Abstract
Background
Oral ulcers represent a full thickness loss of the mucosal epithelium leading to exposure of the submucosal connective tissue. These are common and usually self-limited lesions, although they may sometimes result from neoplasms, most commonly squamous cell carcinoma. Lymphoproliferative disorders may be difficult to diagnose in apthous ulcers since they mimic reactive inflammation.
Methods
This review presents ten rare oral lymphoid proliferations which should not be missed when assessing oral ulcer biopsies.
Results
The ten lesions include several with diagnostic cells which look similar to the histiocytes of a reactive inflammatory ulcer, including Rosai–Dorfman disease, reticulohistiocytoma, Langerhans cell histiocytosis, and traumatic ulcerative granuloma. Other lesions, such as EBV-positive mucocutaneous ulcer, extranodal marginal zone lymphoma of mucosal-associated lymphoid tissue, and plasmablastic lymphoma have lymphoid and/or plasma cell differentiation that mimic the reactive lymphocytes and plasma cells found in reactive ulcers. Two dendritic cell lesions, follicular dendritic cell sarcoma and blastic plasmacytoid dendritic cell neoplasm, both have distinct phenotypes which are required to make an accurate diagnosis.
Conclusion
Each of these lesions are diagnosed by evaluating their histology, along with their phenotypic profile, which is sometimes enhanced by pertinent molecular findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral ulcers are defined as a full thickness loss of the mucosal epithelium leading to exposure of submucosal connective tissue. A common condition with an estimated prevalence in the general population of 6.6% [1], oral ulcer is usually a self-limited lesion related to minor oral trauma that heals within days to weeks and does not necessitate medical attention. Persistent ulcers, on the other hand, deserve examination to determine the underlying cause and offer appropriate management. The differential diagnosis of an oral ulcer is quite wide and includes traumatic, infectious, autoimmune, iatrogenic, and neoplastic conditions [2]. A biopsy is therefore often part of the evaluation for a persistent ulcers in order to narrow this wide differential. In deciding whether to biopsy an oral ulcer, clinicians and dentists look for certain worrisome clinical features that suggest a possibility of malignancy or other underlying condition [3]. These include palpable tumor beneath the ulcer, surrounding leukoplakia or erythroleukoplakia, rolled edges, multifocality, recurrence after previous excision, the presence of blisters, a previous diagnosis of malignancy, and worrisome radiographic findings. The main role of biopsy is to rule out infectious and neoplastic causes of the ulceration.
From the surgical pathologist’s point of view, the evaluation of an oral ulcer biopsy involves histological confirmation of ulceration in the biopsy, evaluation of the ulcer for the presence of a neoplasm, determination of the type of inflammation present, inspection for the presence of features specific to particular ulcerative conditions, and confirmation or exclusion of the presence of specific microorganisms. This evaluation may seem unsatisfactory because, in many cases, the pathologist finds only nonspecific mixed inflammation with ulcer base granulation tissue and no specific diagnostic features, such as microorganisms, neoplasm, or other findings that would allow for a specific diagnosis to be made. Despite this, the biopsy is still valuable for ruling out malignant and infectious causes.
In their assessment for the underlying neoplastic causes of ulceration, the pathologist’s focus is often on ruling out squamous cell carcinoma (SCC) and dysplasia since these are by far the most common neoplastic causes of oral ulcers; up to 50% of oral SCC presents with ulceration [4]. Of the non-squamous neoplasms causing oral ulcers, many are readily apparent when present in the routine histological evaluation. However, lymphoproliferative conditions are often an exception, as they may mimic inflammation in an aphthous ulcer and therefore may be missed if not considered. We present here ten oral lymphoid proliferations which, although uncommonly encountered, should not be missed when present in a biopsy of an oral ulcer.
Rosai–Dorfman Disease
One of the most difficult conditions to recognize in an oral ulcer is Rosai–Dorfman Disease (RDD). First described as “adenitis with lipid excess” by Pierre–Paul Destombes in 1965 [5] and then characterized in detail by Juan Rosai and Ronald Dorfman in 1969 as sinus histiocytosis with massive lymphadenopathy [6], RDD is an uncommon, benign histiocytic disorder that for many years was thought to be non-neoplastic [7]. Recent studies, however, have demonstrated the presence of activating mutations in the MAPK/ERK pathway in approximately half of the cases [8, 9] and the abnormal co-expression of PU.1 and OCT-2 transcription factors in most cases [7], suggesting that this is a histiocytic neoplasm with an intense inflammatory background.
Although described initially as involving lymph nodes and causing lymphadenopathy that mimics malignancy, RDD is now known to affect a variety of extranodal sites, including head and neck sites [10]. While RDD occurs most commonly in young adults and children, patients of any age can be affected [11]. The disease is characterized by proliferation of abnormal large pale histiocytic cells showing engulfment of other immune cells (so-called ‘emperipolesis’) and a characteristic immunoprofile (CD68 + S100 +). The difficulty in its diagnosis is caused by the intense background mixed inflammatory component present in the RDD lesions, which includes neutrophils, plasma cells, lymphocytes, and histiocytes. In the setting of an oral ulcer, this mixed inflammatory component easily mimics the mixed inflammation expected at the base of an ulcer and the presence of the large pale histiocytes can be easily overlooked. Even if noticed, these RDD cells may be assumed to be part of the inflammatory response to the ulcer. Pathologists must think of this diagnosis in order to be able to make it.
The difficulty in the histological diagnosis of RDD is well known and often multiple biopsies are taken before the diagnosis is made, as is illustrated in the following case: A 63-year-old woman was referred to an oral medicine clinic with a history of a persistent, nonhealing, well-delineated ulcer of the ventrolateral tongue (Fig. 1A). The ulcer showed rolled-up borders worrisome for underlying malignancy and a biopsy was performed. The biopsy showed ulceration with dense mixed inflammatory infiltrate extending deep into the underlying tongue skeletal muscle (Fig. 1B–C). The presence of the large pale histiocytes with emperipolesis was not appreciated (Fig. 1D) and no immunohistochemistry was performed. The biopsy was reported as an ulcer with no evidence of malignancy. The ulcer persisted for another 5 months, at which time an excisional biopsy was performed. This specimen showed essentially the same histology (not shown). The consultant pathologist performed immunohistochemistry for S100 protein to rule out an underlying melanoma, but did not appreciate the S100 protein-positive histiocytes present at the base of the ulcer. The lesion recurred again after 6 months, with a second excisional biopsy performed. This biopsy showed a sharply defined ulcer with granulation tissue and pseudoepitheliomatous hyperplasia (Fig. 1E). Beneath the ulcer there was a deep area of fibrosis and mixed inflammatory infiltrate with multiple large pale histiocytes showing emperipolesis (Fig. 1F). Immunohistochemistry showed these cells to be positive for CD68 and S100 protein (Fig. 1G–H), resulting in a diagnosis of RDD.
A Rosai–Dorfman disease of the ventrolateral tongue causing an ulcer of the ventrolateral tongue (photo courtesy of Tanya Rouleaux). B The first biopsy showed an ulcer with dense mixed inflammatory infiltrate in its base (H&E, 20x). C The inflammation extended deep into the skeletal muscle fibers of the intrinsic tongue muscles (H&E, 200x). D Large pale histiocytes with emperipolesis (arrow) are seen within the inflammatory infiltrate (H&E, 600x). E Subsequent excisional biopsy shows an ulcer with a deep fibroinflammatory base (H&E, 20x). F The base of the ulcer contains multiple large pale histiocytes (H&E, 400x), which were positive for CD68 (G, 600x) and S100 protein (H, 600x)
RDD can affect a variety of mucosal sites of the head and neck, including the oropharynx [11], larynx [12], nasopharynx [13], sinonasal cavities [14], and salivary glands [9]. In the oral cavity, the most frequent sites of involvement are the gnathic bones [15, 16, 17, 18]. While oral mucosal involvement is rare, the condition is likely underrecognized. Careful examination of the deepest part of the ulcer for the presence of RDD cells with emperipolesis and the use of immunohistochemistry when such cells are observed will help establish the diagnosis. This is important since the disease may be multifocal and is potentially treatable with MAPK/ERK pathway inhibitors [19].
The differential diagnosis includes inflammatory ulcer, EBV-positive mucocutaneous ulcer, reticulohistiocytosis, and classic Hodgkin lymphoma, amongst others. While inflammatory ulcers may contain histiocytes and occasionally multinucleated giant cells, these cells will not show emperipolesis nor S100 protein positivity. Reticulohistiocytosis shares with RDD the presence of large pale histiocytic cells; however, they tend to be S100 protein negative or at most positive in a minority of cells. The cytology of the reticulohistiocytoma is different with darker, denser cytoplasm and absent emperipolesis. Classic Hodgkin lymphoma shares with RDD the presence of intense mixed inflammatory background; however, eosinophils are not typically seen in RDD but are typical in Hodgkin lymphoma.
Reticulohistiocytoma
Reticulohistiocytoma is another lesion that is underrecognized in the oral cavity due to its rarity and nonspecific histological features. Encountered typically in the skin and mostly in pediatric populations, this tumor is well known to dermatopathologists and pediatric pathologists but causes difficulties for other pathologists. First described in 1950 [20], reticulohistiocytoma is a benign histiocytic lesion of unknown etiology [21]. Although lacking the BRAF p.V600E mutations found in other histiocytoses [22], a recent case study demonstrating pathogenic somatic mutations in RAF1 and TSC2 suggests that reticulohistiocytoma may represent a neoplastic proliferation [23]. While reticulohistiocytoma is seen predominantly in pediatric populations, cases have been reported in patients of all ages [21]. The lesions may be solitary, multiple, or present as a generalized variant known as multicentric reticulohistiocytosis.
The tumor is characterized by a well-demarcated accumulation of epithelioid histiocytes with eosinophilic cytoplasm described variably as “ground-glass,” “two-toned,” or “muddy rose” (Fig. 2A–C). This appearance, although characteristic, is not specific [24]. The tumor cells are often embedded in an inflammatory stroma with variable numbers of neutrophils, lymphocytes, and granulocytes, which increases the lesion’s similarity to non-tumoral histiocyte-rich inflammation (Fig. 2B). Tumor cells may include occasional multinucleated cells with several nuclei, but do not show Touton or osteoclast-type giant cells. A minority of the histiocytes may show spindle cell and/or xanthomatous cytology. The cells express CD68 (Fig. 2D), CD163, lysozyme (variable) and may show some S100 protein positivity in a minority of cells (Fig. 2E), but they are negative for CD1a, CD21, CD30, CD34, HMB45, EMA, and keratins [21]. Membranous CD10 may be seen (Fig. 2F). This immunoprofile, while useful in ruling out other histiocytic lesions, is nonspecific, which contributes to the diagnostic challenge.
