the groups of patients. Potential predictors of MDD severity were assessed by binary logistic regression analysis, considering the group (MD = 0, TRD = 1) as the dependent variable, and CAR and PSQI as the predictor variables. The assessment of the predictive quality of the model was performed using the area under the ROC (Received Operating Characteristic) curve, with its respective specificities and sensitivities. Mann-Whitney tests were used to compare the CAR and the PSQI with respect to the presence or not of comorbidities in the TRD group.
GLM and Mann-Whitney tests were performed using the IBM SPSS Statistics 25 software. Spearman’s correlation, Boruta, logistic regression and figures were performed using the RStudio IDE. Base R functions, pROC and Boruta packages were used for analyses. A post-hoc to determine the achieved power was computed using G*Power. Given the total sample size, assuming a large effect size (f = 0.80), and the F-test (ANCOVA), the power reached was 89.2%. The significance level considered was p \(\le\) 0.05 (two-tailed) in all tests.
Results
A total of 640 patients were screened for eligibility, and 107 met criteria, being 49 allocated to CG, and 58 to PG (MD = 30, TRD = 28) (For CONSORT, see Supplementary Fig. 1). The majority of patients presented comorbidity, such as personality disorder (76%) and anxiety disorder (31%). On average, patients with mild depression (MD) scored 12.57 \(\pm\) 3.11 in HAM-D, while patients with treatment-resistant depression (TRD) scored 21.57 \(\pm\) 5.27. Among all groups, sex was biased towards women (CG: 30 [61.2%]; MD: 16 [53.3%]; TRD: 21 [75%]), although it was not statistically significant (\({\chi }^{2}\left(2\right)\) = 2.98, p = 0.22). The majority of participants were undergraduate in all groups (CG: 32 [66.7%]; MD: 21 [77.8%]; TRD: 23 [82.1%]) but no significant difference was found (\({\chi }^{2}\left(2\right)\) = 2.49, p = 0.29). Age was significantly different between groups (F(2,103) = 30.28 p < 0.001) with patients in TRD (39.86 \(\pm\) 11.44) being older than MD (24.38 \(\pm\) 4.00, LSD = 17.4 p < 0.001) and CG (28.52 \(\pm\) 7.89, LSD = 12.3 p < 0.001), and MD older than CG (LSD = 5.05 p = 0.04). Therefore, age was used as a covariate in GLM analyses. Sociodemographic and clinical characteristics are summarized in Supplementary Table 1.
Salivary cortisol awakening response
The TRD group had a lower CAR than MD (GLM: F(2,95) = 34.69 p < 0.001) (LSD = -0.73 p < 0.001) and CG (LSD = -0.10 p = 0.05). In contrast, CAR was higher in MD compared to CG (LSD = -0.62 p < 0.001) (Fig. 1A and Supplementary Table 1). Age did not have a main effect as covariate (F(1,95) = 0.98 p = 0.32). For patients (TRD + MD) a negative correlation was observed between depressive symptoms (HAMD-17) and CAR (rho = -0.45 p < 0.001) (Fig. 1B).The presence or absence of anxiety or personality disorders as comorbidity did not modulate CAR (Supplementary Table 2).
(A) Salivary cortisol awakening response (CAR) across groups. Control group (C) in blue, mild depressive patients’ group (MD) in red and treatment-resistant depressive patients’ group (TRD) in green (* = p < 0.05; GLM and LSD). (B) Correlation of depressive symptoms, assessed by HAMD- 17, and CAR through TRD and MD. (p < 0.001; rho = -0.45)
Sleep quality
The TRD group had higher PSQI scores compared to MD (GLM: F(2,101) = 66.12 p < 0.001) (LSD = 4.67 p < 0.001) and CG (LSD = 9.25 p < 0.001). The PSQI score was higher for MD compared to CG (LSD = -4.58 p < 0.001) (Fig. 2A and Supplementary Table 1). Age did not have a main effect as covariate (F(1,101) = 0.88 p = 0.35). For patients (TRD + MD) a positive correlation was found between depressive symptoms (HAMD- 17) and PSQI (rho = 0.60 p < 0.001) (Fig. 2B). The presence or absence of anxiety or personality disorders as comorbidity did not modulate sleep quality (Supplementary Table 2).
