Abstract
Purpose
We conducted a systematic review to analyse the performance of the sentinel lymph-node biopsy (SLNB) after the neoadjuvant chemotherapy, compared to axillary lymph-node dissection, in terms of false-negative rate (FNR) and sentinel lymph-node identification rate (SLNIR), sensitivity, negative predictive value (NPV), need for axillary lymph-node dissection (ALND), morbidity, preferences, and costs.
Methods
MEDLINE, Embase, Scopus, and The Cochrane Library were searched. We assessed the quality of the included systematic reviews using AMSTAR2 tool, and estimated the degree of overlapping of the individual studies on the included reviews.
Results
Six systematic reviews with variable quality were selected. We observed a very high overlapping degree across the included reviews. The FNR and the SLNIR were quite consistent (FNR 13–14%; SLNIR ~ 90% or higher). In women with initially clinically node-negative breast cancer, the FNR was better (6%), with similar SLNIR (96%). The included reviews did not consider the other prespecified outcomes.
Conclusions
It would be reasonable to suggest performing an SLNB in patients treated with NACT, adjusting the procedure to the previous marking of the affected lymph node, using double tracer, and biopsy of at least three sentinel lymph nodes. More well-designed research is needed.
PROSPERO registration number: CRD42020114403.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Neoadjuvant chemotherapy (NACT) has become a generalized approach to the treatment of breast cancer, with the aim of reducing the size of the primary tumor and to facilitate performing a conservative surgery. In addition, it will also allow an earlier evaluation of the clinical efficacy and changes in the regimens [1], as well as the conservation of the breast in operable cancers, with higher rates of complete pathological responses [2].
Sentinel lymph-node status is an important prognostic factor and sentinel node biopsy (SLNB) is considered the reference procedure for lymph-node staging of early breast cancer lesions [3, 4]. SLNB is usually undergone before performing the axillary lymph-node dissection (ALND). ALND is a more accurate method to evaluate the spread of the disease to the loco-regional lymph nodes, but is in turn a more complex procedure and is associated with important morbidities in the short and long term such as lymphedema, nerve injury, worse quality of life, etc [4, 5].
There is still a debate about the value of SLNB after neoadjuvant treatment, especially for clinical-positive lymph node initially [6]. There are also concerns about the increase in false-negative rate (FNR) and the decrease in sentinel lymph-node identification rate (SLNIR) after NACT. Although infrequent (near 3%), the occurrence of loco-regional relapses in sentinel lymph-node biopsy negative breast cancer patients is another matter of concern [7].
SLNIR and FNR are considered as the most clinically relevant performance characteristics of this procedure [8]. The SLNIR is defined as the proportion of successfully completed SLNB. FNR represents in turn people who had a negative index test result, but were classified by the reference standard as having the target condition [9]. These patients may be denied, or experience delays in receiving effective treatment.
When synthesizing the available evidence on a given topic, researchers can identify multiple relevant systematic reviews addressing the same (or very similar) clinical questions and that includes many of the same primary studies (overlapping) [10]. The simple sum of data coming from an increasing number of studies/reviews, where primary studies can be counted more than once, will result in an artificial and disproportionate statistical power, and hence, in biased and falsely reliable results [11, 12].
We aimed to assess the performance of SLNB after NACT, in terms of FNR, SLNIR, sensitivity, negative predictive value (NPV), need for axillary lymph-node dissection (ALND), morbidity, preferences, and costs. We sought also to assess the quality of the existing systematic reviews, as well as to know the degree of study overlapping across the published systematic reviews.
Methods
Literature review
To find relevant studies to answer the clinical question, we designed a search strategy in MEDLINE (accessed via PubMed), Scopus, and The Cochrane Library. We also carried out a manual search of relevant reviews and studies, and contacted experts in the field (PS, AP, FJC, and SS) to find out if they were aware of other unpublished or on-going studies. The search was first conducted in December 2018, and lastly updated in November 2020 (see Appendix 1. Search strategy).
Eligibility criteria
Systematic reviews including prospective or retrospective studies evaluating the value of SLNB for decision-making after neoadjuvant chemotherapy, followed by ALND, were considered for inclusion. An attempt was made to identify relevant economic evaluations for the question, as well as studies on the importance given by patients to the outcomes of interest.
Risk of bias
We assessed the quality of the systematic reviews using the AMSTAR 2 tool [13]. We considered the items related to literature search, risk-of-bias assessment/impact, appropriateness of meta-analytical methods, and assessment and impact of publication bias as the most important ones.