A Tongue solitary reticulohistiocytoma causing mucosal ulceration (H&E 40x). B The base of an ulcer shows pale histiocytic cells embedded in mixed inflammatory stroma (H&E, 400x). C The histiocytic cells show two-toned “ground-glass” cytoplasm, large nuclei, and prominent nucleoli. D Tumor cells show CD68 positivity by immunohistochemistry (CD68, 100x). E S100 protein is generally negative with scant-scatted single-positive cells (S100 protein, 200x). F CD10 highlights the cell borders (CD10, 400x)
The importance of the diagnosis lies in its rare association with multicentric reticulohistiocytosis, a rare disorder presenting in adults with destructive polyarthritis and pruritic nodular dermal nodules, caused by diffuse reticulohistiocytoma-like lesions developing in the joints, skin, and other sites [25]. Like RDD, multicentric reticulohistiocytosis may also affect a variety of internal organs and soft tissue sites, including the heart, lungs, gastrointestinal tract, liver, head and neck, and urogenital systems [25]. While oral involvement by solitary reticulohistiocytomas is rare [21], oral lesions have been reported to occur in up to half of patients with multicentric reticulohistiocytosis [25, 26, 27]. While the arthritis typically precedes the cutaneous and mucosal involvement, it is the diagnosis of the skin or oral lesions that may lead to the correct diagnosis of this serious disease.
Follicular Dendritic Cell Sarcoma
Follicular dendritic cell sarcoma (FDCS) is a malignant neoplasm showing follicular dendritic cell differentiation, first described by Monda, Warnke, and Rosai in 1986 in a case series of four patients with cervical lymphadenopathy [28]. As with RDD, this tumor was later shown to also occur outside of lymph nodes, with the first such two cases reported in the oral cavity by Chan et al. [29]. Since then, it has been reported in a variety of head and neck and other body sites [30]. The percentage of reported cases with extranodal lesions, initially low, has been increasing as the familiarity with the tumor and its recognition has increased, and it is now clear that most head and neck cases are extranodal [31, 32, 33]. Most of the extranodal head and neck cases occur in the oropharynx [31, 32, 34, 35] followed by the oral cavity (palate most frequently) [36]. The diagnosis of FDCS in an oral cavity ulcer biopsy may be difficult given the likelihood of superficial partial sampling and the overlapping features with other histiocytic lesions and granulomatous inflammation.
FDCS shows no sex predilection and occurs in all ages, although pediatric cases are rare. Some cases are associated with hyaline-vascular Castleman disease [37], and associations with paraneoplastic pemphigus and myasthenia gravis have also been proposed [33]. The tumor consists of pale, monotonous, oval, and spindle cells with mild nuclear atypia and indistinct borders, occasionally multinucleated, arranged in nesting, swirling, and storiform patterns with a background of mild stromal fibrosis and a sprinkling of small lymphocytes (Fig. 3A–C). Focal necrosis can be seen (Fig. 3D). Perivascular cuffing and multinucleated giant cells have been described in some cases [28, 30, 34, 35, 36]. Immunohistochemistry shows the tumor cells are positive for CD21, CD23, CD35, and D2-40 (Fig. 3E–F); variably and focally positive for CD68, S100 protein, smooth muscle actin, and p16; and negative for CD1a, CD79a, CD3, CD20, CD31, CD34, ALK, myeloperoxidase, and HMB45 [34]. FDCS shows frequent mutations in the NF-κB pathway [38], while rare cases have shown BRAF p.V600E mutations [39].
A Follicular dendritic cell sarcoma shows well-circumscribed borders (H&E, 20x). B Tumor consists of spindle and oval pale eosinophilic cells in storiform pattern (H&E, 200x). C Cells show mild nuclear atypia and occasional mitoses (H&E, 400x). D Focal necrosis can be seen (H&E, 100x). E, F Immunohistochemistry shows the tumor cells are positive for D2-40 (F, 200x) and CD21 (G. 200x)
The differential diagnosis of FDCS includes nonkeratinizing SCC (NKSCC), histiocytic carcinoma, myeloid sarcoma (MS), blastic plasmacytoid dendritic cell neoplasm (BPDCN), and interdigitating dendritic cell sarcoma. Low-grade cytology, lack of connection to the mucosa, and lack of keratin expression allow for differentiation from NKSCC; however, confusion may arise from the frequent expression of p16 in FDCS, which has been mistaken for confirmation of HPV-related NKSCC in the absence of performing other immunohistochemistry. Histiocytic sarcoma, MS, BPDCN, and interdigitating dendritic cell sarcoma all tend to show a higher degree of cytologic atypia, more mitotic activity, and the lack of expression of follicular dendritic cell markers, such as CD21, CD23, and CD35.
Langerhans Cell Histiocytosis
Langerhans cell histiocytosis (LCH) is a disorder characterized by a neoplastic proliferation of immature myeloid dendritic cells in a variety of body sites and with diverse clinical manifestations [40]. LCH commonly involves bone, skin, and lymph nodes, but any site can be affected, including the oral cavity. Clinically, LCH is classified into solitary, multifocal, and multisystem types. Whereas multifocal and multisystem forms affect children and infants, respectively, the solitary form occurs in older children and adults [41, 42]. Patients with single organ involvement, such as skin or bone, have an excellent prognosis; however, multisystem LCH can be life-threatening [40, 42]. MAPK pathway mutations are known drivers of this neoplasm [43], and depending on whether they arise in pluripotent haematopoietic stem cell, a tissue-restricted precursor, or local precursor cell, these mutations cause multisystem, multifocal, or unifocal LCH, respectively [40]. Patients with LCH have an increased risk of developing an associated malignancy, including T cell acute lymphoblastic leukemia (ALL), promyelocytic acute myeloid leukemia (AML), retinoblastoma in children, and lymphoma and thyroid carcinoma in adults [41].
Oral involvement is most common in the gnathic bones, seen in 20% to 30% of cases, but oral mucosal sites can also be involved, including the hard palate and gingiva [42, 44, 45]. These lesions may be ulcerated and, as with other lesions presented in this review, can be easily mistaken for reactive inflammatory ulcer. In some cases, the oral presentation may be the first sign of the disease and lead to the diagnosis of LCH [46].
The biopsy of LCH typically shows accumulation of medium-sized oval cells with eosinophilic cytoplasm and grooved, folded, indented, or lobed nuclei (Fig. 4A–B). These cells show mild atypia, inconspicuous nucleoli, and variable mitotic activity (Fig. 4C). Characteristically, the LCH cells are found within variably rich inflammatory stroma, with prominent eosinophils and variable numbers of plasma cells, histiocytes, neutrophils, and lymphocytes (Fig. 4C). Additional features seen occasionally include foamy macrophages, eosinophilic microabscesses, multinucleated giant cells, and fibrosis. The LCH cells show a characteristic immunoprofile of positivity for CD1a (Fig. 4D), CD68, S100 protein, and CD207 and absence of CD21, CD23, and CD35 staining.
A Langerhans cell histiocytosis of the buccal mucosa shows sheets of monotonous histiocytic cells with a heavy eosinophilic background inflammation (H&E 40x). B, C LCH cells show eosinophilic cytoplasm, oval nuclei, and mild atypia, as well as intense inflammatory background rich in eosinophils and plasma cells with variable numbers of lymphocytes and histiocytes (H&E, B. 400x, C, 600x). D The LCH cells show positivity for CD1a (400x, courtesy of Dr. A. Franko)
The differential diagnosis of oral LCH prior to biopsy varies depending on the clinical presentation. Unilocular LCH in the gnathic bones may simulate periapical dental abscess, odontogenic cysts and tumors, and osteomyelitis, while multilocular lesions may simulate multiple myeloma and brown tumors of hyperparathyroidism. Extraosseous hard palate lesions can mimic necrotizing sialometaplasia and gingival lesions may resemble periodontal disease [42]. The biopsy findings can easily mimic reactive inflammatory ulcer, especially when the background inflammation obscures the neoplastic component. The differential diagnosis includes other histiocytic disorders, including Erdheim–Chester disease, reticulohistiocytoma, RDD, and histiocytic sarcoma, as well as traumatic ulcerative granuloma and mastocytosis. The presence of eosinophil-rich inflammation, the grooved nuclei, and the immunopositivity for CD1a all help to differentiate LCH from the other histiocytic neoplasms. Traumatic ulcerative granuloma shares with LCH the eosinophil-rich mixed inflammation but lacks significant proliferation of Langerhans-like cells. Mast cells seen in oral mastocytosis can mimic LCH cells and both lesions can involve the gnathic bones in children; however, mastocytic lesions typically lack the eosinophilic component and mast cells show a different immunoprofile, lacking CD1a positivity and showing CD117 and tryptase expression. Lastly, a rare possibility of Langerhans cell sarcoma, which has an immunoprofile similar to that of LCH but carries a significantly worse prognosis, should be ruled out by the absence of a high proliferative index, and high-grade features, such as necrosis, and invasive growth [47].
Traumatic Ulcerative Granuloma with Stromal Eosinophilia
Traumatic ulcerative granuloma with stromal eosinophilia (TUGSE) is a benign, self-limited, deep tongue ulcer of unknown etiology. It presents as a rapidly developing ulcer with elevated borders that persists for weeks to months, mimicking squamous cell carcinoma and thus is often biopsied [48]. Although most often found on the lateral tongue, it can also occur in the gingiva, buccal mucosa, vestibules, and retromolar trigone [48]. While TUGSE is well known in the oral medicine world, surgical pathologists are less familiar with it. This lack of recognition is confounded by the variety of names used to describe it in the literature over the decades, including traumatic ulcerative granuloma, eosinophilic granuloma, traumatic granuloma of the tongue, ulcerative eosinophilic granuloma, eosinophilic ulcer, eosinophilic ulcer of the oral mucosa, ulcerated granuloma with eosinophilia, and Riga–Fede disease in infants [49].