(A) Pittsburgh Sleep Quality Index (PSQI) scores across groups. Control group (C) in blue, mild depressive patients’ group (MD) in red and treatment-resistant patients’ group (TRD) in green (* = p < 0.05; GLM and LSD). (B) Correlation of depressive symptoms, assessed by the HAMD- 17, and PSQI through TRD and MD. (p < 0.001; rho = 0.60)
MDD severity
CAR and PSQI were both significant predictors of MDD severity stages (CAR: B = -3.17, OR = 1.04 [1.01, 1.22], p < 0.001; PSQI: B = 0.24, OR = 3.58 [2.88, 4.98], p = 0.02) (Fig. 3A) with a greater effect on sleep quality in the discrimination between MD and TRD. The higher the score on the PSQI was, the worse the quality of sleep, and greater the chance that the individual will be classified as TRD. For CAR, the smaller the area under the curve, that is, the responsiveness, the greater the chance of being classified as TRD. The area under the ROC curve was 90.5% (specificity = 90.0%, sensitivity = 82.1%), suggesting a good model quality (Fig. 3B).
(A) Odds ratio (OR) and 95% confidence interval of salivary cortisol awakening response (CAR) and sleep quality (PSQ) as predictors of depressive disorder severity stages (MD and TRD). (B) ROC curve for CAR and PSQ as predictors of depressive disorder severity, depicting, in parentheses, the value of specificity and sensitivity, respectively
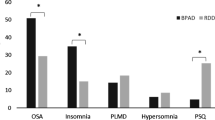
From the seven components of PSQI, the components C6 (use of sleep medication) (I = 33.11) and C4 (habitual sleep efficiency) (I = 5.05) were important to discriminate between the MD and TRD depressive groups. The other components of PSQI were rejected in the Boruta test (C1 = -1.99; C2 = -1.23; C3 = 0.62; C5 = -0.51; C7 = -0.89) (Fig. 4A). The MD group had higher habitual sleep efficiency (MD: µ = 89.87% ± 2.58, TRD: µ = 75.07% ± 4.99; t = 2.64, p = 0.01) (Fig. 4B) and made significantly less use of sleep medication (less than once a week) (approximate use of 1 to 3 times a week) (t = -10.5, p < 0.001; MD: µ = 0.37 ± 0.16, TRD: µ = 2.71 ± 0.16) than the TRD group (Fig. 4C).
(A) The importance of PSQI components in the discrimination of MD and TRD groups. The box plots represent the distribution of PSQI components importance assessed by the Boruta algorithm (dark gray = important components; light gray = non-important components). Components C6 (use of medication to sleep) and C4 (usual sleep efficiency) stood out in the discrimination of depressive groups (MD and TRD). (B) Bar plot of C4 (%) by MD and TRD groups, and (C) Bar plot of C6 (frequency) by MD and TRD. (* = p < 0.05; T test) red = group with mild depression; green = treatment-resistant group)
Discussion
In this study, changes in CAR and in sleep quality were evident in patients with depression in relation to healthy control volunteers, and such changes were correlated with depressive symptoms and predicted the two analyzed MDD severity stages (Initial/mild and advanced/TRD), corroborating our initial hypothesis.
MD patients presented larger CAR in relation to CG, while TRD patients showed a blunted and lower CAR in relation to CG and MD. For patients, we found a negative correlation between depressive symptoms and CAR in the present study, which means that the lower the CAR, the greater the severity of MDD. Major depression has been associated with different altered degrees of activation of the HPA axis, resulting in either increased, decreased, or attenuated cortisol levels (Bremmer et al., 2007; Dedovic & Ngiam, 2015; Stetler & Miller, 2005). More specifically, a pattern of hypocortisolemia, has been found in individuals with severe symptoms of depression, and difficulty of achieving remission (Bremmer et al., 2007; Dedovic & Ngiam, 2015; Stetler & Miller, 2005). In contrast, a profile of hypercortisolemia was observed in patients who generally had mild symptoms or recent illness (Lopez-Duran et al., 2009).
A hypercortisolemic profile in individuals with a recent onset of MDD is related to an acute effect on the hypothalamic-pituitary-adrenal (HPA) axis, with failure of negative feedback (Lopez-Duran et al., 2009). On the other hand, a blunted CAR and a hypocortisolemic pattern have been associated with a disturbance in the self-adjustment of allostatic systems (McEwen et al., 2015), suggesting that such conditions may arise after prolonged periods of stress, HPA axis hyperactivity, and excessive release of glucocorticoids. Another explanation for the etiology of hypocortisolemia suggests the presence of an increased sensitivity of HPA axis negative feedback (Sriram et al., 2012).
