Abstract
Objective
To compare the outcomes of adrenocorticotrophic hormone (ACTH) and Prednisolone therapy in children with West syndrome.
Methods
The study was done at a tertiary health centre for children. The pediatric neurologist at the centre enrolled children into the study based on the inclusion and exclusion criteria. They were evaluated in detail, classified according to etiologic type and then, randomly assigned into two treatment groups, either ACTH or Prednisolone. They were followed at regular intervals till 6 mo.
Results
There was no difference between ACTH and Prednisolone groups with respect to all the outcomes measured. Cessation of spasms was achieved in 6/15 (40%) in Prednisolone group and 9/18 (50%) in ACTH group (p = 0.3906). The average time for achieving cessation was 6.9 and 8 d in ACTH and Prednisolone groups respectively (p = 0.7902). The relapse rates were 18.18 and 50% in ACTH and Prednisolone groups respectively (p = 0.28). The side-effects profile, subsequent epilepsy rates and improvement in milestones were similar in both the treatment groups.
Conclusions
There is no significant difference in children treated with ACTH and Prednisolone. Study results cannot be generalized due to small sample size. However, Prednisolone can be a suitable alternative to ACTH in resource poor settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
West syndrome is a rare epileptic syndrome characterized by triad of infantile spasms, hypsarrhythmia interictal electroencephalogram (EEG) and psychomotor retardation [1, 2]. The incidence has been estimated to be 1.3 to 4.6 per 10,000 live births [2]. The disorder was originally described by Dr. Williams James West in his own son Edwin in 1841 in a letter written to ‘Lancet’- “On a peculiar form of convulsions” [3,4,5].
The causes of West syndrome are multiple and diverse ranging from genetic to structural, metabolic and unknown causes [6,7,8,9]. The pathophysiology is still unclear. The best accepted explanation is Baram’s theory of Corticotrophin Releasing Hormone (CRH) [10]. According to this theory, the multiple causes of West syndrome result in increased release of stress activated mediators in the brain, especially CRH which is responsible for spasms. CRH receptors are abundant in the early neonatal period [11]. CRH has excitant property on many neurons [12] and is a potent convulsant as observed in animal studies [13].
The adrenocorticotrophin (ACTH) and glucocorticoids act by suppressing excessive synthesis and secretion of endogenous convulsant CRH [14]. The efficacy of these drugs in reducing spasms and normalizing EEG in West syndrome has been reported by Sorel and Dusaucy-Bauloye way back in 1958 [10]. ACTH and steroids are also known to have intrinsic anticonvulsant properties [15]. Though newer anticonvulsant drugs have been discovered, most studies advocate hormonal therapy with ACTH and steroids for treatment of infantile spasms.
Vaddi et al. in their KAP study of pediatricians on infantile spasms in India shows that 67% of pediatricians were following Nelson Textbook of Pediatrics for treatment of infantile spasms, which recommends ACTH as the first choice [16, 17]. However, it does not mention about therapeutic option of oral corticosteroids, which also have high quality evidence-base and ease of oral administration [18]. Treatment with ACTH is costly and cumbersome, as it has to be given via intramuscular or subcutaneous routes. On the other hand, Prednisolone is cheaper and easy to administer as it is given orally. If Prednisolone is proven to be equally effective or better than ACTH, it will be very useful for treatment of infantile spasms, especially, in developing countries like India. Hence, the study was conducted with the objective to compare the treatment outcomes in children treated with ACTH against those treated with Prednisolone. The study was apt and pertinent from a developing country perspective.
Material and Methods
The study was done to test the null hypothesis that there is no difference between ACTH and Prednisolone in the treatment of infantile spasms. The study was a randomized interventional study conducted at Indira Gandhi Institute of Child Health, Bangalore, from October 2013 through October 2015. It was approved by the Institutional Ethical Committee. The study included all children with West syndrome aged 2 mo to 5 y. West syndrome was defined as per consensus statement of the West Delphi group [19]. Children who had already received steroids and those in whom steroids were contraindicated were excluded. Cases of West syndrome secondary to Tuberous sclerosis were also excluded.
Informed consent was taken from parents/guardian of children. The details of history and physical examination were recorded in a pre-designed proforma. Interictal EEG was done in all children. Neuroimaging and screening for inborn errors of metabolism was done wherever required.
A random number sequence was generated by the computer and numbers were allocated alternatively to the two interventional groups. The first group received Prednisolone while the second group received ACTH. Blinding of participants and care providers could not be done as the two drugs were given through different routes.