Although long considered to have traumatic and inflammatory etiology, the pathogenesis of TUGSE has continued to puzzle clinicians and researchers. Despite its name, trauma is only rarely linked to this lesion [49, 50]. Rather, the typical ulcer location on the lateral tongue has led clinicians to assume they may result from biting. The slow but eventual resolution of the ulcer seen in almost all cases was interpreted as healing and reinforced this assumption. However, other inflammatory conditions, which have been assumed to be reactive in the past, have been recently shown to harbor specific mutations and were consequently reclassified as neoplastic, including some of the conditions presented in this review. This has raised the possibility that TUGSE may represent a self-limited neoplasm analogous to nodular fasciitis. Several studies demonstrated the presence of CD30-positive atypical mononuclear cells with T cell antigens, suggesting TUGSE may represent a low-grade CD30 + T cell lymphoproliferative disorder [48, 50, 51, 52] similar to cutaneous CD30 + lymphoproliferative disorders [51]. In addition, a few studies reported T cell receptor gene rearrangements [48], although this finding has not been replicated by others [49, 50]. The self-limited nature, lack of recurrence and lack of association with other lymphoproliferative disorders have resulted in the persistent opinion that TUGSE is not itself a lymphoproliferative disorder; however, its etiology and pathogenesis remain unsolved.
The inflammatory infiltrate in TUGSE shows dense sheets of eosinophils, T cells, histiocytes, and occasional plasma cells, involving the mucosa and the underlying intrinsic tongue skeletal muscle (Fig. 5A–B). The neutrophilic component is typically present in the ulcer base but may be absent deeper in the lesion. Atypical large mononuclear cells are seen in approximately half of the cases [49] leading to the label of granuloma given to the lesion. As reported above, these cells often show positivity for CD30 and T cell markers and for this reason TUGSE needs to be distinguished from primary mucosal CD30-positive T cell lymphoproliferative disorder (TLPD), which is a neoplasm of CD30-positive T cells arising mainly in the oral cavity [53, 54, 55]. The main difference between the two is the number of the CD30-positive cells: scant and scattered in TUGSE and dense sheet-like accumulation in TLPD. Both lesions have the background of eosinophilic and neutrophilic inflammation. Other lesions that may resemble TUGSE include Langerhans cell histiocytosis, T cell lymphoma, Hodgkin lymphoma, and EBV-positive mucocutaneous ulcer, but the characteristic histological and molecular findings of these lesions are absent in TUGSE.
Epstein–Barr Virus-positive Mucocutaneous Ulcer
Epstein–Barr virus-positive mucocutaneous ulcer (EBVMCU) is a recently described disorder with a propensity for the oral cavity that may mimic an inflammatory ulcer. The WHO classification of hematopoietic tumors describes EBVMCU as a lymphoproliferative disorder with a polymorphous lymphoid infiltrate including EBV-positive atypical large B cells and/or Hodgkin/Reed–Sternberg (HRS)-like cells that typically involves mucosal and cutaneous sites in patients with immune deficiency/dysregulation [56]. First described in 2011, EBVMCU can involve the skin and any mucosal site, including the oral mucosa, but is also commonly found in the tonsils, palate, and even the gastrointestinal tract [57, 58]. EBVMCU is associated with immune deficiency or dysregulation, both acquired and inborn. In Jaffe’s first series of EBVMCU, all cases were associated with immune dysregulation, including therapy-associated dysregulation from azathioprine, methotrexate, and cyclosporine, but even more people showed age-related senescence. With further study of EBVMCU, the immune dysregulation has been shown to be caused by many other medical therapies, autoimmune conditions including HIV infection, and post-transplantation.
EBV has been detected in all cases of EBVMCU, with the atypical cells positive for EBER in situ hybridization (ISH), thought to be an important factor related to the etiology of this disease. EBV viral load measurements in blood is not sufficient for diagnosis. EBV has been associated with several lymphoproliferative disorders, including but not limited to Burkitt lymphoma, classic Hodgkin lymphoma, primary effusion lymphoma, and angioimmunoblastic T cell lymphoma. Most adults have had a primary infection with EBV at a relatively young age that persistently infects the B cells of adults. In cases of EBVMCU, patients usually present with a well-circumscribed painful ulcer but lack systemic symptoms, lymphadenopathy, or bone marrow involvement.
EBVMCU forms ulcers of the mucosa, especially in the mouth, which are circumscribed, shallow, and that contain a mixed inflammatory polymorphous infiltrate (Fig. 6A). The infiltrate typically contains lymphocytes that can range from small to large in size. The larger atypical cells are the neoplastic component of the lesion, and the atypical cells can have a single central nucleolus resembling immunoblasts or can be binucleate with distinct nucleoli looking like Reed–Sternberg cells (Fig. 6B). Besides the atypical cells, there are varying numbers of plasma cells, eosinophils, small lymphocytes, and histocytes. Necrosis and angioinvasion can sometimes be identified and apoptotic cells may also be present. In tissues with a squamous mucosa, pseudoepitheliomatous hyperplasia and reactive epithelial atypia may be identified.
EBV-positive mucocutaneous ulcer of the oral cavity (EBVMCU). A Scanning magnification of EBVMCU shows that there is a lymphoid neoplasm underneath the oral mucosa which could easily be mistaken for a reactive inflammatory ulcer. B At high magnification, the atypical cells can have a single central nucleolus resembling immunoblasts or can be binucleate with distinct nucleoli looking like Reed–Sternberg cells. C High magnification shows that the neoplastic cells are PAX5 positive indicating that this is a B cell lymphoproliferative disorder. D This is a 600 × magnification of a CD30 +-stained slide showing that the neoplastic cells express CD30, which is seen in most cases of EBVMCU. E This EBER ISH test at high magnification shows that the neoplastic cells of EBVMCU are positive as would be expected
The atypical lymphocytes in EBVMCU are B cells and will express pan B cell markers, like CD20 and PAX5 (Fig. 6C). They will also typically be immunoreactive for CD30, but only a subset of cases will express CD15, which helps distinguish them from classic Hodgkin lymphoma (Fig. 6D). The atypical B cells always express EBV markers, particularly EBER (Fig. 6E). The background lymphoid infiltrate is a mix of CD20 + B cells and CD3 + T cells, and there are often bands of T cells at the periphery of the lesion. EBVMCU is a B cell lymphoproliferative disorder where B cells are infected with EBV, in which IGH gene rearrangement has been detected in approximately 50% of the cases, proving that they are a clonal B cell process. TRB and TRG gene rearrangements have also been reported in some cases, and the presence of T cell receptor gene rearrangement should not be misleading for a T cell lymphoma.
EBVMCU may be easily mistaken for a reactive inflammatory ulcer or another type of lymphoma based on hematoxylin and eosin stain examination alone. EBVMCU may resemble the nonspecific inflammation of a reactive inflammatory ulcer which show a mixed inflammatory infiltrate and can have increased numbers of plasma cells and eosinophils, but this polymorphous infiltrate is also seen in EBVMCU. EBVMCU can sometimes have large numbers of small lymphocytes that can be similar in appearance to those seen in a reactive inflammatory ulcer. In contrast to most reactive inflammatory ulcers, EBVMCU will have larger atypical cells and will express B cell markers, CD30, and EBER, so careful evaluation of an oral lesion with the appropriate ancillary tests should readily separate EBVMCU from a reactive inflammatory ulcer [59]. Many cases of EBVMCU have large numbers of atypical B cells similar to diffuse large B cell lymphoma (DLBCL), and the two entities can be difficult to differentiate based on morphology alone. However, the clinical presentation of EBVMCU as an ulcerated mucosal-based lesion in a patient with immunodysregulation will typically distinguish it from DLBCL. EBER expression and CD30 positivity can sometimes be seen in DLBCL. Cases lacking EBER-positive cells should represent a different lymphoma and not EBVMCU. Classic Hodgkin-like, mucosal-associated lymphoid tissue-like, and plasmablastic differentiation have been described in cases of EBVMCU, and must be differentiated from classic Hodgkin lymphoma, marginal zone lymphoma, and plasmablastic lymphoma in some instances [60]. The tumor cells in classic Hodgkin lymphoma often coexpress CD30, CD15, MUM1, PAX5, and EBER ISH, but these markers will often not all be expressed in EBVMCU. CD45RB is often positive in EBVMCU, but frequently negative in classic Hodgkin lymphoma. It is thought that EBER is positive only in very large cells in Hodgkin lymphoma and DLBCL but can be seen in a variety of sizes of cells in EBVMCU.
The prognosis of EBVMCU differs from that of other EBV-associated lymphomas since it often shows spontaneous regression without any systemic chemotherapy treatment. EBVMCU is considered a low-grade B cell lymphoproliferative disorder because it is a clonal B cell process, however, is not as aggressive as most other B cell lymphomas. Correct identification of EBVMCU and its distinction from other B cell lymphomas is important to decrease the use of unnecessary chemotherapy [61, 62]. Some cases may relapse but typically do not progress.
Myeloid Sarcoma
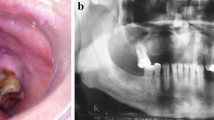
Like EBVMCU, MS can be difficult to diagnose when it presents as an oral ulcer. Previously called chloroma, MS is an extramedullary tumor found in any anatomic site, most common in the oral cavity in head and neck sites, composed of myeloid blasts and frequently containing maturing myeloid elements [63]. In a series on head and neck MS, 71% of cases involved the oral cavity, including gingiva (most common), lips, buccal mucosal, palate, tongue, and maxillary and mandible ridge [64, 65].
Most people with MS also have concurrent or previous AML in the blood or bone marrow, although fewer than 10% of people with AML will develop MS. In fact, isolated de novo MS with a normal bone marrow is rare and is reportedly detected in less than 2% of patients with AML [66]. MS may also present in patients with other myeloid neoplasms, including myelodysplastic syndrome, myeloproliferative neoplasms, or myelodysplastic syndrome/myeloproliferative neoplasm.