Here, we found that treatment-resistant MDD patients, besides showing hypocortisolemia, also had worse sleep quality than the MD group, which in turn had an inferior sleep quality when compared to the control group. For patients, a positive correlation was found between depressive symptoms and PSQI score, which implies a poor quality of sleep for this group. The correlation between sleep disorders and depressive symptoms is mainly studied regarding the onset of depressive symptoms, in incidence studies (Alvaro et al., 2013; Kalmbach et al., 2017; Zhai et al., 2015), while few studies have investigated this relationship along MDD severity, as we have performed here (de Menezes Galvão et al., 2021; Urrila et al., 2012).
As supposed, here, both CAR and PSQI were predictors of depression severity, with sleep quality having the greater effect for discriminating between MD and TRD. However, differently from our hypothesis, among the sleep disorders, insomnia (assessed in this study by sleep latency and sleep duration in the PSQI) was not the main discriminant of MDD severity stages. Habitual sleep efficiency, which is the ratio between hours slept and spent in bed with the intention to sleep, and the use of sleep medications were the elements of sleep that exhibited more importance in discriminating between the two tested clinical severity stages of MDD. In this context, the TRD group of patients presented lower sleep efficiency and, in parallel, disclosed a greater use of medications for sleep induction. We can make a bridge with current literature when we notice that the subset of MDD patients with depression associated with suicidal tendencies presented the lowest rates of habitual sleep efficiency (Ağargün, Kara & Solmaz, 1997; Gallo et al., 2020).
Currently, there are few studies like this one evaluating the relationship between sleep and CAR in patients with MDD, some studies have evaluated such a relationship in a distinct sample such as women in perimenopause (Gordon et al., 2021) or caregivers of patients with chronic illnesses (Ljubičić et al., 2020; Knorr et al., 2012). For MDD, a related study described a positive correlation considering the cortisol response to the dexamethasone suppression test and depression severity while also describing a negative correlation of these two with rapid eye movement (REM) sleep (Hubain et al., 1998). However, since they have used distinct techniques for measuring cortisol and sleep, the causal associations with the present discussion are difficult to establish.
Concerning the limitations of this study, due to a modest sample size of patients (n = 58) it was not possible to form 3 patient groups with mild, moderate, and severe depressive symptoms. Thus, another strategy was applied, and patients were categorized in two subgroups according to the time of the diagnosis, comorbidities, and use of antidepressants. Therefore, these results encourage further studies with a larger population sample, in order to better validate these findings on more accurate subsets of patients, including at least 3 groups with mild (or initial phase major depression), moderate, and severe symptoms. Another limitation is the absence of repetitive CAR dosages for 2 to 3 days which could provide a more accurate panel of cortisol secretion in depressive patients and should also be addressed in future studies. Regarding the sleep profile of patients, the complexity of data collection and analysis, and the use of polysomnography, despite the costs, could also aggregate useful information about sleep profiles according to the severity or progression of the disease in the different subsets of patients. Finally, we did not consider comorbidities other than those from the major depression diagnosis, especially those related to personality disorders. Due to the high chance of these comorbidities in the TRD population, it is difficult to obtain a sample covering only exclusively depressed patients, and we would need a much larger population sample to perform such a study with this aim.
Given the impact of major depressive disorder on individuals and the difficulties faced in its treatment, a better comprehension of the relationship of its clinical course, CAR, and sleep quality, may help in the management of the current treatment or in the search for new alternatives for a MDD therapeutic approach. Despite sleep impairment being a key symptom of major depression described in DSM-5 (American Psychiatric Association, 2013), these results suggest that attention should be given particularly to sleep efficiency in the investigation of the MDD severity along with the clinical course of this disease, which according to our results looks to have more value than the analysis of insomnia by itself. This may help to improve the current clinical practice on treatment planning, through psychotherapy or drug therapy, focused on improving sleep disorders associated with MDD.