Examining overlapping
We used the approach described by Pieper y cols. [11], and included only the prospective studies identified in the reviews. We calculated the measure of the “covered area” (CA) according to the formulae
where N is the number of included publications (including double counting), r is the number of rows (studies), and c is the number of columns (reviews). We then calculated the “corrected cover area” (CCA), a measure that takes into consideration the differences in the number of studies included by every separate review, using the formulae
Summary of findings
We elaborated a narrative synthesis of the results of the reviews and the studies obtained from the search of the literature. The main characteristics of the included reviews/studies are provided, as well as the main findings of the reviews for each of the outcomes of interest.
Results
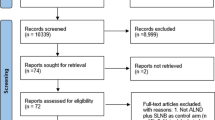
The last search was performed in November 2020. After the analysis of the abstracts and the potentially relevant full-text articles, we selected six systematic reviews [14,15,16,17,18,19]. Consultation with experts did not yield any other additional information (see Table 1 Characteristics of the included systematic reviews).
Quality assessment
Using AMSTAR 2 tool, four reviews [15, 16, 18, 19] reached a good quality assessment. One review [17] failed to report data on a previous protocol and the potential deviations from it, as well as the impact of the risk of bias assessment on the results of the review. In the other systematic review [14], literature search was limited to PubMed/Ovid, and provided not enough information about the impact of the risk of bias on both the individual studies. (See Table 2 AMSTAR 2 Assessment, and Fig. 1 AMSTAR 2 Assessment).
Risk of bias
The main flaws of the included studies were found in the domains “Patient selection” and “Index test”, where most of the studies were qualified as high risk of bias; therefore, we judged the quality of the evidence as low.
Overlapping
The six systematic reviews included in total 107 prospective studies, corresponding to 51 primary studies. Using the method described by Pieper et al. [11] we calculated a CA of 35.0%, and a CCA of 22%, showing a very high overlapping across the five included reviews.
Outcomes of interest
See Table 3. Summary of findings.
False-negative rate (FNR)
FNR were very similar across five systematic reviews [14, 16,17,18,19], ranging between 13 and 17%.
El Hage Chehade 2016 found that the pooled estimate for the FNR was 13% (95% CI 10.8–15.6%). In this review, median age, tumor histology, tumor size, receptor status, and chemotherapy regimen had no effect on pCR, although authors describe a cN1 disease marginally associated with an increased pCR rate when compared with N2 or N3 disease (p = 0.06).
In Mocellin 2016, the calculated FNR was 14.2% (95% CI 12.5–16.0%). No statistically significant differences were found between patients with clinically negative nodes before NACT (FNR 23.5%, 95% CI 15.8–33.5%) and patients with nodes clinically positive before NACT (FNR 15.2%, 95% CI 12.4–18.5%).
The systematic review by Tee et al. found a pooled estimate of 14% for the FNR (95% CI 11–17%). No differences were found when FNR was analysed according to the mapping technique (single mapping: 19% [95% CI 1–27%], dual mapping: 11% [95% CI 6–15%], I2 = 40.5% [moderate heterogeneity], p = 0.12). The review did find differences in the FNR when the analysis was performed according to the number of lymph nodes removed (one lymph node removed: 20% [95% CI 13–27%], two lymph nodes removed: 12% [IC 95% 5–19%], three or more nodes removed: 4% [95% CI 0–9%] [I2 = 78.2% [high heterogeneity]; p = 0.00]).
In the meta-analysis by Shirzadi 2019, the pooled FNR was 13% (95% CI 7–18%). In the subgroup analysis considering the number of tracers used, the pooled FNR for single and dual tracers was 9% (95% CI 3–15%) and 14% (95% CI 10–19%), respectively, (I2 = 91.3%, high heterogeneity). Egger’s test showed evidence of publication bias.
Finally, Simons 2019 reported an FNR of 17% (95% CI 14–20%). No differences were observed with the use of single tracer when compared to dual tracers (16 vs 13%; p = 0.53), or when immunohistochemistry (IHC) analysis was used or not (15 vs 17%; p = 0.47). Removal of at least 3 SLNs was associated with a lower FNR, when compared to < 3 SLNs (8 vs 22%; p < 0.0001).