When MS is the first manifestation of disease it can be difficult to diagnose. One of the difficulties in diagnosing this disease is that the clinical presentation is often nonspecific. These lesions can present as ulcers, skin lesions, masses, bleeding, jaw pain, sore throat, skin rash, mouth pain, sinus pressure, or gingival irritation after tooth extraction. Biopsy and diagnosis can be delayed clinically because it often presents like an inflammatory reactive process and can mimic ulcer, periodontal abscess, pyogenic granuloma, or peripheral giant cell granuloma [67, 68]. These oral cases of MS, especially if they present as an ulcer, can be very subtle and difficult to diagnose.
By morphology, MS consists of immature myeloid cells and there may or may not also be mature granulocytic cells present (Fig. 7A). The immature cells can be seen in aggregates and form masses. Many, but not all cases of MS in the oral cavity, will show monocytic differentiation both by morphology and by expression of monocytic markers. MS is a disease of myeloid blasts with or without other maturing granulocytes that are positive for multiple granulocytic and monocytic markers. The immature cells may also form a single file pattern. The immature cells can be identified by their fine blastic chromatin, usually with distinct nucleoli, and frequently with high nuclear:cytoplasmic ratios. Auer rods can be seen in a subset of cases. If there is monocytic differentiation, then cells may show the folded nuclei common to promonocytes. More mature granulocytes are also often present and frequently contain cytoplasmic granules. If there are eosinophilic myelocytes or metamyelocytes, there may be eosinophilic cytoplasm. Dysplasia can also be seen in granulocytic cells, erythroid cells, or megakaryocytes.
Myeloid sarcoma (MS). A This image (H&E stain, 400x) from a biopsy from a patient with MS shows a neoplastic population of cells with immature chromatin as well as cells with clumps of myeloid granules, as are often seen in these tumors. B This image (MPO stain, at high power 600x) shows strong staining in the cells of MS. Other antibodies such as CD15 and CD33 were also positive, confirming that this is a myeloid neoplasm (not shown). C This CD34 image (400x) is negative in a case of MS with monocytic differentiation. MS with monocytic differentiation is common in the oral cavity and frequently lacks CD34
Immunophenotyping is necessary for accurate diagnosis and can be done by immunohistochemistry or flow cytometry. MS should express myeloid antigens, such as CD13, CD33, CD43, and myeloperoxidase, but also often expresses monocyte antigens, such as CD4, CD14, CD68, CD163, and lysozyme [69, 70, 71] (Fig. 7B). The immature cells should also express at least one of the markers of immaturity, such as CD34, CD117, CD123, CD99, or TdT (not often positive and often only dim), although CD34 is often negative in the monocytic cells that are commonly found in gingival specimens (Fig. 7C). CD19 is known to be found in cases that have t(8;21). CD45RB is also positive and CD56 is expressed in most cases. CD43 and CD68 are thought to be some of the most sensitive markers for MS but are not specific [72]. MPO is one of the most specific myeloid markers and CD14 is one of the most specific monocytic markers, with both potentially expressed in these lesions. It is best to deploy a broad panel of markers with multiple immature antigens, multiple myeloid antigens, and multiple monocytic antigens.
MS shares the same range of genetic alterations that can be seen in AML. De novo MS cases must have molecular testing done for treatment and prognostic purposes and molecular testing is also often performed in cases where there has been an antecedent diagnosis of AML [73]. Cytogenetic karyotyping, next-generation sequencing (NGS), and multiprobe FISH testing for changes seen in myeloid neoplasms are commonly done in these tumors to determine their genetic alterations. Molecular abnormalities detected by FISH or cytogenetics are identified in about half of the cases and alterations are found in the vast majority of cases by NGS. There are many different molecular abnormalities and translocations that can be identified such as t(8; 21) RUNX1::RUNX1T1. MS with monocytic differentiation will often have NPM1 and KMT2A rearrangements.
The differential diagnosis of oral MS presenting as a mucosal ulcer is diverse. As these lesions can present in the mouth as a mass or ulcer, they must in all cases be distinguished from a reactive inflammatory ulcer. Reactive inflammatory ulcers are often difficult to distinguish from MS due to the abundant neutrophils, eosinophils, plasma cells and the mixed inflammatory infiltrate appearance often seen in either entity. Identification of blasts by their morphology along with their phenotype is necessary to differentiate MS from reactive ulcer. To accurately diagnose MS, the immature myeloid cells must be recognized even though they may reside in a mixed inflammatory background. If the patient has a history of a myeloid neoplasm, such as AML, myelodysplastic syndrome, or myeloproliferative neoplasm, then a MS should always be excluded in an oral lesion. Immaturity markers such as CD34 or TdT detected in MS are not found in reactive ulcers. However, CD117, another marker of immature cells, can be positive in mature mast cells. Mast cells can sometimes be seen in reactive ulcers, so there can be some CD117 expression in reactive ulcers which should not be mistaken for blasts. The molecular alterations seen in MS would not be expected to be found in reactive ulcers. MS can also be mistaken for a plasmacytoma when plasma cells are numerous or form aggregates, but the kappa and lambda light chains would be polytypic in MS and monotypic in plasmacytoma, helping differentiate between the two.
The prognosis of patients with MS is like that of patients with AML and is based on clinical as well as molecular parameters. Patient with MS and patients with AML are treated with similar therapeutic regimens. It is thought that patients with isolated MS without evidence of concurrent AML may have a more favorable clinical course [74, 75].
Blastic Plasmacytoid Dendritic Cell Neoplasm
Often misdiagnosed as a MS, BPDCN is another hematopoietic neoplasm that can involve the oral cavity and rarely present as an ulcer. Although both are neoplasms of immature cells, BPDCN is thought to differ because it consists of immature cells with plasmacytoid dendritic cell differentiation [76]. The disease frequently first presents with cutaneous or, less frequently, mucosal involvement and then progresses to systemic involvement. In the series of cases reported by Julia et al., 6% of cases involved the mucosa, particularly the oral cavity, and it was postulated that there may be more cases that are underdiagnosed, especially if they present as an ulcer instead of a large mass [77]. Hashikawa et al., reported 2 of 26 cases that were located in the pharynx in their series of Japanese patients [78]. Skin lesions may present with ulcers, patches, plaques, or nodules, and are most commonly found in the head and neck, upper extremities, and torso. BPDCN can also involve lymph nodes or may present as leukemia only showing blood and bone marrow involvement, with CNS progression in some advanced cases [79]. Like those with MS, BPDCN patients may also have a history of or have concurrent myelodysplastic syndrome, myelodysplastic syndrome/myeloproliferative neoplasm, or clonal hematopoiesis (occurring in up to 30% of patients with BPDCN) [80].
Plasmacytoid dendritic cells are thought to be the origin of BPDCN. TCF4 is a transcription factor thought to play a central role in BPDCN, especially in its relationship to BRD4 [81]. TCF4 functions in the development of plasmacytoid dendritic cells from other dendritic cells. These tumors have aberrant type I interferon signaling and have been shown to have activation of the NF-kB pathway [82].
By morphology, these tumors frequently show a monotonous infiltrate of immature blastic cells, usually with fine chromatin, one or more distinct nucleoli, eccentric nuclei with somewhat irregular nuclear contours, and scant cytoplasm (Fig. 8A). They can be variable in size, but most cases are more intermediate in size. The cytoplasm may have vacuoles or cytoplasmic protrusions [83], but lack Auer rods. These cells often cannot be distinguished from myeloblasts or lymphoblasts by morphology alone. Necrosis and mitotic figures are commonly detected.
Blastic plasmacytoid dendritic cell neoplasm (BPDCN). A This image (40×) from a case of BPDCN involving the oral cavity shows immature cells that are indistinguishable for MS on H&E alone. B CD123 is a marker of plasmacytic dendritic cells (PDCs) and is positive in this case of BPDCN (400x). CD123 can be negative in a subset of these cases. C This TCL1 stain is positive in a case of BPDCN and is another marker of PDCs (400x). These cells should be used in panels, so that multiple markers can be assessed. D CD4 is also positive in the case of BPDCN (400x). E The tumor is also strongly positive for CD56. (400x). BPDCN was previously called CD4 + CD56 + hematodermic neoplasm, although this term is no longer used. F MPO is negative in this case of BPDCN (400x). MPO can help distinguish MS from BPDCN as it is usually positive in MS, but negative in BPDCN
Since these cells do not have a specific morphology distinct from those of other high-grade tumors, phenotyping by immunohistochemistry and or flow cytometry is required. CD123, TCF4, TCL1, CD2AP, SPID, CD303, CD304, E-cadherin, and MX1 are all considered markers of plasmacytoid dendritic cells and several of these markers should be expressed in cases of BPDCN [84, 85]. CD123 represents the interleukin-3 recenter alpha chain and is the most commercially available of plasmacytoid dendritic cell antibodies, although it may be negative in some cases, so should not be the sole marker used to diagnose BPDCN (Fig. 8B). CD4 and CD56 are usually expressed; BCL2, CD2, CD5, CD7, CD13, CD33, CD34, CD48, CD43, CD79a, CD117, and TDT can also be positive (Fig. 8C-–E). CD3, CD19, lysozyme, and myeloperoxidase should all be negative [86, 87] (Fig. 8F).
The molecular alterations of BPDCN are evolving, with most cases showing complex karyotypes by cytogenetic testing and deletion of 12p13/ETV6 in most cases [88]. ASXL1, TET2, SF3B1, SRSF2, U2AF1, and ZRSR2 are genes that are either epigenetic regulators or RNA splicing genes that are often altered in BPDCN. ATM, DNMT3A, KRAS, NRAS, and NPM1 have also been reported as have 8q24 MYC translocations [89, 90].
When BPDCN presents as an ulcer in the mouth, it must be differentiated from a reactive inflammatory ulcer, as both lesions may not present with an obvious mass lesion and may present without systemic symptoms and with only localized disease. It is not until the blastic immature cells are identified by histology and by phenotype that BPDCN can be diagnosed. Furthermore, background inflammatory cells, especially reactive histiocytes, neutrophils, and lymphocytes in BPDCN can sometimes obscure the blastic immature cells. However, the presence of immature blasts that express plasmacytoid dendritic cell markers (CD123, TCL1, CD303) as well as other markers (CD4, CD56) would be diagnostic for BPDCN and effectively exclude an inflammatory ulcer. Some cases of AML overlap with BPDCN in their clinical presentation, their histology, and their immunophenotype. These cases of AML/MS may also express CD4, CD56, and CD123, mimicking BPDCN. Only with further phenotyping and a broad panel of immunohistochemistry can BPDCN be definitively diagnosed. Expression of other plasmacytoid dendritic cell markers (CD303, TCL1, TCF4) supports a diagnosis of BPDCN, but the tumor cells should also lack myeloid specific markers, such as myeloperoxidase.