Additionally, our results also suggest the relevance of CAR investigation along the MDD severity in patients with sleep dysfunction. Despite there are many studies investigating CAR in depression, in clinical practice, the salivary cortisol evaluation has not been widely used. In the last years, the field of precision psychiatry has been increasing and some advances in biomarkers for psychiatric disease have been shown (Lozupone et al., 2017; Rush & Ibrahim, 2018), such as the value of cortisol changes for MDD diagnosis and its severity evaluation (Dziurkowska & Wesolowski, 2021; Galvão et al., 2021). However, as MDD is a complex and multifactorial disease, more robust studies with larger and diverse sample size, as well as comprising the investigation of multiple biopsychosocial parameters are necessary to validate biomarkers use in clinical practice (Gómez-Carrillo et al., 2023). This study supports CAR use as a potential complementary easy-collection tool to support the clinical investigation of MDD severity stages, however further studies should focus on looking for its value as a biomarker of disease severity or progression, which is not the present goal, aiming to apply it in clinical practice.
In sum, this study contributed to better understand how the relationship between sleep quality and cortisol changes in CAR behave along the MDD severity stages supporting future and larger studies that aim to apply this knowledge in the context of precision psychiatry or treatments for MDD.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aǧargün, M. Y., Kara, H., & Solmaz, M. (1997). Subjective sleep quality and suicidality in patients with major depression. Journal of Psychiatric Research, 31(3), 377–381. https://doi.org/10.1016/S0022-3956(96)00037-4.
Alvaro, P. K., Roberts, R. M., & Harris, J. K. (2013). A systematic review assessing bidirectionality between sleep disturbances, anxiety, and Depression. Sleep, 36(7), 1059–1068. https://doi.org/10.5665/sleep.2810.
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association. https://doi.org/10.1176/appi.books.9780890425596.
Bernardi, G., Siclari, F., Yu, X., Zennig, C., Bellesi, M., Ricciardi, E., Cirelli, C., Ghilardi, M. F., Pietrini, P., & Tononi, G. (2015). Neural and behavioral correlates of extended training during Sleep Deprivation in humans: Evidence for local, Task-Specific effects. Journal of Neuroscience, 35(11), 4487–4500. https://doi.org/10.1523/JNEUROSCI.4567-14.2015.
Bertolazi, A. N., Fagondes, S. C., Hoff, L. S., Dartora, E. G., da Silva Miozzo, I. C., de Barba, M. E. F., & Barreto, M., S. S (2011). Validation of the Brazilian Portuguese version of the Pittsburgh Sleep Quality Index. Sleep Medicine, 12(1), 70–75. https://doi.org/10.1016/j.sleep.2010.04.020.
Bremmer, M. A., Deeg, D. J. H., Beekman, A. T. F., Penninx, B. W. J. H., Lips, P., & Hoogendijk, W. J. G. (2007). Major Depression in Late Life is Associated with both hypo- and Hypercortisolemia. Biological Psychiatry, 62(5), 479–486. https://doi.org/10.1016/j.biopsych.2006.11.033.
Cepeda, M. S., Reps, J., & Ryan, P. (2018). Finding factors that predict treatment-resistant depression: Results of a cohort study. Depression and Anxiety, 35(7), 668–673. https://doi.org/10.1002/da.22774.
Chellappa, S. L., & Araujo, J. F. (2007). Qualidade subjetiva do sono em pacientes com transtorno depressivo. Estudos De Psicologia (Natal), 12(3), 269–274. https://doi.org/10.1590/S1413-294X2007000300009.
Chida, Y., & Steptoe, A. (2009). Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology, 80(3), 265–278. https://doi.org/10.1016/j.biopsycho.2008.10.004.
Corsi-Cabrera, M., Rosales-Lagarde, A., del Río-Portilla, Y., Sifuentes-Ortega, R., & Alcántara-Quintero, B. (2015). Effects of selective REM sleep deprivation on prefrontal gamma activity and executive functions. International Journal of Psychophysiology, 96(2), 115–124. https://doi.org/10.1016/j.ijpsycho.2015.02.027.
de Menezes Galvão, A. C., Almeida, R. N., de Sousa, G. M., Leocadio-Miguel, M. A., Palhano-Fontes, F., de Araujo, D. B., Lobão-Soares, B., Maia-de-Oliveira, J. P., Nunes, E. A., Hallak, J. E. C., Schuch, F. B., Sarris, J., & Galvão-Coelho, N. L. (2021). Pathophysiology of Major Depression by Clinical stages. Frontiers in Psychology, 12(August), 1–12. https://doi.org/10.3389/fpsyg.2021.641779.