The systematic review and meta-analysis by Geng et al., which included only women with initially clinically node-negative breast cancer, found a pooled FNR of 6% (95% CI 3–8%). No significant differences were found between studies with and without IHC staining (p = 0.241) (only H&E staining: 11% (95% CI 4–18%; six studies); H&E combined with IHC staining: 4% (95% CI 1–7%; six studies).
Sentinel lymph-node identification rate (SLNIR)
Sentinel lymph-node identification rates (SLNIR) were also very similar across the six systematic reviews, ranging from 89 to 96%.
El Hage Chehade 2016 found a pooled estimate of 90.9% (95% CI 87.6–93.4%). Mocellin 2016 reported an SLNIR of 89.6% (95% CI 87.8–91.2), while the systematic review by Tee et al. found that pooled SLNIR was 90% (95% CI 87–93%), with a high heterogeneity (I2 = 75.2%).
The systematic review by Geng 2016 found a pooled SLNIR of 96% (95% C: 95–97%). No significant differences were found when different mapping methods were used (p = 0.18) (only blue dye mapping: 96% (95% CI 91–100%, three studies; only radiocolloid: 96% (95% CI 94–99%; four studies; both blue dye and radiocolloid: 97% (95% CI 96–98%; six studies).
In the meta-analysis performed by Shirzadi 2019, the pooled SLNIR was 89% (95% CI 85–94%). The subgroup analysis according to the type of tracer showed that the pooled SLNIR for single and dual tracers was 92% (95% CI 87–96%) and 89% (95% CI 80–98%), respectively (I2 = 80.5%; high heterogeneity).
Finally, the systematic review by Simons et al. found an SLNIR of 89% (95% CI 87–92%).
Evidence on the use of resources
We identified a study about costs conducted in Hong Kong, which evaluated the resource needs derived from performing a sentinel lymph-node biopsy using gammagraphy [20]. However, this study “excluded patients who had undergone neoadjuvant chemotherapy, because there is still an open discussion on the influence of neoadjuvant chemotherapy on sentinel node identification”. Therefore, these findings are not applicable to the population of interest for this review.
Discussion
In the last years, SLNB has gained prominence in patients with non-metastatic breast cancer, as a minimally invasive alternative to ALND.
The overview included six systematic reviews focused mainly on the false-negative rate and the sentinel node identification rate, with fairly consistent results for both outcomes in five of them (FNR 13–14%; SLNIR ~ 90% or higher). The rest of the outcomes of interest intended to investigate were not considered in the individual reviews.
The identification rates showed in general acceptable values (~ 90% or higher). False-negative rates were also consistent (13–14%), although several authors agree that values below 10% would be advisable. In the subgroup analyses of two of the included reviews [18, 19], there were no significant differences according to the use of single and dual tracers, with a trend to higher FNRs when dual tracers are used in Shirzadi 2019. These results contrast with those from the ACOSOG Z1071 trial, where the clip placement in the biopsy-proven positive node at time of initial diagnosis and removal of this clipped node during axillary surgery showed to be an effective intervention to decrease the FNR from 12.6 to 6.8% [21]. In the study by Caudle et al., the use of Targeted Axillary Dissection (TAD) led to an FNR of 2%, compared to 10% when SLNB was performed alone [22]. Is important to notice the lack of randomized trials aimed to assess the role of the marking the affected lymph node to guide the clinical practice.
Subgroup analyses showed that FNR was also lower when more than one node was removed [17, 19]. A recent article by Classe et al. [23], a report from the GANEA2 study, found an overall FNR of 11.9% (95% CI 7.3–17.9%) in women with pN1 sentinel nodes, with significant differences according to the number of resected SLNs (19.3% for cases of one SLN versus 7.8% for cases of two or more SLNs; p = 0.041). Despite these findings, it is not clear yet how to manage patients for which metastases in less than 3 sentinel nodes are identified. Two on-going studies, the POSNOC trial [24] and the SENOMAC trial [25] will include women with no more than two metastatic sentinel nodes, and will contribute to shed light on this particular group of patients.
From the included systematic reviews, there is limited evidence about using single or double mapping. Tee 2018 found lower FNRs when dual mapping was used, but results came from only four studies. In a recent study by Arjunan et al. [26], in 44 women, most of them (86.3%) classified as N1 at diagnosis, and found a higher FNR with the single method of SLN mapping (33–50%), compared to the use of both method simultaneously (11%). The study reported also better results for the SLNIR when the dual method was used (100%) compared to 66.7% each when only the single method of SLN mapping was performed. To reduce the rate of false negatives in initially N1 tumors, it is recommended to map them prior to initiating neoadjuvant chemotherapy, although in some cases, the identified node is not the sentinel node, or the FNR is unacceptably high [27].