BPDCN is an aggressive disease with a poor prognosis and median survival usually less than 2 years. Cases involving the skin or mucosa do not have a different prognosis than those that are disseminated. Treatment is usually with combination chemotherapy or targeted therapy, such as tagraxofusp, an anti-CD123 therapy that has improved response rates [91].
Plasmablastic Lymphoma
DLBCL is one of the aggressive B cell lymphomas not uncommonly diagnosed in oral lesions. They can present as ulcers, especially when they arise from an underlying lymph node or form a mass that extends to the surface, causing surface erosion or ulcer. Because of their aggressive clinical course, these lymphomas must be differentiated from reactive oral ulcers. Since plasmablastic lymphoma is a DLBCL with a particular propensity for the head and neck, including the oral cavity, it will be the focus of this section.
Plasmablastic lymphoma is a rare entity, typically presenting as an asymptomatic rapidly growing mass or swelling of the oral cavity (the most common site, specifically gingiva and palate) with or without ulceration and bleeding. Pain and symptoms of oropharyngeal obstruction are also reported [92, 93, 94]. This neoplasm is prevalent in the adult HIV-positive population and may be the initial presentation of HIV infection [92, 93]. Plasmablastic lymphoma can also be seen, although less commonly, in severely immunosuppressed patients, such as those with solid organ transplants, while only rarely seen in immunocompetent patients [56, 95, 96]. Studies have demonstrated a male:female predominance of approximately 3 to 4:1 [93, 95]. Unfortunately, cases may be initially misdiagnosed as oral abscess or other primary malignancy of bone or soft tissue [92, 94, 97]. Radiographically, these lesions can exhibit destructive bone loss and soft tissue involvement adding to the diagnostic difficulty [92, 94, 97]. Patients rarely present with B symptoms or other signs of systemic involvement, with lymph node involvement present in less than 10% of cases [56].
Histologically, this neoplasm is typically composed of large, high-grade, pleomorphic cells with features of both plasmablastic and immunoblastic differentiation (Fig. 9A). They have variable amphophilic cytoplasm, paranuclear clearing (hof), and large, irregular, and commonly eccentrically located nuclei with coarse chromatin and prominent nucleoli. The cells exhibit sheet-like growth with tingible body macrophages and apoptotic bodies, often described as a starry-sky pattern. Frequent mitoses and areas of necrosis can also be seen [98].
Plasmablastic lymphoma (PBL) and extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (EMZL). A This high magnification shows large, atypical cells with a plasmacytoid morphology in a case of PBL. B PBL will show plasma cell differentiation and this 600× magnification of a CD138 immunostain shows that the neoplastic cells are positive for plasma cell/plasmacytoid cell markers. C This MUM1 immunostain is also positive in PBL and is another marker of plasma cells/plasmacytoid cells. D This CD20 immunostain is negative in PBL and CD20 is a pan B cell marker that is often lost in cases with plasmacytoid differentiation. E This medium powered, 200× magnification of a case of EMZL shows small lymphocytes with increased clear cytoplasm or so-called monocytoid change. F At 400x, this EMZL shows colonization of a reactive follicle where neoplastic B cells enter the follicle and disrupt it
Immunophenotypically, plasmablastic lymphoma will show phenotype overlap with plasma cells. Plasmablastic lymphoma is positive for plasma cell markers CD38, CD138, IRF4/MUM1, PRDM1, and cytoplasmic IgG with a light chain restriction (Fig. 9B–C). Since these tumors have plasmacytic differentiation, they will be negative or weakly positive for CD45 as well as B cell markers CD20 and PAX5 (Fig. 9D). CD79a is considered a pan B cell marker that is often positive in plasma cells and can be positive in up to 40% of cases of plasmablastic lymphoma. CD56 is negative in most cases arising from the oral mucosa. The neoplastic cells frequently express EMA and CD30, with a Ki67 proliferation index greater than 90% and EBER positivity in up to 80% of cases [95, 98]. Characteristically, they should be negative for HHV8 [95]. The molecular profile of plasmablastic lymphoma is different from that of DLBCL, NOS, and plasma cell myeloma. MYC rearrangement may be an important molecular finding and can be detected in approximately half of the cases of plasmablastic lymphoma.
Plasmablastic lymphoma needs to be differentiated from other lymphomas, as well as distinguished from reactive inflammatory ulcers. Other lymphoid neoplasms that should be considered in the differential diagnosis are other large B cell lymphomas (ALK-positive large B cell lymphoma and Burkitt’s lymphoma) and other plasma cell neoplasms, such as multiple myeloma and anaplastic plasmacytoma [93, 94]. Plasmablastic lymphoma differs from the other mature B cell lymphomas by showing dark and diffuse staining with pan B cell markers, like CD20. The high-grade polymorphic B cells of plasmablastic lymphoma may be set in a mixed inflammatory background with many lymphocytes, eosinophils, apoptotic cells, and histiocytes that can mimic an inflammatory ulcer. Inflammatory ulcers will often have a large number of plasma cells which appear similar to the plasmacytic cells of plasmablastic lymphoma. However, the tumor cells in plasmablastic lymphoma are often higher grade and more pleomorphic in appearance. Detection of very large, atypical cells would favor a diagnosis of plasmablastic lymphoma. Although both reactive ulcer and plasmablastic lymphoma have cells with a plasmacytoid morphology, light chain restriction by flow cytometry or immunohistochemistry should only be identified in plasmablastic lymphoma, while polytypic in an inflammatory ulcer. As a clonal B cell lymphoproliferative disorder, plasmablastic lymphoma should show IGH gene rearrangements, which are not typically found in a reactive inflammatory ulcer. Sheets of CD30 + cells may be detected in plasmablastic lymphoma, but only rare, scattered CD30 + cells would be seen in reactive ulcers. EBER is also typically positive in the tumor cells of plasmablastic lymphoma, but may be positive in cases of reactive ulcers associated with infectious mononucleosis or immunosuppression.
Plasmablastic lymphoma is a highly aggressive B cell-derived lymphoma with 5-year survival rates no greater than 33.5% [94], with a mean survival of 11–13 months [93], often due to high tumor stage. Cases with MYC translocations reportedly have a worse prognosis [95]. Current treatment regimens, although not standardized, generally involve high-dose chemotherapy, often with methotrexate prophylaxis and aggressive highly active antiretroviral therapy (HAART) to control underlying HIV infection in HIV-positive patients. EBV-targeted therapies such as zidovudine, ganciclovir, and interleukin-2 also show promise [95, 99]. Disseminated disease is common at initial diagnosis, with bone marrow involvement in up to 75% of HIV-positive patients and up to 50% of patients with transplant-related immunosuppression.
Extranodal Marginal Zone Lymphoma of Mucosa-Associated Lymphoid Tissue
Primary lymphomas of the oral cavity account for only about 2% of all extranodal lymphomas, of which extranodal marginal zone lymphoma (EMZL) represents about 13% [100]. EMZL of the oral cavity/oropharynx generally manifests as localized areas of asymptomatic swelling with or without ulceration; pain is sometimes reported [101]. These lesions can be found arising from the tonsils, palate, buccal mucosa, gingiva, tongue, floor of mouth, salivary glands, retromolar region, and uvula [101, 102]. The neoplastic cells are derived from mucosal-associated lymphoid tissue (MALT) or from inflammatory tissue within the salivary glands, most commonly the parotid gland. Neoplastic change is believed to be related to continuous antigenic stimulation caused by proinflammatory states, such as chronic infection or autoimmune disease [56, 103]. In the head and neck, EMZL most frequently arises from the parotid gland in association with Sjögren syndrome. Patients with Sjögren syndrome have a risk of lymphoma 14–18-fold higher than that of the general population. EMZL typically arise in the 7th decade of life, with head and neck cases demonstrating a female predominance of approximately 2:1 [103].
Histologically, EMZL is predominantly composed of small- to medium-sized mature B cells often with ample pale cytoplasm, nuclei with minimally irregular contours, dispersed chromatin, and inconspicuous nucleoli resembling centrocytes. These cells will often demonstrate a monocytoid morphology with increased clear/pale cytoplasm; however, plasma cell differentiation can also be seen [102] (Fig. 9E). Cases with plasmacytoid differentiation often have eccentric nuclei and ample pink cytoplasm. Dutcher bodies and occasionally amyloid deposition can also be seen. Scattered larger centroblast- and immunoblast-like cells are routinely admixed. The neoplastic cells arise in the marginal zone just outside the mantle of reactive follicles. As the neoplastic cells proliferate, they expand the marginal zone overtaking adjacent follicles and infiltrating into nearby epithelial structures. In some cases of EMZL, reactive follicles are still identified. The neoplastic small B cells can infiltrate into the reactive follicles, a process known as follicular colonization (Fig. 9F). Lymphoepithelial lesions are defined as clusters of three or more neoplastic marginal zone cells permeating into epithelial tissues with associated acute damage or alteration to the tissue [56].
Immunophenotypically, EMZL expresses B cell markers including CD20 and CD79a and is commonly positive for IgM heavy chain with a light chain restriction. IgG and IgA heavy chain expression do occur, although infrequently. CD21, CD23, and CD35 will highlight residual follicular dendritic networks that have been colonized, with the follicles typically expanded in EMZL. The neoplastic cells are classically negative for CD5, CD10, BCL-6, cyclin D1, and CD23, with variable expression of CD43 and CD11a [104]. Rare CD5-positive cases are seen and may be associated with more aggressive behavior and increased likelihood of dissemination. IRTA1 and MNDA positivity also help support a diagnosis of EMZL. EBV has rarely been identified in patients with immunosuppression. Some common genetic alterations include trisomies of chromosomes 3 and 18 and abnormalities of TNFAIP3 on chromosome 6q23. Several translocations can be seen in MALT lymphomas, of which t(14;18) is the most frequently identified in EMZL of the salivary gland.