Dedovic, K., & Ngiam, J. (2015). The cortisol awakening response and major depression: Examining the evidence. Neuropsychiatric Disease and Treatment, 11, 1181–1189. https://doi.org/10.2147/NDT.S62289.
Dziurkowska, E., & Wesolowski, M. (2021). Cortisol as a Biomarker of Mental Disorder Severity. Journal of Clinical Medicine, 10(21), 5204. https://doi.org/10.3390/jcm10215204.
Eek, F., Karlson, B., Garde, A. H., Hansen, Å. M., & Ørbæk, P. (2012). Cortisol, sleep, and recovery – some gender differences but no straight associations. Psychoneuroendocrinology, 37(1), 56–64. https://doi.org/10.1016/j.psyneuen.2011.05.003.
Fang, H., Tu, S., Sheng, J., & Shao, A. (2019). Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. Journal of Cellular and Molecular Medicine, 23(4), 2324–2332. https://doi.org/10.1111/jcmm.14170.
Gallo, J. J., Hwang, S., Truong, C., Reynolds, C. F., & Spira, A. P. (2020). Role of persistent and worsening sleep disturbance in depression remission and suicidal ideation among older primary care patients: The PROSPECT study. Sleep, 43(10). https://doi.org/10.1093/sleep/zsaa063.
Galvão, A. C., de Almeida, M., Silva, R. N., do., E. A., Freire, S., Palhano-Fontes, F. A. M., Onias, F., Arcoverde, H., Maia-de-Oliveira, E., de Araújo, J. P., Lobão-Soares, D. B., B., & Galvão-Coelho, N. L. (2018). Cortisol modulation by Ayahuasca in patients with treatment resistant depression and healthy controls. Frontiers in Psychiatry, 9(MAY), 1–10. https://doi.org/10.3389/fpsyt.2018.00185.
Gómez-Carrillo, A., Paquin, V., Dumas, G., & Kirmayer, L. J. (2023). Restoring the missing person to personalized medicine and precision psychiatry. Frontiers in Neuroscience, 17, https://doi.org/10.3389/fnins.2023.1041433.
Gordon, J. L., Halleran, M., Beshai, S., Eisenlohr-Moul, T. A., Frederick, J., & Campbell, T. S. (2021). Endocrine and psychosocial moderators of mindfulness-based stress reduction for the prevention of perimenopausal depressive symptoms: A randomized controlled trial. Psychoneuroendocrinology, 130, 105277. https://doi.org/10.1016/j.psyneuen.2021.105277.
Guze, S. B. (1995). Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV). American Journal of Psychiatry, 152(8), 1228–1228. https://doi.org/10.1176/ajp.152.8.1228.
Hamilton, M. (1960). A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry, 23(1), 56–62. https://doi.org/10.1136/jnnp.23.1.56.
Hubain, P. P., Staner, L., Dramaix, M., Kerkhofs, M., Papadimitriou, G., Mendlewicz, J., & Linkowski, P. (1998). The dexamethasone suppression test and sleep electroencephalogram in nonbipolar major depressed inpatients: A multivariate analysis. Biological Psychiatry, 43(3), 220–229. https://doi.org/10.1016/S0006-3223(97)80434-9.
Jansson-Fröjmark, M., & Lindblom, K. (2008). A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. Journal of Psychosomatic Research, 64(4), 443–449. https://doi.org/10.1016/j.jpsychores.2007.10.016.
Kalmbach, D. A., Arnedt, J. T., Song, P. X., Guille, C., & Sen, S. (2017). Sleep disturbance and Short Sleep as risk factors for Depression and Perceived Medical errors in First-Year residents. Sleep, 40(3). https://doi.org/10.1093/sleep/zsw073.
Kaneita, Y., Yokoyama, E., Harano, S., Tamaki, T., Suzuki, H., Munezawa, T., Nakajima, H., Asai, T., & Ohida, T. (2009). Associations between sleep disturbance and mental health status: A longitudinal study of Japanese junior high school students. Sleep Medicine, 10(7), 780–786. https://doi.org/10.1016/j.sleep.2008.06.014.
Knorr, U., Vinberg, M., Gether, U., Winkel, P., Gluud, C., Wetterslev, J., & Kessing, L. V. (2012). The effect of escitalopram versus placebo on perceived stress and salivary cortisol in healthy first-degree relatives of patients with depression—A randomised trial. Psychiatry Research, 200(2–3), 354–360. https://doi.org/10.1016/j.psychres.2012.05.015.