Initial screening tests were done to rule out infections. Treatment followed the guidelines of the United Kingdom Infantile Spasms Study protocol except daily ACTH instead of alternate days [3]. ACTH was given at a dose of 100 units per body surface area through intramuscular route daily for 2 wk on outpatient basis. Prednisolone was given at a high dose of 4 mg/kg/d (Max. dose of 60 mg/d) orally for 2 wk. The response was assessed at the end of 2 wk. After 2 wk, the drugs were tapered and stopped over a period of 3–4 wk. Patients were followed at 2 wk, 3 mo and 6 mo from the day of initiation of intervention. The primary outcomes measured were cessation of spasms and time taken for cessation. Cessation of spasms was defined as no reported spasms for at least 48 h including days 13 and 14 after randomization. Time taken for cessation of spasms was defined as number of consecutive days free of spasms preceding and including day 14. The secondary outcomes measured were relapses, if any; time taken for relapse; side-effects, if any; and subsequent epilepsy rates. An adverse reaction was defined as any untoward or unintended response thought to be related to trial treatments [19]. An adverse reaction was judged serious if it was life-threatening, caused death, resulted in persistent or substantial disability, or required admission to hospital. The treating clinician determined the causality.
The cessation of spasms, relapse of spasms, occurrence of complications of the intervention, and subsequent epilepsy rate were measured as dichotomous variable. The time taken for cessation of spasms and the relapse of the spasms were measured as continuous variable.
Sample size calculation based on a prospective randomized single blinded study by Baram et al., estimated a minimum sample size of 90 (45 in each group) to be required to detect a difference in improvement rate (both clinical and EEG) of 61.6% between ACTH and Prednisolone groups (25% vs. 86.6%) with a power of 80% using a two-sided test (α = 0.05) with an estimated drop out of 30% and after adjusting for regional differences [20].
All the data collected were tabulated and descriptive and inferential statistics were carried out. For children whose outcome data was missing, “last observation carried forward” method was used for outcome analysis. Chi square test and Fisher exact tests were used for categorical data. Independent Student t-test was used for quantitative data with continuous variables. To prevent overestimation of statistical significance for small data, Yates correction for continuity was applied for Chi square tests. A p value <0.05 was considered statistically significant. All statistical tests were done using Medical Statistical Software.
Results
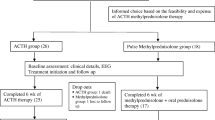
A total of 34 children were included, 18 were randomized to ACTH therapy and 16 to Prednisolone therapy. The enrolments, treatment allocation, follow-up and analysis of outcome have been outlined in the flowchart in Fig. 1. The various baseline characteristics were equally distributed in the two treatment arms (Table 1).
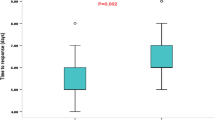
At the first follow-up on day 14, nine out of 18 children who received ACTH achieved cessation of spasm (50%), while it was 5 out of 15 in the Prednisolone group (33.33%). EEG became normal in 4 and 7 of the Prednisolone and ACTH group respectively. At the second follow-up after 3 mo, 2 children died in the ACTH group. One was a case of Aicardi syndrome with severe neurological impairment and other child due to aspiration pneumonia. Eleven of the remaining 16 children responded (61.11%). In the Prednisolone group, 1 child died of surgical and anesthetic complications following gastrostomy for feeding difficulties. Six of the remaining 14 children showed spasm cessation (40%). The time taken for cessation of spasm was on average 6.9 d in ACTH group and 8 d in the Prednisolone group (p > 0.1). Table 2 depicts the various outcome measures of the study and their level of significance.
Two out of 11 children in the ACTH group (18.18%) and 3 out of 6 in the Prednisolone group (50%) had relapses of spasms (p > 0.1). Time taken for relapse was on average 166.18 d in the ACTH group and 127.6 d in the Prednisolone group.
There was no significant difference between the two groups with respect to side-effects profile and development of subsequent epilepsy. Side-effects noted in Prednisolone group was 20% (3) compared to 16.6% in ACTH group. None of the side-effects observed were serious enough requiring discontinuation of therapy.
Discussion
Ever since the description of infantile spasms, various drugs have been investigated for its treatment. But, till date, no optimal or standard treatment has been established. The present study provides insight into a developing country perspective in treating infantile spasms with particular reference to ACTH and Prednisolone as first line therapy.