When EMZL arises in the oral cavity it must be differentiated from a reactive inflammatory ulcer as well as from the other low-grade B cell lymphomas. These oral cases of EMZL often extend from a salivary gland MZL and may present in the oral cavity as an ulcer. Especially in small biopsy specimens, these EMZL of the mouth can be difficult to separate from reactive inflammatory ulcers. Reactive inflammatory ulcers can have large numbers of small lymphocytes as well as plasma cells that look similar to the small B cells and monocytoid/plasmacytoid change of EMZL. Both EMZL and reactive inflammatory ulcers can contain reactive follicles. However, the follicle colonization by small neoplastic B cells and expansion of the follicular dendritic cell meshworks seen in EMZL are not seen in reactive ulcers. Monocytoid change is another feature suggesting a diagnosis of EMZL. In rare cases, reactive ulcers may show marginal zone hyperplasia which can look like the marginal zone B cells of EMZL. B cell co-expression of CD43 would also favor EMZL. Features of small B cell lymphoma including light chain restriction by flow cytometry or immunohistochemistry as well as IGH, IGK, or IGL gene rearrangement would support a diagnosis of EMZL.
Although rare, primary MALT lymphoma of the oral cavity is typically indolent with slow disease progression, as with most other MALT lymphomas. The overall 5-year survival rates are between 86 and 95% [100]. Multifocal disease and bone marrow involvement do not portend a significantly worse prognosis [105].
There is no consensus on treatment of EMZL, with treatment dependent on the stage of disease, extent of tissue involvement, and the activity of any contributory inflammatory or autoimmune conditions, such as Sjögren’s syndrome. Treatment options include watchful waiting, surgical excision, radiation therapy, monoclonal antibody administration, chemotherapy, or any combination thereof [105]. For patients with underlying inflammatory or autoimmune disease, maintaining adequate control of these conditions helps to limit disease progression. There is a low frequency of transformation to higher-grade malignancy, such as DLBCL.
Careful histological examination of oral ulcers, with pertinent immunohistochemistry evaluation, occasionally combined with additional ancillary studies will allow for a meaningful separation of these uncommon, but frequently deadly disorders from benign, reactive conditions.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Cebeci AR, Gülşahi A, Kamburoglu K, Orhan BK, Oztaş B (2009) Prevalence and distribution of oral mucosal lesions in an adult Turkish population. Med Oral Patol Oral Cir Bucal 14(6):E272–E277
Fitzpatrick SG, Cohen DM, Clark AN (2019) Ulcerated lesions of the oral mucosa: clinical and histologic review. Head Neck Pathol 13(1):91–102. https://doi.org/10.1007/s12105-018-0981-8
Compilato D, Cirillo N, Termine N, Kerr AR, Paderni C, Ciavarella D et al (2009) Long-standing oral ulcers: proposal for a new “S-C-D classification system.” J Oral Pathol Med 38(3):241–253. https://doi.org/10.1111/j.1600-0714.2008.00722.x
Allon I, Allon DM, Anavi Y, Kaplan I (2013) The significance of surface ulceration as a sign of malignancy in exophytic oral mucosal lesions: myth or fact? Head Neck Pathol 7(2):149–154. https://doi.org/10.1007/s12105-012-0413-0
Destombes P (1965) Adenitis with lipid excess, in children or young adults, seen in the Antilles and in Mali (4 cases). Bull Soc Pathol Exot Filiales 58(6):1169–1175
Rosai J, Dorfman RF (1969) Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol 87(1):63–70
Kiruthiga KG, Younes S, Natkunam Y (2022) Strong coexpression of transcription factors PU.1 and Oct-2 in Rosai-Dorfman disease. Am J Clin Pathol. https://doi.org/10.1093/ajcp/aqac119
Garces S, Medeiros LJ, Patel KP, Li S, Pina-Oviedo S, Li J et al (2017) Mutually exclusive recurrent KRAS and MAP2K1 mutations in Rosai-Dorfman disease. Mod Pathol 30(10):1367–1377. https://doi.org/10.1038/modpathol.2017.55
Shanmugam V, Margolskee E, Kluk M, Giorgadze T, Orazi A (2016) Rosai-Dorfman disease harboring an activating KRAS K117N missense mutation. Head Neck Pathol 10(3):394–399. https://doi.org/10.1007/s12105-016-0709-6
Goodnight JW, Wang MB, Sercarz JA, Fu YS (1996) Extranodal Rosai-Dorfman disease of the head and neck. Laryngoscope 106(3 Pt 1):253–256. https://doi.org/10.1097/00005537-199603000-00002
Silver AL, Farkash EA, Pitman MB, Rocco JW (2011) Rosai-Dorfman disease presenting in the oropharynx. Head Neck 33(11):1660–1663. https://doi.org/10.1002/hed.21446
Illing EA, Halum SL (2012) Rosai-Dorfman disease with isolated laryngeal involvement. Ear Nose Throat J 91(10):439–440
Di Dier K, Lemmerling M, De Vos G (2022) Nasal and nasopharyngeal Rosai-Dorfman disease. J Belg Soc Radiol 106(1):86. https://doi.org/10.5334/jbsr.2896
Ojha J, Rawal YB, Hornick JL, Magliocca K, Montgomery DR, Foss RD et al (2020) Extra nodal Rosai-Dorfman disease originating in the nasal and paranasal complex and gnathic bones: a systematic analysis of seven cases and review of literature. Head Neck Pathol 14(2):442–453. https://doi.org/10.1007/s12105-019-01056-8
Cardoso CL, Damante JH, Santos PS, Taveira LA, Fernandes LM, Pigatti FM et al (2012) Rosai-Dorfman disease with widespread oral-maxillofacial manifestations: a case report. J Oral Maxillofac Surg 70(11):2600–2604. https://doi.org/10.1016/j.joms.2011.12.015
Alawi F, Robinson BT, Carrasco L (2006) Rosai-Dorfman disease of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102(4):506–512. https://doi.org/10.1016/j.tripleo.2005.10.071
Kademani D, Patel SG, Prasad ML, Huvos AG, Shah JP (2002) Intraoral presentation of Rosai-Dorfman disease: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93(6):699–704. https://doi.org/10.1067/moe.2002.123495
Keskin A, Genç F, Günhan O (2007) Rosai-Dorfman disease involving maxilla: a case report. J Oral Maxillofac Surg 65(12):2563–2568. https://doi.org/10.1016/j.joms.2006.10.003
Jacobsen E, Shanmugam V, Jagannathan J (2017) Rosai-Dorfman disease with activating KRAS mutation - response to cobimetinib. N Engl J Med 377(24):2398–2399. https://doi.org/10.1056/NEJMc1713676
Zak FG (1950) Reticulohistiocytoma (“ganglioneuroma”) of the skin. Br J Dermatol Syph 62(9):351–355. https://doi.org/10.1111/j.1365-2133.1950.tb15475.x
Miettinen M, Fetsch JF (2006) Reticulohistiocytoma (solitary epithelioid histiocytoma): a clinicopathologic and immunohistochemical study of 44 cases. Am J Surg Pathol 30(4):521–528. https://doi.org/10.1097/00000478-200604000-00014
Hoyt BS, Yan S, Linos KD, Momtahen S, Sriharan A, Tran TN et al (2019) BRAF V600E mutations are not an oncogenic driver of solitary xanthogranuloma and reticulohistiocytoma: testing may be useful in screening for Erdheim-Chester disease. Exp Mol Pathol 111:104320. https://doi.org/10.1016/j.yexmp.2019.104320
Bahrani E, Fernandez-Pol S, Wang JY, Aasi SZ, Brown RA, Novoa RA (2020) Reticulohistiocytoma (solitary epithelioid histiocytoma) with mutations in RAF1 and TSC2. J Cutan Patho 47:985–987
Agarwala S, Hafeez F (2021) Letter to editor: characterizing the lack of specificity of the histopathological findings seen in multicentric reticulohistiocytosis and reticulohistiocytoma. Ann Clin Lab Sci 51(6):894–895
Selmi C, Greenspan A, Huntley A, Gershwin ME (2015) Multicentric reticulohistiocytosis: a critical review. Curr Rheumatol Rep 17(6):511. https://doi.org/10.1007/s11926-015-0511-6
Yoshimura Y, Sugihara T, Kishimoto H, Nagaoka S (1987) Multicentric reticulohistiocytosis accompanied by oral and temporomandibular joint manifestations. J Oral Maxillofac Surg 45(1):84–86. https://doi.org/10.1016/0278-2391(87)90094-2
Katz RW, Anderson KF (1988) Multicentric reticulohistiocytosis. Oral Surg Oral Med Oral Pathol 65(6):721–725. https://doi.org/10.1016/0030-4220(88)90017-5
Monda L, Warnke R, Rosai J (1986) A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol 122(3):562–572
Chan JK, Tsang WY, Ng CS, Tang SK, Yu HC, Lee AW (1994) Follicular dendritic cell tumors of the oral cavity. Am J Surg Pathol 18(2):148–157. https://doi.org/10.1097/00000478-199402000-00004
Biddle DA, Ro JY, Yoon GS, Yong Y-WH, Ayala AG, Ordonez NG (2002) Extranodal follicular dendritic cell sarcoma of the head and neck region: three new cases, with a review of the literature. Mod Pathol 15(1):50–58. https://doi.org/10.1038/modpathol.3880489
Li J, Zhou ML, Zhou SH (2015) Clinical and pathological features of head and neck follicular dendritic cell sarcoma. Hematology 20(10):571–583. https://doi.org/10.1179/1607845415y.0000000008
Pang J, Mydlarz WK, Gooi Z, Waters KM, Bishop J, Sciubba JJ et al (2016) Follicular dendritic cell sarcoma of the head and neck: Case report, literature review, and pooled analysis of 97 cases. Head Neck 38(Suppl 1):E2241–E2249. https://doi.org/10.1002/hed.24115