Kursa, M. B., & Rudnicki, W. R. (2010). Feature selection with the Boruta Package. Journal of Statistical Software, 36(11). https://doi.org/10.18637/jss.v036.i11.
Lee, E. K., & Douglass, A. B. (2010). Sleep in Psychiatric disorders: Where are we now? The Canadian Journal of Psychiatry, 55(7), 403–412. https://doi.org/10.1177/070674371005500703.
Li, L., Wu, C., Gan, Y., Qu, X., & Lu, Z. (2016). Insomnia and the risk of depression: A meta-analysis of prospective cohort studies. Bmc Psychiatry, 16(1), 375. https://doi.org/10.1186/s12888-016-1075-3.
Lin, C. H., Park, C., & McIntyre, R. S. (2019). Early improvement in HAMD-17 and HAMD-7 scores predict response and remission in depressed patients treated with fluoxetine or electroconvulsive therapy. Journal of Affective Disorders, 253, 154–161. https://doi.org/10.1016/j.jad.2019.04.082.
Ljubičić, M., Baković, L., Ćoza, M., Pribisalić, A., & Kolčić, I. (2020). Awakening cortisol indicators, advanced glycation end products, stress perception, depression and anxiety in parents of children with chronic conditions. Psychoneuroendocrinology, 117, 104709. https://doi.org/10.1016/j.psyneuen.2020.104709.
Lopez-Duran, N. L., Kovacs, M., & George, C. J. (2009). Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology, 34(9), 1272–1283. https://doi.org/10.1016/j.psyneuen.2009.03.016.
Lozupone, M., Seripa, D., Stella, E., La Montagna, M., Solfrizzi, V., Quaranta, N., Veneziani, F., Cester, A., Sardone, R., Bonfiglio, C., Giannelli, G., Bisceglia, P., Bringiotti, R., Daniele, A., Greco, A., Bellomo, A., Logroscino, G., & Panza, F. (2017). Innovative biomarkers in psychiatric disorders: A major clinical challenge in psychiatry. Expert Review of Proteomics, 14(9), 809–824. https://doi.org/10.1080/14789450.2017.1375857.
McEwen, B. S., Bowles, N. P., Gray, J. D., Hill, M. N., Hunter, R. G., Karatsoreos, I. N., & Nasca, C. (2015). Mechanisms of stress in the brain. Nature Neuroscience, 18(10), 1353–1363. https://doi.org/10.1038/nn.4086.
Nicolaides, N. C., Vgontzas, A. N., Kritikou, I., & Chrousos, G. (2000). HPA Axis and Sleep. In Endotext. http://www.ncbi.nlm.nih.gov/pubmed/25905298.
Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. https://doi.org/10.1016/S0306-4530(02)00108-7.
Riemann, D., Berger, M., & Voderholzer, U. (2001). Sleep and depression — results from psychobiological studies: An overview. Biological Psychology, 57(1–3), 67–103. https://doi.org/10.1016/S0301-0511(01)00090-4.
Rush, A. J., & Ibrahim, H. M. (2018). A clinician’s perspective on biomarkers. FOCUS, 16(2), 124–134. https://doi.org/10.1176/appi.focus.20170044.
Santiago, G. T. P., de Menezes Galvão, A. C., de Almeida, R. N., Mota-Rolim, S. A., Palhano-Fontes, F., Maia-de-Oliveira, J. P., de Araújo, D. B., Lobão-Soares, B., & Galvão-Coelho, N. L. (2020). Changes in Cortisol but not in Brain-Derived Neurotrophic Factor Modulate the Association between Sleep Disturbances and Major Depression. Frontiers in Behavioral Neuroscience, 14, https://doi.org/10.3389/fnbeh.2020.00044.
Sculthorpe, L. D., & Douglass, A. B. (2010). Sleep pathologies in Depression and the clinical utility of Polysomnography. The Canadian Journal of Psychiatry, 55(7), 413–421. https://doi.org/10.1177/070674371005500704.
Sriram, K., Rodriguez-Fernandez, M., & Doyle, F. J. (2012). Modeling cortisol dynamics in the neuro-endocrine axis distinguishes normal, depression, and post-traumatic stress disorder (PTSD) in humans. PLoS Computational Biology, 8(2). https://doi.org/10.1371/journal.pcbi.1002379.
