Abstract
Diffuse large B cell lymphoma, not otherwise specified (DLBCL, NOS) is the most frequent non-Hodgkin lymphoma subtype. This aggressive neoplasm may variably express the CD30 protein, which may be used as a therapeutic target for this tumor. However, CD30 expression in DLBCL NOS arising from the oral cavity and the oropharynx has not been investigated. Therefore, this study aims to determine the frequency of CD30 expression and its prognostic significance for patients affected by oral/oropharyngeal DLBCL NOS. Fifty cases were retrieved from pathology files and submitted to immunohistochemistry against CD30. Reactivity was accessed by two oral pathologists using two cut-off values (> 0% and > 20% of tumor cells) to determine positivity in each case. Clinical data were obtained from the patients’ medical files to investigate the prognostic potential of the protein. Seven high-grade B cell lymphomas and two EBV-positive DLBCL NOS were identified. We found one CD30-positive case in each of these two groups of lymphomas. Among the remaining 41 DLBCL NOS, other four cases (three in the oral cavity and one in the oropharynx) were positive for CD30, but only two expressed the protein in > 20% of tumor cells, both in the oral cavity. Survival analysis demonstrated that CD30-positive cases had a higher five-year overall survival rate (75%) than CD30-negative cases (32.3%), although a statistically significant result was not achieved (p = 0.19). Only a minor subset of oral and oropharyngeal DLBCL NOS express CD30 and these patients seems to have a higher survival rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the International Agency for Research on Cancer approximately 18.1 million new cancer cases were estimated for 2018, with approximately 9.6 million cancer deaths worldwide. Non-Hodgkin lymphomas represent one of the most common human cancers accounting for 509,590 (2.8%) cases and 248,724 deaths (2.6%) [1]. Diffuse large B cell lymphoma, not otherwise specified (DLBCL NOS) is the most common microscopic subtype, which carries a complex prognosis and molecular background that may also significantly differ according to the primary anatomical location affected [2, 3]. Although it more often affects the lymph nodes, over 40% of the cases may develop in extranodal sites, usually the gastrointestinal tract [2, 4]. In the oral and oropharyngeal region, DLBCL NOS is also the most frequent lymphoma subtype, representing 75% of the lymphomas involving the Waldeyer’s ring area [5] and ranging from 37.3% to 67% in the oral cavity [6, 7], and these patients still carry a poor prognosis [8]. Therefore, investigating biomarkers to better understand the pathogenesis of primary oral/oropharyngeal cases and that could be used as therapeutic targets are desired to improve the clinical management of these individuals.
The transmembrane protein CD30 is a molecule that has been used for the treatment of different types of lymphoproliferative disorders through the commercially available monoclonal antibody Brentuximab vedotin. This 120 kDa weighted protein was originally described in 1982 and belongs to the tumor necrosis factor receptor superfamily [9]. In addition to a small subset of B and T lymphocytes, CD30 is often expressed by Reed-Sternberg cells, being ordinarily found in Hodgkin lymphoma and anaplastic large cell lymphoma, while it is variably expressed in DLBCL more frequently as focal stains, although some cases may demonstrate stronger and diffuse membrane reactivity [10, 11]. CD30 ligation leads to nuclear factor-kappa B (NFκB) and/or mitogen-activated protein kinase (MAPK) pathways, ultimately leading to survival of neoplastic cells [12].
Several studies have evaluated the frequency, clinicopathological and prognostic potential of CD30 in DLBCL NOS [13,14,15] and we have recently integrated these data into a systematic review with meta-analysis demonstrating that its expression is associated with a higher survival rate [16]. However, previous studies did not attempt to investigate possible differences in CD30 expression according to primary lymphoma sites and most authors only compared a lower versus a higher extranodal involvement, therefore, the expression pattern of this protein and its clinical significance for patients affected by oral and oropharyngeal DLBCL NOS remains unknown. With a null hypothesis that CD30 expression is not significantly associated with the prognosis of DLCBCL NOS affecting these locations, the aim of this study is to determine the prognostic potential of CD30 expression in oral and oropharyngeal DLBCL NOS.
Material and Methods
This study was approved by the Ethical Committee of the Piracicaba Dental School, University of Campinas, Piracicaba, Brazil (process no. 44647421.1.0000.5418). All procedures followed the ethical standards of the Helsinki Declaration of 1975, as revised in 2008.