The forms, doses and duration of ACTH varied in different studies. In a randomized trial by Baram et al., the efficacy of prednisone (2 mg/kg/d) was inferior to that of high dose ACTH (150 U/d) [20]. However, similar efficacy was seen when Hrachovy et al. [21] administered ACTH at lower doses in study. UKISS, a large Class III randomized controlled study (n = 55), compared a high dose oral prednisolone protocol (40–60 mg/d) with synthetic ACTH therapy. The short-term results were nearly similar efficacy for both interventions (ACTH 76% vs. prednisolone 70%). AAN concluded that currently the evidence is insufficient to recommend the use of oral steroids as being as effective as ACTH for short-term treatment of infantile spasms. There is limited evidence in literature on optimum dose and duration of ACTH therapy. The minimum effective dose of ACTH remains unclear. A Class I study by Hrachovy et al. revealed similar efficacy between low-dose (20–30 IU), short-duration and high-dose natural ACTH (150 IU/m2), long duration [22]. However, this study had small sample size (n = 50) and lacked equivalence study design. Similarly, few other small sample sized-inadequate powered studies had shown near similar efficacy. However, the efficacy of low-dose ACTH in terms of response rate varied from 42 to 75% in RCTs. In a small sample sized (n = 15) study by Baram et al., high efficacy of high-dose ACTH up to 86.6% was observed [20].
This study finds no difference between two drugs with respect to cessation of spasms. The result is comparable to studies done by Hrachovy et al. [21], Azam et al. [23], Kossoff et al. [24] and Kalra et al. [25]. However, two other studies (Baram et al. [20], Snead et al. [26]) have shown ACTH to be superior to Prednisolone. Another recent study by Wanigasinghe et al. [27] has found Prednisolone to be superior to ACTH.
The time taken for cessation of spasms is similar in both groups in the present study. However, the study by Wanigasinghe et al. [27] has shown that time taken is less in Prednisolone therapy against ACTH therapy. This has not been statistically analyzed in most other studies. The common finding in all studies is that most children, who responded, did so within 4–8 d of initiation of therapy.
The improvement in milestones following therapy is similar in both groups in the present study. In all other studies comparing the two treatments, length of follow-up period is insufficient for analyzing cognitive and developmental outcome of therapy, unlike, the present study, where children have been followed up to 6 mo. In the present study, a total of 12 children definitely showed improvement by gaining new milestones, stressing the importance of treatment in infantile spasms.
The major limitation of the study is small sample size. The other limitation is absence of blinding, owing to which, observer and analyzer bias could not be eliminated. Children had been followed up to 6 mo only and hence; the study fails to determine the final developmental outcome in long term follow-up.
In conclusion, the present study did not find any difference in the outcome of children with West syndrome treated with ACTH or Prednisolone in terms of cessation of spasms, time taken for cessation of spasms, relapse rates, developmental outcome, side-effects and subsequent epilepsy rates. However, due to small sample size, results cannot be generalized. Prednisolone can be suitable alternative, particularly in resource poor settings. Further, more studies are needed to prove efficacy of either drug and also to know the best dose and duration of treatment to maximize the response and to decrease the side-effects. As idiopathic cases are few in the present study as well as most other studies, a prospective multicentre collaborative effort is needed to study the effect of these drugs particularly in idiopathic variety of West syndrome.
References
Dulac O. Review article – what is West syndrome? Brain Dev J. 2001;23:447–52.
Riikonen R. Long-term evolution of epileptic encephalopathies. In: Marina N, Pierre G, Anne S, editors. West syndrome. John Libbey Eurotext; 2009. p. 13–28. (Topics in Epilepsy series, Vol 1). [Internet] Available at: https://books.google.co.in/books?id=xMc63-y4afAC. Accessed 4 Oct 2015.
Lux AL. Review article – West & son: the origins of West syndrome. Brain Dev J. 2001;23:443–6.
Eling P, Renier WO, Pomper J, Baram TZ. The mystery of the Doctor’s son, or the riddle of West syndrome. Neurology. 2002;58:953–5.
Pies NJ, Beardsmore CW. West & West syndrome – a historical sketch about the eponymous doctor, his work and his family. Brain Dev J. 2003;25:84–101.
Shorvon SD. The etiologic classification of epilepsy. Epilepsia. 2011;52:1052–7.
Pellock JM, Hrachovy R, Shinnar S, et al. Infantile spasms: a U.S. consensus report. Epilepsia. 2010;51:2175–89.