Staner, L., Duval, F., Haba, J., Mokrani, M. C., & Macher, J. P. (n.d.). Disturbances in hypothalamo pituitary adrenal and thyroid axis identify different sleep EEG patterns in major depressed patients. Journal of Psychiatric Research, 37(1), 1–8. https://doi.org/10.1016/s0022-3956(02)00068-7.
Stetler, C., & Miller, G. E. (2005). Blunted cortisol response to awakening in mild to moderate depression: Regulatory influences of sleep patterns and social contacts. Journal of Abnormal Psychology, 114(4), 697–705. https://doi.org/10.1037/0021-843X.114.4.697.
Urrila, A. S., Karlsson, L., Kiviruusu, O., Pelkonen, M., Strandholm, T., & Marttunen, M. (2012). Sleep complaints among adolescent outpatients with major depressive disorder. Sleep Medicine, 13(7), 816–823. https://doi.org/10.1016/j.sleep.2012.04.012.
Varela, Y. M., de Almeida, R. N., Galvão, A. C., de Sousa, M., de Lima, G. M., da Silva, A. C. L., Leocadio-Miguel, N. G., Lobão-Soares, M. A., Hallak, B., Maia-de-Oliveira, J. E. C., J. P., & Galvão-Coelho, N. L. (2021). Psychophysiological responses to group cognitive-behavioral therapy in depressive patients. Current Psychology. https://doi.org/10.1007/s12144-020-01324-9.
Verleger, R., Rose, M., Wagner, U., Yordanova, J., & Kolev, V. (2013). Insights into sleep’s role for insight: Studies with the number reduction task. Advances in Cognitive Psychology, 9(4), 160–172. https://doi.org/10.2478/v10053-008-0143-8.
Vreeburg, S. A., Hoogendijk, W. J. G., DeRijk, R. H., van Dyck, R., Smit, J. H., Zitman, F. G., & Penninx, B. W. J. H. (2013). Salivary cortisol levels and the 2-year course of depressive and anxiety disorders. Psychoneuroendocrinology, 38(9), 1494–1502. https://doi.org/10.1016/j.psyneuen.2012.12.017.
Wang, X., Cheng, S., & Xu, H. (2019). Systematic review and meta-analysis of the relationship between sleep disorders and suicidal behaviour in patients with depression. Bmc Psychiatry, 19(1), 303. https://doi.org/10.1186/s12888-019-2302-5.
Waters, F., Chiu, V., Atkinson, A., & Blom, J. D. (2018). Severe sleep deprivation causes hallucinations and a gradual progression toward psychosis with increasing Time Awake. Frontiers in Psychiatry, 9. https://doi.org/10.3389/fpsyt.2018.00303.
Zhai, L., Zhang, H., & Zhang, D. (2015). SLEEP DURATION AND DEPRESSION AMONG ADULTS: A META-ANALYSIS OF PROSPECTIVE STUDIES. Depression and Anxiety, 32(9), 664–670. https://doi.org/10.1002/da.22386.
Acknowledgements
We would like to thank Kedma Valnice Freire Oliveira for contributing to the data collection.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
NG-C, FP-F, BL-S, ML-M, and DA conducted the clinical trial. AM and RA performed the dosing of biomarkers. GS and AM carried out the statistical analyses. LT and YM wrote the first version of the manuscript. All authors drafted and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors report no conflicts of interest.
Ethics approval
The study was approved by the Hospital Universitário Onofre Lopes (HUOL) Medical Research Ethics Committee (Registration number 579.479), and the Human Research Ethics Committee of the Federal University Rio Grande do Norte (UFRN) (Registration number 2.628.202). All volunteers signed a consent form in which they state that they are aware of the study methods, and that guarantees that they have complete freedom to interrupt their participation in the study, without any harm being done. Moreover, this study fulfills the ethical standards of the relevant national and institutional committees for human experimentation and with the Declaration of Helsinki of 1975, revised in 2008.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Torres, L.H.S.F., Medeiros, Y.K.M., de Sousa, G.M. et al. Use of sleep quality questionary and cortisol awakening response as complementary tools for the evaluation of major depression progression. Curr Psychol 43, 19820–19829 (2024). https://doi.org/10.1007/s12144-024-05786-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12144-024-05786-z