Study Sample
Fifty cases diagnosed as oral and oropharyngeal DLBCL, NOS between January 2001 and December 2021 were obtained from the pathology files of seven Brazilian institutions [Piracicaba Dental School of the University of Campinas (Piracicaba); School of Dentistry of the Universidade Federal de Minas Gerais (Belo Horizonte); Oral Pathology Service of the João de Barros Barreto University Hospital (Belém); Federal University of Rio Grande do Sul (Porto Alegre); Dental School of the Rio de Janeiro State University (Rio de Janeiro) and Private Pathology Service (Natal)]. Formalin-fixed, paraffin-embedded tissues (FFPE) were obtained and diagnostic confirmation was done following the current WHO Classification of Lymphoid Neoplasms [4]. The clinicopathological features retrieved included age, sex, tumour location, treatment employed, status at last follow-up (dead or alive), and follow-up period. The overall survival rate was defined as the time from the date of diagnosis to the date of the patients’ last follow-up or death.
Immunohistochemistry
Immunohistochemical reactions done for diagnosis confirmation were detailed described previously [17]. To investigate CD30 expression, reactions were carried out in 3 µm sections of FFPE tissues that were dewaxed with xylene and hydrated in a descending ethanol series. The endogenous peroxidase activity was blocked with 10% hydrogen peroxide in a single bath for 15 min. After washing in PBS buffer (pH 7.4), the sections were incubated for 2 h with primary antibody (anti-human mouse monoclonal CD30, clone Ber-H2, diluton 1:200, DAKO cytomation, Carpendia, CA, USA) and then exposed to high-sensitive horseradish peroxidase reagents (ADVANCE, Dako, Capinteria, CA, USA) and diaminobenzidine tetrahydrochloride (DAB, Sigma-Aldrich, St Louis, MO, USA). The slides were counterstained with Carazzi haematoxylin for 3 min. Histological sections of normal tonsils were used as positive control, while the negative control was acquired by omitting the specific primary antibody. Two observers jointly evaluated the immunohistochemical reactions that were descriptively evaluated according to the cellular compartment and cellular population expressing CD30 protein and used the cut-off values of > 0% and > 20% of neoplastic cells expressing CD30 to determine positivity for this marker. Using both cut-off values will allow that our results are compared with previous studies.
In situ Hybridization (ISH) and Fluorescence in situ Hybridization (FISH)
All cases were submitted to ISH to detect EBV as described previously [17] using a fluorescein-labelled peptide nucleic acid probe (PNA) complementary to 2 nuclear encoded RNAs (EBER) (Y5200, Dako, Glostrup, Denmark), while to detect MYC, BCL2 and BLC6 genes’ translocations, FISH analysis was performed in 41 cases using a dual-colour break-apart probes (MYC/8q24 and BCL6/3q27) and dual-colour fusion translocation probes (BCL2/18q21) (Abbott Laboratories, Des Plaines, IL). Analysis was done using an Olympus BX 41fluorescence microscope and using Case Data Manager 5.0 program 100 cells/case were randomly counted regarding the probe signals in each allele of the gene investigated.
Statistical Analysis
Fisher’s exact test was used to investigate the association between CD30 protein and clinicopathological features. Kaplan–Meier method was used to calculate survival curves, whereas differences between the curves were investigated using the Log-Rank univariate test to identify potential prognostic factors. Because the number of CD30 positive cases was limited, we did not investigate the prognostic importance of this marker using a multivariate model. The software SPSS version 22.0 was used, and a p-value ≤ 0.05 was considered statistically significant.
Results
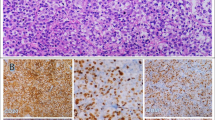
Most of the cases used in this analysis (49/50 cases) were obtained from our previous study on DLBCL and high-grade B cell lymphoma (HGBL) [17]. The oral/oropharyngeal tumours were the only manifestations of the disease in the moment of the patients’ initial clinical examination and no patient had received any type of treatment. Seven cases represented HGBL (four double-hit and three triple-hit) and one of these cases positively stained for CD30 in more than 20% of the neoplastic cells (Fig. 1A-B). Another two cases were EBV-positive DLBCL NOS and one of these cases also diffusely stained for CD30 protein (Fig. 1C-D). Considering all 50 cases (41 DLBCL NOS, 7 HGBL and 2 EBV-positive DLBCL NOS) initially investigated, we found no statistically significant correlation between CD30 expression with clinicopathological parameters and the overall survival (p > 0.05); however, we observed a clear difference between the survival curves of CD30-positive and CD30-negative patients, with a higher survival of cases expressing the protein, using both cut-off values (Fig. 2A-C).