It is important to note that decisions about which cN + patients should be treated with SLNB after NAC must rely not only on the performance of the procedure but also on other methods, like lymphoscintigraphy [28], TAD [22], ultrasound-guided biopsy [29], determination of molecular subtypes [30], and breast pCR [31].
There are several clinical practice guidelines that address this important topic, and have issued recommendations about the SLNB in the NACT context. For example, the consensus of the Working Group of Radioguided Surgery of the Spanish Society of Nuclear Medicine and Molecular Imaging [32] states that, in patients with breast cancer undergoing neoadjuvant treatment, SNB is an alternative to avoid performing unnecessary axillary emptying. It states that, in patients node-positive at diagnosis, patients should be carefully selected according to the TN status (T1-3, N1), using a combined technic for lymph mapping (radiotracer plus staining), placing a clip on the pathological node and removing it during the biopsy, and completing the ALND, even if the SNB results in isolated tumor cells or micrometastases.
In the same line, the American Society of Clinical Oncology Clinical Practice Guideline [33] recommends that SNB should be offered to women with operable breast cancer receiving preoperative/neoadjuvant treatment. Such recommendation is based on an updated review of the literature, including randomized controlled trials, systematic reviews, meta-analyses, and clinical practice guidelines.
Finally, a more recent multidisciplinary guidance elaborated by the Association of Breast Surgery, Faculty of Clinical Oncology of the Royal College of Radiologists, UK Breast Cancer Group, National Coordinating Committee for Breast Pathology, and British Society of Breast Radiology [34] recommends for women with clinically node-positive axilla (cN1), that patients can be safely considered for SNB after NACT, and that four nodes should be removed using dual mapping.
Strengths
To perform this overview, we developed a structured and extensive bibliographic search complemented with manual search in relevant articles and reviews, and several recognized experts in the field were consulted for potentially relevant studies.
Key steps like article selection, data extraction, and risk-of-bias assessment were performed independently by two authors with experience in systematic review methodology, and the interpretation of the data and the conclusions were discussed and agreed upon with a panel of experts with extensive clinical and research experience.
In the analysis of the information, we implemented a useful method to detect the degree of overlapping among the reviews, which contributed to the knowledge of the real value of the sentinel lymph-node biopsy in the context of NACT.
Limitations
The six systematic reviews included a heterogeneous mix of women, i.e., women either with negative or positive SLNs before NACT, or with positive SLNs that became negative or remained positive after the treatment, and even some of them with an unknown status before and/or after the therapy. It is valid to consider that the outcomes would be different for these varied groups of patients; therefore, this fact precludes drawing firm conclusions about the applicability, accuracy, and safety of the procedure.
One significant limitation is that was not possible to make a separate analysis of the performance of SLNB for different subtypes of breast cancer (HER2 and triple-negative, very sensitive to neoadjuvant treatment) and luminal or hormonal (not very sensitive).
The very high degree of overlapping detected means that several studies were considered simultaneously in two or more of the reviews, giving rise to apparently consistent results and to a supposedly high certainty from the available evidence. This fact is one of the classically described limitations of overviews, which has been recognized as resulting in a fictitious increase in statistical power [11, 12]. In this case, we decided to describe the overlap and recognize it as a possible limitation of the study, instead of adopting formal statistical approaches to deal with this issue.
Another potential flaw to consider is the lack of information on important outcomes for patients, such as satisfaction with treatment and costs. Given this limitation, the decisions on the most convenient procedure for an individual case should be taken along with patients, after providing them with the available information on the accuracy and possible adverse events associated with each procedure.
As implications for research, it is advisable that future studies develop new axillary markers, easier to locate in the operating room and not requiring a nuclear medicine service. Thresholds for residual tumor burden in lymph nodes that are considered susceptible to treatment by radiotherapy and without performing lymphadenectomy should be also established.