Saygin C, Uzunaslan D, Ozguroglu M, Senocak M, Tuzuner N (2013) Dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol 88(2):253–271. https://doi.org/10.1016/j.critrevonc.2013.05.006
Duan GJ, Wu F, Zhu J, Guo DY, Zhang R, Shen LL et al (2010) Extranodal follicular dendritic cell sarcoma of the pharyngeal region: a potential diagnostic pitfall, with literature review. Am J Clin Pathol 133(1):49–58. https://doi.org/10.1309/ajcp7u8yisbuavnw
Hu T, Wang X, Yu C, Yan J, Zhang X, Li L et al (2013) Follicular dendritic cell sarcoma of the pharyngeal region. Oncol Lett 5(5):1467–1476. https://doi.org/10.3892/ol.2013.1224
Martins MT, Witzel AL, Sugaya NN, Kowalski LP, de Araujo VC (2004) Dendritic cell sarcoma of the oral cavity. Oral Oncol 40(3):341–347. https://doi.org/10.1016/j.oraloncology.2003.08.012
Facchetti F, Lorenzi L (2016) Follicular dendritic cells and related sarcoma. Semin Diagn Pathol 33(5):262–276. https://doi.org/10.1053/j.semdp.2016.05.002
Massoth LR, Hung YP, Ferry JA, Hasserjian RP, Nardi V, Nielsen GP et al (2021) Histiocytic and dendritic cell sarcomas of hematopoietic origin share targetable genomic alterations distinct from follicular dendritic cell sarcoma. Oncologist 26(7):e1263–e1272. https://doi.org/10.1002/onco.13801
Go H, Jeon YK, Huh J, Choi SJ, Choi YD, Cha HJ et al (2014) Frequent detection of BRAF(V600E) mutations in histiocytic and dendritic cell neoplasms. Histopathology 65(2):261–272. https://doi.org/10.1111/his.12416
Allen CE, Merad M, McClain KL (2018) Langerhans-cell histiocytosis. N Engl J Med 379(9):856–868. https://doi.org/10.1056/NEJMra1607548
Bagnasco F, Zimmermann SY, Egeler RM, Nanduri VR, Cammarata B, Donadieu J et al (2022) Langerhans cell histiocytosis and associated malignancies: a retrospective analysis of 270 patients. Eur J Cancer 172:138–145. https://doi.org/10.1016/j.ejca.2022.03.036
Faustino ISP, Fernandes PM, Pontes HAR, Mosqueda-Taylor A, Santos-Silva AR, Vargas PA et al (2021) Langerhans cell histiocytosis in the oral and maxillofacial region: an update. J Oral Pathol Med 50(6):565–571. https://doi.org/10.1111/jop.13207
Chakraborty R, Hampton OA, Shen X, Simko SJ, Shih A, Abhyankar H et al (2014) Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood 124(19):3007–3015. https://doi.org/10.1182/blood-2014-05-577825
AbdullGaffar B, Awadhi F (2020) Oral manifestations of langerhans cell histiocytosis with unusual histomorphologic features. Ann Diagn Pathol 47:151536. https://doi.org/10.1016/j.anndiagpath.2020.151536
Neves-Silva R, Fernandes DT, Fonseca FP, Rebelo Pontes HA, Brasileiro BF, Santos-Silva AR et al (2018) Oral manifestations of langerhans cell histiocytosis: a case series. Spec Care Dentist 38(6):426–433. https://doi.org/10.1111/scd.12330
Bedran NR, Carlos R, de Andrade BAB, Bueno APS, Romañach MJ, Milito CB (2018) Clinicopathological and immunohistochemical study of head and neck langerhans cell histiocytosis from Latin America. Head Neck Pathol 12(4):431–439. https://doi.org/10.1007/s12105-017-0867-1
Howard JE, Masterson L, Dwivedi RC, Jani P (2016) Langerhans cell sarcoma of the head and neck. Crit Rev Oncol Hematol 99:180–188. https://doi.org/10.1016/j.critrevonc.2015.12.017
Salisbury CL, Budnick SD, Li S (2009) T-cell receptor gene rearrangement and CD30 immunoreactivity in traumatic ulcerative granuloma with stromal eosinophilia of the oral cavity. Am J Clin Pathol 132(5):722–727. https://doi.org/10.1309/ajcpx3s5msovvlop
Hirshberg A, Amariglio N, Akrish S, Yahalom R, Rosenbaum H, Okon E et al (2006) Traumatic ulcerative granuloma with stromal eosinophilia: a reactive lesion of the oral mucosa. Am J Clin Pathol 126(4):522–529. https://doi.org/10.1309/afha406gbt0n2y64
Aizic A, Raiser V, Solar I, Aharon Z, Shlomi B, Kaplan I (2019) Traumatic ulcerative granuloma with stromal eosinophilia: CD30 analysis and clonality for T cell receptor gene re-arrangement. Acta Histochem 121(8):151450. https://doi.org/10.1016/j.acthis.2019.151450
Alobeid B, Pan LX, Milligan L, Budel L, Frizzera G (2004) Eosinophil-rich CD30+ lymphoproliferative disorder of the oral mucosa. A form of “traumatic eosinophilic granuloma.” Am J Clin Pathol 121(1):43–50. https://doi.org/10.1309/jqfx-pnd6-dblf-6b9u
Agarwal M, Shenjere P, Blewitt RW, Hall G, Sloan P, Pigadas N et al (2008) CD30-positive T-cell lymphoproliferative disorder of the oral mucosa–an indolent lesion: report of 4 cases. Int J Surg Pathol 16(3):286–290. https://doi.org/10.1177/1066896907313755
Rosenberg A, Blesma DH, Sie-Go DMDS, Slootweg PJ (1996) Primary extranodal CD30-positive T-cell non-Hodgkin’s lymphoma of the oral mucosa: report of two cases. Int J Oral Maxillofac Surg 25(1):57–59. https://doi.org/10.1016/S0901-5027(96)80013-0
Sciallis AP, Law ME, Inwards DJ, McClure RF, Macon WR, Kurtin PJ et al (2012) Mucosal CD30-positive T-cell lymphoproliferations of the head and neck show a clinicopathologic spectrum similar to cutaneous CD30-positive T-cell lymphoproliferative disorders. Mod Pathol 25(7):983–992. https://doi.org/10.1038/modpathol.2012.38
Wang W, Cai Y, Sheng W, Lu H, Li X (2014) The spectrum of primary mucosal CD30-positive T-cell lymphoproliferative disorders of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol 117(1):96–104. https://doi.org/10.1016/j.oooo.2013.10.002
WHO Classification of tumours of haematopoietic and lymphoid tissues (2017). In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, (eds) Revised 4th edn, vol 2
Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES (2010) EBV positive mucocutaneous ulcer–a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol 34(3):405–417. https://doi.org/10.1097/PAS.0b013e3181cf8622
Castro Limo JD, Sierra Bernal CA, Martínez ZE (2021) Epstein-Barr virus-positive mucocutaneous ulcer mimicking sigmoid colon adenocarcinoma. Rev Esp Enferm Dig 113(3):225–226. https://doi.org/10.17235/reed.2020.7272/2020
Prieto-Torres L, Eraña I, Gil-Redondo R, Gómez de la Riva I, Manso R, Pajares R et al (2019) The spectrum of EBV-positive mucocutaneous ulcer: a study of 9 cases. Am J Surg Pathol 43(2):201–210. https://doi.org/10.1097/pas.0000000000001186
Ikeda T, Gion Y, Sakamoto M, Tachibana T, Nishikori A, Nishimura MF et al (2020) Clinicopathological analysis of 34 Japanese patients with EBV-positive mucocutaneous ulcer. Mod Pathol 33(12):2437–2448. https://doi.org/10.1038/s41379-020-0599-8
Attard AA, Praveen P, Dunn PJ, James GJ (2012) Epstein-Barr virus-positive mucocutaneous ulcer of the oral cavity: the importance of having a detailed clinical history to reach a correct diagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol 114(2):e37–e39. https://doi.org/10.1016/j.oooo.2012.04.003
Natkunam Y, Goodlad JR, Chadburn A, de Jong D, Gratzinger D, Chan JK et al (2017) EBV-positive B-cell proliferations of varied malignant potential: 2015 SH/EAHP workshop report-part 1. Am J Clin Pathol 147(2):129–152. https://doi.org/10.1093/ajcp/aqw214
Neiman RS, Barcos M, Berard C, Bonner H, Mann R, Rydell RE et al (1981) Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer 48(6):1426–1437. https://doi.org/10.1002/1097-0142(19810915)48:6%3c1426::aid-cncr2820480626%3e3.0.co;2-g
Zhou J, Bell D, Medeiros LJ (2013) Myeloid sarcoma of the head and neck region. Arch Pathol Lab Med 137(11):1560–1568. https://doi.org/10.5858/arpa.2012-0537-OA
Pau M, Beham-Schmid C, Zemann W, Kahr H, Kärcher H (2010) Intraoral granulocytic sarcoma: a case report and review of the literature. J Oral Maxillofac Surg 68(10):2569–2574. https://doi.org/10.1016/j.joms.2009.09.040
Abbas HA, Reville PK, Geppner A, Rausch CR, Pemmaraju N, Ohanian M et al (2021) Clinical and molecular characterization of myeloid sarcoma without medullary leukemia. Leuk Lymphoma 62(14):3402–3410. https://doi.org/10.1080/10428194.2021.1961235
Papamanthos MK, Kolokotronis AE, Skulakis HE, Fericean AM, Zorba MT, Matiakis AT (2010) Acute myeloid leukaemia diagnosed by intra-oral myeloid sarcoma. A case report Head Neck Pathol 4(2):132–135. https://doi.org/10.1007/s12105-010-0163-9
Xie Z, Zhang F, Song E, Ge W, Zhu F, Ja Hu (2007) Intraoral granulocytic sarcoma presenting as multiple maxillary and mandibular masses: a case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103(6):e44–e48. https://doi.org/10.1016/j.tripleo.2006.12.028
Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M et al (2007) Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 21(2):340–350. https://doi.org/10.1038/sj.leu.2404491
Wilson CS, Medeiros LJ (2015) Extramedullary manifestations of myeloid neoplasms. Am J Clin Pathol 144(2):219–239. https://doi.org/10.1309/ajcpo58ywibubesx
Amador-Ortiz C, Hurley M, Ghahramani G, Frisch S, Klco J, Lind A et al (2011) Use of classic and novel immunohistochemical markers in the diagnosis of cutaneous myeloid sarcoma. J Cutan Pathol 38:945–953. https://doi.org/10.1111/j.1600-0560.2011.01809.x