Histologic and immunohistochemical aspects of the six cases that stained positively for CD30 in the current study. A A HGBL (triple hit) demonstrating large and median sized neoplastic cells. B Diffuse staining of CD30 in > 20% of the neoplastic cells, although only a faint staining was observed. C An EBV + DLBCL NOS composed by large neoplastic B cells. D CD30 positivity was high and stained more than 20% of the tumor cells. E Oral DLBCL NOS (BCL2 neg., BCL6 neg. and MYC neg.) showing a diffuse growth pattern of neoplastic large B cells. F Less than 20% of tumor cells were faintly positive for CD30. G Oropharyngeal DLBCL NOS (BCL2 neg., BCL6 neg. and MYC neg.) containing large neoplastic cells and areas of necrosis and atypical mitotic figures. H This oropharyngeal DLBCL NOS was the only positive for CD30, but in only scattered neoplastic cells. I Oral DLBCL NOS (BCL2 neg., BCL6 neg. and MYC neg.) showing large neoplastic cells with one of more evident nucleoli and atypical mitotic figures. J CD30 was found in > 20% of neoplastic cells, although the staining intensity was weak in the majority of the neoplasm. K Oral DLBCL NOS (BCL2 not evaluated, BCL6 not evaluated and MYC not evaluated) showing a diffuse growth of neoplastic B cells. L CD30 strongly and diffusely stained > 20% of the tumor cells
Survival analyses of the sample investigated and the prognostic impact of CD30 expression using Log-rank univariate analysis. A Overall survival curve obtained for the whole sample (DLBCL NOS, HGBL and EBV + DLBCL NOS) demonstrating a five-year survival of 35.8%. B Univariate analysis showed that CD30-positive patients had five-year old overall survival rate of 60%, while CD30-negative patients was 32.3%. However, tis difference was not statistically significant (p = 0.28). C Investigating the prognostic impact of CD30 expression higher than 20% it was not found a statistically significant result either (p = 0.32), but the CD30-positive patients also showed a better prognosis (five-year survival 66.7 vs 32.3%, respectively). D Evaluating the overall survival of the DLBCL NOS cases only, we found a five-year survival rate of 38.2%. E The expression of CD30 in this group was not significantly associated with a higher survival (p = 0.19), but CD30-positive cases had a higher five-year survival (75%) than CD30-negative cases (32.3%). F A similar result was found when CD30 positivity in > 20% of the tumor cells was considered, although significance could not be achieved (p = 0.17) (CD30-positive 100% vs CD30-negative 32.3%)
Regarding only the 41 DLBCL NOS cases, the detailed clinicopathological data of these patients is available in Table 1 and Table 2. Briefly, most of the cases affected the oral cavity (31 cases) with an equal sex distribution (21 females:20 males) and 61% of the patients older than 60 years. Six patients were treated with CHOP regimen, four with R-CHOP and 10 with other approaches, while 21 patients did not have available data. Follow-up period was available for 22 cases, achieving a mean of 20.6 months (range from 1 to 83 months), whereas outcome was available for 24 patients (10 individuals alive and 14 dead). Another four CD30-positive cases (two cases > 20%) were found in this series of 41 DLBCL, NOS (9.8%). Three affected the oral cavity (9.6%) (two stained > 20% and one stained > 0% of tumor cells) and one affected the oropharynx (10%) (stained > 0% of tumor cells) (Fig. 1E-L). None of the four cases expressed CD10, three were positive for Bcl6, MUM1 and Bcl2 each, and two were positive for c-Myc. Two of the four cases exhibited simultaneous c-Myc and Bcl2 immunoexpression. Three CD30-positive cases were classified as non-GCB DLBCL NOS. Two cases exhibited a Ki67 staining higher than the mean value and just one case showed a BCL2 translocation, whereas BCL6 and MYC translocations were not found in any of the four CD30-positive cases (Table 3). We found no statistically significant association between CD30 expression and the clinicopathological variables investigated (Table 4). Patients expressing CD30 had a five-year overall survival rate of 75%, while CD30-negative patients achieved 32.3%, although there is a clear difference between both groups, a statistical significance was not achieved (Fig. 2D-F), using both cut-off values (p = 0.19 and p = 0.17, respectively) (Table 5). See Fig. 3