Conclusions
From the analysis of the six available systematic reviews, it would be reasonable to suggest performing a sentinel lymph-node biopsy in patients treated with NACT. However, for patients with positive LN initially, it is advisable to adjust the procedure to a number of technical requirements (previous marking of the affected lymph node, using double tracer, and biopsy of at least three sentinel lymph nodes), having established a correct staging of the tumor [32]. As the evidence from well-designed studies is still limited, such recommendation is based on the results of two clinical trials (ACOSOG Z1071, [21] SN FNAC [31]) which have shown a significant reduction in the false-negative rate with the biopsy of at least three lymph nodes, previously marking the affected lymph node and using a double tracer. It is important to have a precise definition of the indication, for example according to the staging proposed by the recommendations of the Radioguided Surgery Working Group of the Spanish Society of Nuclear Medicine and Molecular Imaging, [32] which addresses the feasibility of marking possible positive lymph nodes and their subsequent identification.
References
Rubovszky G, Horváth Z. Recent advances in the neoadjuvant treatment of breast cancer. J Breast Cancer. 2017;20(2):119–31. https://doi.org/10.4048/jbc.2017.20.2.119.
Killelea BK, Yang VQ, Mougalian S, Horowitz NR, Pusztai L, Chagpar AB, Lannin DR. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the National Cancer Database. J Am Coll Surg. 2015;220(6):1063–9. https://doi.org/10.1016/j.jamcollsurg.2015.02.011.
Esposito E, Di Micco R, Gentilini OD. Sentinel node biopsy in early breast cancer. A review on recent and ongoing randomized trials. Breast. 2017;36:14–9.
Manca G, Rubello D, Tardelli E, Giammarile F, Mazzarri S, Boni G, Chondrogiannis S, Marzola MC, Chiacchio S, Ghilli M, Roncella M, Volterrani D, Colletti PM. Sentinel lymph node biopsy in breast cancer: indications, contraindications, and controversies. Clin Nucl Med. 2016;41(2):126–33.
Bing AU, Kerr GR, Jack W, Chetty U, Williams LJ, Rodger A, Dixon JM. Pooled long-term outcomes from two randomized trials of axillary node sampling with axillary radiotherapy versus axillary node clearance in patients with operable node-positive breast càncer. Br J Surg. 2016;103(1):81–7.
Liu J, Mao K, Jiang S, Jiang W, Chen K, Kim BY, Liu Q, Jacobs LK. The role of postmastectomy radiotherapy in clinically node-positive, stage II–III breast cancer patients with pathological negative nodes after neoadjuvant chemotherapy: an analysis from the NCDB. Oncotarget. 2016;7(17):24848–59.
Falco M, Masojć B, Kram A. Locoregional relapse is a strong prognostic indicator of distant metastatic progression in breast cancer patients after negative sentinel lymph node biopsy. Breast J. 2020. https://doi.org/10.1111/tbj.14118.
Kelly AM, Dwamena B, Cronin P, Carlos RC. Breast cancer sentinel node identification and classification after neoadjuvant chemotherapy-systematic review and meta-analysis. Acad Radiol. 2009;16(5):551–63. https://doi.org/10.1016/j.acra.2009.01.026 (PMID: 19345896).
Deeks JJ, Bossuyt PM. Chapter 3: Evaluating diagnostic tests. Draft version (27 July 2021) for inclusion. In: Deeks JJ, Bossuyt PM, Leeflang MM, Takwoingi Y, (eds) Cochrane handbook for systematic reviews of diagnostic test accuracy version 2. Cochrane, London
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29.
Pieper D, Antoine SL, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67(4):368–75.
Ballard M, Montgomery P. Risk of bias in overviews of reviews: a scoping review of methodological guidance and four-item checklist. Res Synth Methods. 2017;8(1):92–108. https://doi.org/10.1002/jrsm.1229 (Epub 2017 Jan 10 PMID: 28074553).
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;21(358):j4008.
El Hage CH, Headon H, El Tokhy O, Heeney J, Kasem A, Mokbel K. Is sentinel lymph node biopsy a viable alternative to complete axillary dissection following neoadjuvant chemotherapy in women with node-positive breast cancer at diagnosis? An updated meta-analysis involving 3398 patients. Am J Surg. 2016;212(5):969–81.
Geng C, Chen X, Pan X, Li J. The feasibility and accuracy of sentinel lymph node biopsy in initially clinically node-negative breast cancer after neoadjuvant chemotherapy: a systematic review and meta-analysis. PLoS ONE. 2016;11(9):e0162605.
Mocellin S, Goldin E, Marchet A, Nitti D. Sentinel node biopsy performance after neoadjuvant chemotherapy in locally advanced breast cancer: a systematic review and meta-analysis. Int J Cancer. 2016;138:472–80.