Osborne JP, Lux AL, Edwards SW, et al. The underlying etiology of infantile spasms (west syndrome): information from the United Kingdom infantile spasms study (UKISS) on contemporary causes and their classification. Epilepsia. 2010;51:2168–74.
Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsy: report of the ILAE commission on classification and terminology, 2005-2009. Epilepsia. 2010;51:676–85.
Baram TZ. Pathophysiology of massive infantile spasms: perspective on the putative role of the brain adrenal axis. Ann Neurol. 1993;33:231–6.
Insel TR, Battaglia G, Fairbanks DW, De Souza EB. The ontogeny of brain receptors for corticotrophin-releasing factor and the development of their functional association with adenylate cyclase. J Neurosci. 1988;8:4151–8.
Marrosu F, Fratta W, Carcangiu P, Giagheddu M, Gessa GL. Localized epileptiform activity induced by murine CRF in rats. Epilepsia. 1988;29:369–73.
Baram TZ, Hirsch E, Snead OC III, Schultz L. CRH induced seizures in the infant brain originate in the amygdala. Ann Neurol. 1992;31:488–94.
Jingami H, Matsukura S, Numa S, Imura H. Effects of adrenalectomy and dexamethasone administration on the level of pre pro corticotrophin releasing factor messenger-RNA in the hypothalamus and adrenocorticotrophin/beta-lipotropin precursor mRNA in the pituitary in rats. Endocrinology. 1985;117:1314–20.
Joels M, de Kloet ER. Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 1992;15:25–9.
Vaddi VK, Sahu JK, Dhawan SR, Suthar R, Sankhyan N. Knowledge, attitude and practice (KAP) study of pediatricians on infantile spasms. Indian J Pediatr. 2018. https://doi.org/10.1007/s12098-018-2630-3.
Kliegman R, Behrman RE, Nelson WE. The nervous system. In: Kliegman R, Behrman RE, Nelson WE, editors. Nelson textbook of pediatrics, vol. 2. 20th ed. Phialdelphia: Elsevier; 2016. p. 2840–1.
Sahu JK. Infantile spasms – evidence based medical management. Indian J Pediatr. 2014;81:1052–5.
Lux AL, Osborne JP. A proposal for case definitions and outcome measures in studies of infantile spasms and west syndrome: consensus statement of the West Delphi group. Epilepsia. 2004;45:1416–28.
Baram TZ, Mitchell WG, Tournay A, Snead OC, Hanson RA, Horton EJ. High dose corticotrophin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. 1996;97:375–9.
Hrachovy RA, Frost JD, Kellaway R, Zion TE. Double blind study of ACTH vs. prednisone therapy in infantile spasms. J Pediatr. 1983;103:641–5.
Hrachovy RA, Frost JD Jr, Glaze DG. High-dose, long-duration versus low-dose, short-duration corticotropin therapy for infantile spasms. J Pediatr. 1994;124:803–6.
Azam M, Bhatti N, Krishin J. Use of ACTH and prednisolone in infantile spasms: experience from a developing country. Seizure. 2005;14:552–6.
Kossoff EH, Hartman AL, Rubenstein JE, Vining EP. High dose prednisolone for infantile spasms: an effective and less expensive alternative to ACTH. Epilepsy Behav. 2009;14:674–6.
Kalra V, Gulati S, Pandey RM, Menon S. West syndrome and other epileptic encephalopathies – Indian hospital experience. Brain Dev. 2002;24:130–9.
Snead OC III, Benton JW, Myers GJ. ACTH and prednisone in childhood seizure disorders. Neurology. 1983;33:966–70.
Wanigasinghe J, Phila M, Arambepola C, Ranganathan SS, Sumanasena S, Attanapola G. Randomized, single-blind, parallel clinical trial on efficacy of oral prednisolone versus intramuscular corticotrophin on immediate and continued spasm control in West syndrome. Pediatr Neurol. 2015;53:193–9.
Author information
Authors and Affiliations
Contributions
VKG: Concept and designed the study; VN: Collected the data and helped in data analysis; SKS: Analyzed the data and drafted the manuscript; NB: Concept of study and supervised seizure control; AB: Edited and refined the draft. VKG will act as guarantor for this paper.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Rights and permissions
About this article
Cite this article
Gowda, V.K., Narayanaswamy, V., Shivappa, S.K. et al. Corticotrophin-ACTH in Comparison to Prednisolone in West Syndrome – A Randomized Study. Indian J Pediatr 86, 165–170 (2019). https://doi.org/10.1007/s12098-018-2782-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-018-2782-1