Schematic representation of CD30 signals. The presence of the ligand that activates CD30 in the cell membrane can lead to the activation of the MAPK pathway and its components, culminating in the activation of the NF-κB gene in the neoplastic cell nucleus (left). Activation of tumor necrosis factor receptor-associated factor TRAF2 and TRAF5 in tumor necrosis factor receptor TNFR1 signaling, leads to the recruitment and activation of a series of factors. This pathway leads to IKK activation through IKKβ phosphorylation, allowing the translocation of NF-κB to the nucleus, favoring cell survival (central). Both lead to activation of the nuclear factor-kappa B, which triggers the transcription of anti-apoptotic factors, leading to cell proliferation and/or differentiation. Thus, activation of CD30 promotes neoplastic development in a series of human neoplasms. Due to its biological importance, the use of Brentuximab vedotin, an antibody–drug conjugate, releases into the cell anti-microtubule agent monomethyl auristatin E (MMAE), which induces cell cycle suspension and consequent apoptosis (right)
Discussion
The use of CD30 as a molecular target for treating HL and ALCL is well established [18], while its expression has been associated with a better outcome for patients affected by DLBCL, although the therapeutic efficiency of Brentuximab vedotin remains to be validated to these individuals [13, 14]. Moreover, the molecular basis of DLBCL may differ depending on the primary site affected, which is more pronounced when immune-privileged sites are compared with other lymphoid tissues [19]. The expression pattern of CD30 has not been detailed described in DLBCL arising from specific extranodal regions, being usually investigated in nodal vs extranodal diseases. In the current study, we observed that CD30 expression in oral and oropharyngeal DLBCL NOS is very uncommon, but patients expressing this protein had a clear trend towards a better outcome.
CD30 was first described in 1982 in Germany and latter demonstrated to be a transmembrane glycoprotein receptor with intracellular, transmembrane and extracellular domains [9]. It is considered a member of the tumor necrosis factor receptor super family 8 and its ligand (CD30L, TNFSF8 or CD153) is a membrane bound cytokine, while a soluble CD30 is also known [12]. CD30 activates several intracellular molecular pathways, especially NFkB and MAPK pathways, leading to anti-apoptotic and pro-survival features in human neoplasms [9, 12]. In addition to HL and ALCL, CD30 is frequently found in other lymphoma subtypes and in several lymphoproliferative disorders, significantly associated with the prognosis of these diseases, whereas it is only uncommonly found in normal B, T and NK lymphocytes, becoming an attractive therapeutic biomarker [18].
Brentuximab vedotin is an antibody–drug conjugate (ADC) that combines a chimeric anti-CD30 antibody to the anti-microtubule agent monomethyl auristatin E (MMAE) approved by the Food and Drug Administration for treating relapsed and refractory HL and systemic ALCL [11, 18]. Upon binding to CD30 receptor, the ADC undergoes endocytosis and is degraded in lysosomes, leading to MMAE binding to microtubules, causing cell cycle arrest and apoptosis, with related toxicities mostly of grade 1 or 2 [18].
Although the most effective tumor concentration of CD30 for the most effective activity of Brentuximab vedotin is not clear, it is known that even very lower immunoexpression patterns are associated with some therapeutic response [20]. Of note, MMAE may have an antineoplastic effect also on surrounding cells due to its diffusion capacity into the nearby stroma, which would be beneficial for those cases where tumor cells do not properly internalize the ADC [18]. Therefore, the search for the most appropriate cut-off value to determine positivity for CD30 has been debated and Xu et al. (2020) [12] recently discussed a practical approach for reporting CD30 detection, but it still represents a major pitfall since studies use different parameters (> 0%, > 20%, > 40% for example), although the expression of > 20% of tumor cells is usually the most commonly applied. Therefore, depending on what cut-off is used, either > 0% or > 20% of tumor cells, the frequency of CD30 expression ranges from 3.5% to 59.1% and 2.5% to 36.7%, respectively [17]. In our sample, only two cases were positive in > 20% of DLBCL NOS cells, representing 4.9% of the cases (2 out of 41 cases), with none of the 10 oropharyngeal cases expressing the protein in > 20% of the cells, demonstrating the rare expression of this receptor in oral/oropharyngeal DLBCL NOS.