Roth MJ, Medeiros LJ, Elenitoba-Johnson K, Kuchnio M, Jaffe ES, Stetler-Stevenson M (1995) Extramedullary myeloid cell tumors. An immunohistochemical study of 29 cases using routinely fixed and processed paraffin-embedded tissue sections. Arch Pathol Lab Med 119(9):790–798
Zhou T, Bloomquist MS, Ferguson LS, Reuther J, Marcogliese AN, Elghetany MT et al (2020) Pediatric myeloid sarcoma: a single institution clinicopathologic and molecular analysis. Pediatr Hematol Oncol 37(1):76–89. https://doi.org/10.1080/08880018.2019.1683107
Begna KH, Kittur J, Yui J, Gangat N, Patnaik MM, Al-Kali A et al (2021) De novo isolated myeloid sarcoma: comparative analysis of survival in 19 consecutive cases. Br J Haematol 195(3):413–416. https://doi.org/10.1111/bjh.17742
Tsimberidou AM, Kantarjian HM, Wen S, Keating MJ, O’Brien S, Brandt M et al (2008) Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer 113(6):1370–1378. https://doi.org/10.1002/cncr.23691
Li Y, Sun V, Sun W, Pawlowska A (2020) Blastic plasmacytoid dendritic cell neoplasm in children. Hematol Oncol Clin North Am 34(3):601–612. https://doi.org/10.1016/j.hoc.2020.01.008
Julia F, Petrella T, Beylot-Barry M, Bagot M, Lipsker D, Machet L et al (2013) Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol 169(3):579–586. https://doi.org/10.1111/bjd.12412
Hashikawa K, Niino D, Yasumoto S, Nakama T, Kiyasu J, Sato K et al (2012) Clinicopathological features and prognostic significance of CXCL12 in blastic plasmacytoid dendritic cell neoplasm. J Am Acad Dermatol 66(2):278–291. https://doi.org/10.1016/j.jaad.2010.12.043
Pemmaraju N, Wilson NR, Khoury JD, Jain N, Daver N, Pierce S et al (2021) Central nervous system involvement in blastic plasmacytoid dendritic cell neoplasm. Blood 138(15):1373–1377. https://doi.org/10.1182/blood.2021011817
Khanlari M, Yin CC, Takahashi K, Lachowiez C, Tang G, Loghavi S et al (2022) Bone marrow clonal hematopoiesis is highly prevalent in blastic plasmacytoid dendritic cell neoplasm and frequently sharing a clonal origin in elderly patients. Leukemia 36(5):1343–1350. https://doi.org/10.1038/s41375-022-01538-9
Khoury JD (2018) Blastic plasmacytoid dendritic cell neoplasm. Curr Hematol Malig Rep 13(6):477–483. https://doi.org/10.1007/s11899-018-0489-z
Sapienza MR, Fuligni F, Agostinelli C, Tripodo C, Righi S, Laginestra MA et al (2014) Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia 28(8):1606–1616. https://doi.org/10.1038/leu.2014.64
Feuillard J, Jacob M-C, Valensi F, Maynadié M, Gressin R, Chaperot L et al (2002) Clinical and biologic features of CD4+CD56+ malignancies. Blood 99(5):1556–1563. https://doi.org/10.1182/blood.V99.5.1556
Herling M, Teitell MA, Shen RR, Medeiros LJ, Jones D (2003) TCL1 expression in plasmacytoid dendritic cells (DC2s) and the related CD4+ CD56+ blastic tumors of skin. Blood 101(12):5007–5009. https://doi.org/10.1182/blood-2002-10-3297
Sukswai N, Aung PP, Yin CC, Li S, Wang W, Wang SA et al (2019) Dual expression of TCF4 and CD123 is highly sensitive and specific for blastic plasmacytoid dendritic cell neoplasm. Am J Surg Pathol 43(10):1429–1437. https://doi.org/10.1097/pas.0000000000001316
Marafioti T, Paterson JC, Ballabio E, Reichard KK, Tedoldi S, Hollowood K et al (2008) Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood 111(7):3778–3792. https://doi.org/10.1182/blood-2007-10-117531
Wang W, Khoury JD, Miranda RN, Jorgensen JL, Xu J, Loghavi S et al (2021) Immunophenotypic characterization of reactive and neoplastic plasmacytoid dendritic cells permits establishment of a 10-color flow cytometric panel for initial workup and residual disease evaluation of blastic plasmacytoid dendritic cell neoplasm. Haematologica 106(4):1047–1055
Tang Z, Tang G, Wang SA, Lu X, Young KH, Bueso-Ramos CE et al (2016) Simultaneous deletion of 3’ETV6 and 5’EWSR1 genes in blastic plasmacytoid dendritic cell neoplasm: case report and literature review. Mol Cytogenet 9:23. https://doi.org/10.1186/s13039-016-0232-1
Sakamoto K, Katayama R, Asaka R, Sakata S, Baba S, Nakasone H et al (2018) Recurrent 8q24 rearrangement in blastic plasmacytoid dendritic cell neoplasm: association with immunoblastoid cytomorphology, MYC expression, and drug response. Leukemia 32(12):2590–2603. https://doi.org/10.1038/s41375-018-0154-5
Sapienza MR, Abate F, Melle F, Orecchioni S, Fuligni F, Etebari M et al (2019) Blastic plasmacytoid dendritic cell neoplasm: genomics mark epigenetic dysregulation as a primary therapeutic target. Haematologica 104(4):729–737. https://doi.org/10.3324/haematol.2018.202093
Pemmaraju N, Lane AA, Sweet KL, Stein AS, Vasu S, Blum W et al (2019) Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med 380(17):1628–1637. https://doi.org/10.1056/NEJMoa1815105
Corti M, Minué G, Campitelli A, Narbaitz M, Gilardi L (2015) An aggressive plasmablastic lymphoma of the oral cavity as primary manifestation of acquired immunodeficiency syndrome: case report and literature review. Int Arch Otorhinolaryngol. 19(4):354–358. https://doi.org/10.1055/s-0034-1397335
Vale DAD, Rogado CM, Carvalho DLC, Trierveiler M, Ortega KL (2017) Oral plasmablastic lymphoma as the first manifestation of AIDS. An Bras Dermatol 92(5 Suppl 1):110–112. https://doi.org/10.1590/abd1806-4841.20175417
Zizzo M, Zanelli M, Martiniani R, Sanguedolce F, De Marco L, Martino G et al (2020) Oral plasmablastic lymphoma: a case report. Medicine (Baltimore) 99(39):e22335. https://doi.org/10.1097/md.0000000000022335
Bailly J, Jenkins N, Chetty D, Mohamed Z, Verburgh ER, Opie JJ (2022) Plasmablastic lymphoma: an update. Int J Lab Hematol 44(Suppl 1):54–63. https://doi.org/10.1111/ijlh.13863
Lin L, Zhang X, Dong M, Li L, Wang X, Zhang L et al (2017) Human immunodeficiency virus-negative plasmablastic lymphoma: a case report and literature review. Medicine (Baltimore) 96(7):e6171. https://doi.org/10.1097/md.0000000000006171
Bagul N, Mamatha GS, Mahalle A (2012) Plasmablastic lymphoma of gingiva mimicking a reactive lesion: a case report. Case Rep Dent 2012:259307. https://doi.org/10.1155/2012/259307
Hansra D, Montague N, Stefanovic A, Akunyili I, Harzand A, Natkunam Y et al (2010) Oral and extraoral plasmablastic lymphoma: similarities and differences in clinicopathologic characteristics. Am J Clin Pathol 134(5):710–719. https://doi.org/10.1309/ajcpjh6keusecqlu
Liu M, Liu B, Liu B, Wang Q, Ding L, Xia C et al (2015) Human immunodeficiency virus-negative plasmablastic lymphoma: a comprehensive analysis of 114 cases. Oncol Rep 33(4):1615–1620. https://doi.org/10.3892/or.2015.3808
Yonal-Hindilerden I, Hindilerden F, Arslan S, Turan-Guzel N, Dogan IO, Nalcaci M (2016) Primary B-cell mucosa-associated lymphoid tissue lymphoma of the hard palate and parotid gland: report of one case and review of the literature. J Clin Med Res 8(11):824–830. https://doi.org/10.14740/jocmr2733w
Iversen L, Eriksen PRG, Andreasen S, Clasen-Linde E, Homøe P, Wessel I et al (2021) Lymphoma of the uvula: clinical, morphological, histopathological, and genetic characterization. A nationwide Danish study from 1980 to 2019. Front Surg 8:675279. https://doi.org/10.3389/fsurg.2021.675279
Ma S, Jug R, Shen S, Zhang WL, Xu HT, Yang LH (2018) Marginal zone lymphoma of palatine tonsil with prominent plasmacytic differentiation: A CARE-compliant article and review of literature. Medicine (Baltimore) 97(2):e9648. https://doi.org/10.1097/md.0000000000009648
Khalil MO, Morton LM, Devesa SS, Check DP, Curtis RE, Weisenburger DD et al (2014) Incidence of marginal zone lymphoma in the United States, 2001–2009 with a focus on primary anatomic site. Br J Haematol 165(1):67–77. https://doi.org/10.1111/bjh.12730
Tanaka T, Kitabatake K, Iino M, Goto K (2011) Immunohistochemical comparison of CD5, lambda, and kappa expression in primary and recurrent buccal mucosa-associated lymphoid tissue (MALT) lymphomas. Diagn Pathol 6:82. https://doi.org/10.1186/1746-1596-6-82
Alderuccio JP, Florindez JA, Reis IM, Zhao W, Lossos IS (2021) Treatments and outcomes in stage I extranodal marginal zone lymphoma in the United States. Cancers (Basel). https://doi.org/10.3390/cancers13081803
Acknowledgements
The views expressed by the authors are theirs and do not reflect the official policy of the Army/Navy/Air Force, Department of Defense, or U.S. Government.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study informed consent was not required.
Consent for Publication
For this type of study consent for publication was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hyrcza, M.D., Lindenmuth, T.R. & Auerbach, A. Top Ten Lymphoproliferative Lesions Not to Miss When Evaluating Oral Ulcer Biopsies. Head and Neck Pathol 17, 99–118 (2023). https://doi.org/10.1007/s12105-023-01532-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-023-01532-2