Tee SR, Devane LA, Evoy D, Rothwell J, Geraghty J, Prichard RS, McDermott EW. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br J Surg. 2018;105(12):1541–52.
Shirzadi A, Mahmoodzadeh H, Qorbani M. Assessment of sentinel lymph node biopsy after neoadjuvant chemotherapy for breast cancer in two subgroups: initially node negative and node positive converted to node negative—a systemic review and meta-analysis. J Res Med Sci. 2019;25(24):18.
Simons JM, van Nijnatten TJA, van der Pol CC, Luiten EJT, Koppert LB, Smidt ML. Diagnostic accuracy of different surgical procedures for axillary staging after neoadjuvant systemic therapy in node-positive breast cancer: a systematic review and meta-analysis. Ann Surg. 2019;269(3):432–42.
Co M, Kwong A. Preoperative sentinel node mapping in sentinel node biopsy in early breast cancers—is it cost-effective? Clin Breast Cancer. 2017;17(2):134–8.
Boughey JC, Ballman KV, Hunt KK, McCall LM, Mittendorf EA, Ahrendt GM, Wilke LG, Le-Petross HT. Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: results from the American College of Surgeons Oncology Group Z1071 trial (Alliance). J Clin Oncol. 2015;33(30):3386–93. https://doi.org/10.1200/JCO.2014.57.8401 (Epub 2015 Feb 2. PMID: 25646192; PMCID: PMC4606058).
Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, Bedrosian I, Hobbs BP, DeSnyder SM, Hwang RF, Adrada BE, Shaitelman SF, Chavez-MacGregor M, Smith BD, Candelaria RP, Babiera GV, Dogan BE, Santiago L, Hunt KK, Kuerer HM. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072–8. https://doi.org/10.1200/JCO.2015.64.0094 (Epub 2016 Jan 25. PMID: 26811528; PMCID: PMC4933133).
Classe JM, Loaec C, Gimbergues P, Alran S, de Lara CT, Dupre PF, Rouzier R, Faure C, Paillocher N, Chauvet MP, Houvenaeghel G, Gutowski M, De Blay P, Verhaeghe JL, Barranger E, Lefebvre C, Ngo C, Ferron G, Palpacuer C, Campion L. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat. 2019;173(2):343–52.
Goyal A, Mann GB, Fallowfield L, Duley L, Reed M, Dodwell D, Coleman RE, Fakis A, Newcombe R, Jenkins V, Whitham D, Childs M, Whynes D, Keeley V, Ellis I, Fairbrother P, Sadiq S, Monson K, Montgomery A, Tan W, Vale L, Homer T, Badger H, Haines RH, Lewis M, Megias D, Nabi Z, Singh P, Caraman A, Miles E, POSNOC Trialists. POSNOC-positive sentinel node: adjuvant therapy alone versus adjuvant therapy plus clearance or axillary radiotherapy: a randomised controlled trial of axillary treatment in women with early-stage breast cancer who have metastases in one or two sentinel nodes. BMJ Open. 2021;11(12):e054365. https://doi.org/10.1136/bmjopen-2021-054365 (PMID: 34857578; PMCID: PMC8640630).
de Boniface J, Frisell J, Andersson Y, Bergkvist L, Ahlgren J, Rydén L, Olofsson Bagge R, Sund M, Johansson H, Lundstedt D, SENOMAC Trialists’ Group. Survival and axillary recurrence following sentinel node-positive breast cancer without completion axillary lymph node dissection: the randomized controlled SENOMAC trial. BMC Cancer. 2017;17(1):379. https://doi.org/10.1186/s12885-017-3361-y (PMID: 28549453; PMCID: PMC5446737).
Arjunan R, Ramamani TA, Ramachandra C, Swamyvelu K, Chunduri S, Althaf S, Usha A, Namrata R. Sentinel lymph node biopsy in locally advanced breast cancer after neoadjuvant chemotherapy-an Indian perspective. Indian J Surg Oncol. 2020;11(2):242–7.
Li J, Chen X, Qi M, Li Y. Sentinel lymph node biopsy mapped with methylene blue dye alone in patients with breast cancer: a systematic review and meta-analysis. PLoS ONE. 2018;13(9): e0204364. https://doi.org/10.1371/journal.pone.0204364.