A recent systematic review investigated CD30 expression across primary cutaneous lymphomas, showing the variability of its expression and its association with better outcomes in many studies [9]. Travaglino et al. (2021) [21] has also showed a better outcome for transformed Mycosis fungoid. Similarly, we have recently integrated the prognostic significance of CD30 expression in DLBCL in a systematic review, which also demonstrated CD30 to be significantly associated with a higher survival [16]. However, the studies included in our systematic review did not attempt to investigate the protein expression in DLBCL developing in specific locations. In the current study, evaluating only oral/oropharyngeal DLBCL NOS, CD30 expression was not significantly associated with the overall survival, but there was a clear trend towards significance of a higher survival, which could be achieved if the sample size were increased, as also reported by Salas et al. (2020) [15] in DLBCL and by Kawamoto et al. (2017) [10] in extranodal NK/T cell lymphomas.
A small subset of DLBCL may be associated with the presence of EBV, and in these cases CD30 seems to be more frequently expressed, as demonstrated by Ok et al. (2014) [22] that also observed the combination of CD30 and EBV to be associated with a worse prognosis for DLBCL. Moreover, Salas et al. (2020) [15] observed CD30 expression in 71% of their cases, although the reasons for this higher expression of CD30 in the presence of EBV remains unknown. In our series, out of the six positive cases initially observed, one of them represented an EBV-positive DLBCL, while another EBV-positive case was negative for CD30. We have also observed one CD30-positive case being reclassified as a HGBL, which represents a very aggressive subset of lymphomas characterized by mutations in MYC and BCL2 and/or BCL6 genes. Zhang et al. (2020) [23] recently reported four positive cases out of 28 double-hit (MYC/BCL6) HGBL, suggesting that this aberrant expression together with other parameters could contribute for diagnostic accuracy of this uncommon subset of high-grade lymphomas. Two of our four CD30-positive DLBCL NOS exhibited simultaneous expression of c-Myc and Bcl2 proteins, which characterizes double-expressor DLBCL NOS and is associated with an inferior survival [4]. Moreover, cell of origin classification of DLBCL NOS based on Hans algorithm has been used in diagnostic practice with a prognostic purpose, despite its limited reproducibility and accuracy. GCB lymphomas are associated with a better outcome, whereas non-GCB cases usually demonstrate a lower survival. In our series, we had a similar distribution of GCB and non-GCB DLBCL NOS, while three of our four CD30-positive cases were classified as non-GCB lymphomas.
Although we used a relatively large number of cases specifically affecting the oral cavity and the oropharynx, it still represents a statistically small sample size that precluded stronger results and multivariate analyses, and future studies may benefit from the use of larger multi-institutional samples. Some clinical data with prognostic value like IPI, ECOG, bone marrow involvement and B-symptoms were not available for consultation and would be important if a multivariate analysis was intended. Moreover, although the oral/oropharyngeal tumors were the only clinical manifestation of the disease in the moment of their diagnoses, a more detailed clinical, imaging and laboratorial examination would be necessary to confirm that there were no other manifestations outside the head and neck.
In conclusion, we demonstrated that only a minor subset of oral and oropharyngeal DLBCL NOS express CD30 protein, what is even more rarely found if a cut-off value of > 20% is used. Moreover, we further demonstrated that patients expressing CD30 have a higher survival rate, although we did not achieve statistical significance in the current sample.
Data Availability
The manuscript has no further associated data in a data repository.
Code Availability
Not applicable.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Nagakita K, Takata K, Taniguchi K, Miyata-Takata T, Sato Y, Tari A, et al. Clinicopathological features of 49 primary gastrointestinal diffuse large B-cell lymphoma cases; comparison with location, cell-of-origin, and frequency of MYD88 L265P. Pathol Int. 2016;66(8):444–52. https://doi.org/10.1111/pin.12439.
Lontos K, Tsagianni A, Yuan JM, Normolle D, Boyiadzis M, Hou JZ, et al. Location matters in early stage nodal diffuse large B-cell lymphoma. Leuk Lymphoma. 2019;60:250–3. https://doi.org/10.1080/10428194.2018.1471600.
Swerdlow SH, Campo E, Harris NL 2017 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th, editor
Vega F, Lin P, Medeiros LJ. Extranodal lymphomas of the head and neck. Ann Diagn Pathol. 2005;9(6):340–50. https://doi.org/10.1016/j.anndiagpath.2005.09.020.
de Arruda JAA, Schuch LF, Conte Neto N, de Souza LL, Rodrigues-Fernandes CI, Abreu LG, et al. Oral and oropharyngeal lymphomas: a multi-institutional collaborative study. J Oral Pathol Med. 2021;50(6):603–12. https://doi.org/10.1111/jop.13211.
Solomides CC, Miller AS, Christman RA, Talwar J, Simpkins H. Lymphomas of the oral cavity: histology, immunologic type, and incidence of Epstein-Barr virus infection. Hum Pathol. 2002;33(2):153–7. https://doi.org/10.1053/hupa.2002.30721.
Rodrigues-Fernandes CI, de Souza LL, Santos-Costa SFD, Pontes HAR, de Almeida OP, Vargas PA, et al. Clinicopathological analysis of oral diffuse large B-cell lymphoma, NOS: a systematic review. J Oral Pathol Med. 2019;48(3):185–91. https://doi.org/10.1111/jop.12802.
Kampa F, Mitteldorf C. A review of CD30 expression in cutaneous neoplasms. J Cutan Pathol. 2021;48:495–510. https://doi.org/10.1111/cup.13894.
Kawamoto K, Miyoshi H, Suzuki T, Sasaki Y, Yamada K, Yanagida E, et al. Frequent expression of CD30 in extranodal NK/T-cell lymphoma: potential therapeutic target for anti-CD30 antibody-based therapy. Hematol Oncol. 2018;36:166–73. https://doi.org/10.1002/hon.2482.
Horwitz S, O’Connor OA, Pro B, Illidge T, Fanale M, Advani R, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229–40. https://doi.org/10.1016/S0140-6736(18)32984-2.
Xu ML, Gabali A, His ED, Fedoriw Y, Vij K, Salama ME, et al. Practical approaches on CD30 detection and reporting in lymphoma diagnosis. Am J Surg Pathol. 2020;44(2):e1-14. https://doi.org/10.1097/PAS.0000000000001368.
Hu S, Xu-Monette ZY, Balasubramanyam A, Manyam GC, Visco C, Tzankov A, et al. CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: a report from the International DLBCL Rituximab-CHOP consortium program study. Blood. 2013;121(14):2715–24. https://doi.org/10.1182/blood-2012-10-461848.
Gong QX, Wang Z, Liu C, Li X, Lu TX, Liang JH, et al. CD30 expression and its correlation with MYC and BCL2 in de novo diffuse large B-cell lymphoma. J Clin Pathol. 2018;71(9):795–801. https://doi.org/10.1136/jclinpath-2018-205039.
Salas MQ, Climent F, Tapia G, Domènech ED, Mercadal S, Oliveira AC, et al. Clinicopathologic features and prognostic significance of CD30 expression in de novo diffuse large B-cell lymphoma (DLBCL): results in a homogeneous series from a single institution. Biomarkers. 2020;25(1):69–75. https://doi.org/10.1080/1354750X.2019.1691656.
Rodrigues-Fernandes CI, Abreu LG, Radhakrishnan R, Perez DEC, Amaral-Silva GK, Gondak RO, et al. Prognostic significance of CD30 expression in diffuse large B cell lymphoma: a systematic review with meta-analysis. J Oral Pathol Med. 2021;50(6):587–93. https://doi.org/10.1111/jop.13208.
Rodrigues-Fernandes CI, Junior AG, Soares CD, Morais TML, do Amaral-Silva GK, de Carvalho MGF, et al. Oral and oropharyngeal diffuse large B-cell lymphoma and high-grade B-cell lymphoma: a clinicopathologic and prognostic study of 69 cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(4):452–62. https://doi.org/10.1016/j.oooo.2020.11.005.