Hamdy O, Farouk O, El-Badrawy A, Denewer A, Setit A. Sentinel lymph node biopsy in breast cancer guided by CT lymphography; history, evolution and current applications. Breast Dis. 2021;40(4):219–25. https://doi.org/10.3233/BD-201046 (PMID: 33935052).
Arjmandi F, Mootz A, Farr D, Reddy S, Dogan B. New horizons in imaging and surgical assessment of breast cancer lymph node metastasis. Breast Cancer Res Treat. 2021;187(2):311–22. https://doi.org/10.1007/s10549-021-06248-x (Epub 2021 May 12 PMID: 33982209).
Bi Z, Liu J, Chen P, Liu Y, Zhao T, Wang C, Zhang Z, Sun X, Qiu P, Cong B, Song X, Wang Y. Neoadjuvant chemotherapy and timing of sentinel lymph node biopsy in different molecular subtypes of breast cancer with clinically negative axilla. Breast Cancer. 2019;26(3):373–7. https://doi.org/10.1007/s12282-018-00934-3 (Epub 2019 Jan 21 PMID: 30666563).
Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, Meterissian S, Arnaout A, Brackstone M, McCready DR, Karp SE, Trop I, Lisbona A, Wright FC, Younan RJ, Provencher L, Patocskai E, Omeroglu A, Robidoux A. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64. https://doi.org/10.1200/JCO.2014.55.7827 (Epub 2014 Dec 1 PMID: 25452445).
Ruano-Pérez R, Rebollo-Aguirre AC, García-Talavera San Miguel P, Díaz-Expósito R, Vidal-Sicart S, Cordero-García JM, Carrera-Salazar D, Rioja-Martín ME. Actualización de la biopsia del ganglio centinela tras quimioterapia neoadyuvante en el cáncer de mama sin y con afectación ganglionar al diagnóstico. Rev Esp Med Nucl Imagen Mol. 2018;37(1):63–70.
Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35:561–4.
Gandhi A, Coles C, Makris A, Provenzano E, Goyal A, Maxwell AJ, Doughty J. Axillary surgery following neoadjuvant chemotherapy—multidisciplinary guidance from the association of breast surgery, faculty of clinical oncology of the Royal College of Radiologists, UK Breast Cancer Group, National Coordinating Committee for Breast Pathology and British Society of Breast Radiology. Clin Oncol (R Coll Radiol). 2019;31(9):664–8.
Funding
The authors received funding from GEICAM Spanish Breast Cancer Group. This work was developed within a project aimed to update the GEICAM previous clinical guidelines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article. Juan Carlos Vazquez is PhD candidate Program in Biomedical Research Methodology and Public Health, Universitat Autònoma de Barcelona, Spain.
Ethical approval
Not applicable.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1: MEDLINE/EMBASE search strategy
Appendix 1: MEDLINE/EMBASE search strategy
MEDLINE
PubMed
#1 “Breast Neoplasms”[Mesh].
#2 breast[tiab].
#3 #1 OR #2
#4 “Sentinel Lymph Node Biopsy”[Mesh].
#5 sentinel lymph node*[tiab].
#6 sentinel node biops*[tiab].
#7 SLNB[tiab].
#8 SLN[tiab].
#9 lymph node positivity[tiab].
#10 #4 OR #5 OR #6 OR #7 OR #8 OR #9
#11 node positive[tiab].
#12 positive lymph node*[tiab].
#13 axillary[tiab].
#14 ALND[tiab].
#15 ALN[tiab].
#16 #11 OR #12 OR #13 OR #14 OR #15
#17 “Neoadjuvant Therapy”[Mesh].
#18 neo adjuvant chemotherapy[tiab].
#19 neoadjuvant chemotherapy[tiab].
#20 NAC[tiab].
#21 preoperative chemotherapy[tiab].
#22 pre operative chemotherapy[tiab].
#23 primary[ti].
#24 #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23
#25 #3 AND #9 AND #15 AND #24
EMBASE
#1 (neoadjuvan* NEAR/5 (chemotherapy OR treatment)):ti,ab.
#2 (sentinel NEXT/5 node* NEXT/2 biops*):ti,ab.
#3 #1 AND #2
Rights and permissions
About this article
Cite this article
Vázquez, J.C., Piñero, A., de Castro, F.J. et al. The value of sentinel lymph-node biopsy after neoadjuvant therapy: an overview. Clin Transl Oncol 24, 1744–1754 (2022). https://doi.org/10.1007/s12094-022-02824-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02824-9