Minich SS. Brentuximab Vedotin: a new age in the treatment of Hodgkin lymphoma and anaplastic large cell lymphoma. Ann Pharmacother. 2012;46:377–83. https://doi.org/10.1345/aph.1Q680.
Twa DDW, Mottok A, Savage KJ, Steidl C. The pathobiology of primary testicular diffuse large B-cell lymphoma: implications for novel therapies. Blood Rev. 2018;32:249–55. https://doi.org/10.1016/j.blre.2017.12.001.
Bartlett NL, Smith MR, Siddiqi T, Advani RH, O’Connor OA, Sharman JP, et al. Brentuximab vedotin activity in diffuse large B-cell lymphoma with CD30 undetectable by visual assessment of conventional immunohistochemistry. Leuk Lymphoma. 2017;58(7):1607–16. https://doi.org/10.1080/10428194.2016.1256481.
Travaglino A, Russo D, Varricchio S, Pignatiello S, Baldo A, Picardi M, et al. Prognostic significance of CD30 in transformed mycosis fungoides. Am J Clin Pathol. 2021. https://doi.org/10.1093/ajcp/aqaa261.
Ok CY, Li L, Xu-Monette ZY, Visco C, Tzankov A, Manyam GC, et al. Prevalence and clinical implications of Epstein-Barr virus infection in de novo diffuse large B-cell lymphoma in western countries. Clin Cancer Res. 2014;20(9):2338–49. https://doi.org/10.1158/1078-0432.CCR-13-3157.
Zhang J, Weng Z, Huang Y, Li M, Wang F, Wang Y, et al. High-grade B-cell lymphoma with MYC, BCL2, and/or BCL6 translocations/rearrangements clinicopathologic features of 51 cases in a single institution of south China. Am J Surg Pathol. 2020;44:1602–11. https://doi.org/10.1097/PAS.0000000000001577.
Funding
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil, Finance Code 001), the São Paulo State Research Foundation (FAPESP/Brazil) (FAPESP #17/14880–3), the Minas Gerais State Research Foundation (FAPEMIG #APQ-00623–18) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil #406452/2018–0). Manoela Domingues Martins, Pablo Agustin Vargas, Fábio Ramôa Pires and Felipe Paiva Fonseca are fellows of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil).
Author information
Authors and Affiliations
Contributions
Study concepts: Felipe Paiva Fonseca; Study design: Felipe Paiva Fonseca and Ciro Dantas Soares; Data acquisition: Ana Luísa Morais-Perdigão, Carla Isabelly Rodrigues-Fernandes, Gabriela Ribeiro Araújo, Bruno Augusto Benevenuto de Andrade; Quality control of data and algorithms: Carla Isabelly Rodrigues-Fernandes, Manoela Domingues Martins; Data analysis and interpretation: Felipe Paiva Fonseca, Ana Luísa Morais-Perdigão, Carla Isabelly Rodrigues-Fernandes, Fábio Ramôa Pires; Statistical analysis:: Felipe Paiva Fonseca, Ana Luísa Morais-Perdigão, Rommel Mario Rodríguez Burbano; Manuscript preparation: Ana Luísa Morais-Perdigão; Manuscript editing: Ana Luísa Morais-Perdigão, Hélder Antônio Rebelo Pontes; Manuscript review: Felipe Paiva Fonseca, Carla Isabelly Rodrigues-Fernandes, Pablo Agustin Vargas.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical Approval
This study was approved by the Ethical Committee of the Piracicaba Dental School, University of Campinas, Piracicaba, Brazil (process no. 44647421.1.0000.5418). All procedures followed the ethical standards of the Helsinki Declaration of 1975, as revised in 2008.
Consent to Participate
Informed consent was obtained from the individual participants included in the study.
Consent for Publication
Patients signed informed consent regarding publishing their data. No clinical photograph is used in this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morais-Perdigão, A.L., Rodrigues-Fernandes, C.I., Araújo, G.R. et al. CD30 Expression in Oral and Oropharyngeal Diffuse Large B Cell Lymphoma, not Otherwise Specified. Head and Neck Pathol 16, 476–485 (2022). https://doi.org/10.1007/s12105-021-01387-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-021-01387-5