Abstract
Purpose
Peritoneal carcinomatosis (PC) from colorectal cancer (CRC) has poor survival. Multi-modal treatment including systemic chemotherapy, cytoreductive surgery (CRS), and hyperthermic intraperitoneal chemotherapy (HIPEC) can be used in selected patients with curative intent. The majority published works consider PC of CRC origin as a homogenous disease. Aim of this study is to stress the different biological behaviors and survival of PC according to colonic or rectal origin.
Methods
Data of CRS and HIPEC procedures for PC of CRC origin performed at MD Anderson Cancer Center-Madrid (Spain) have been collected, dividing patients into two groups according to colonic or rectal PC. Clinical, operatory, and postoperatory variables of the two groups have been analyzed to compare survival-related rates and PC origin.
Results
In the years 2004–2015, 114 procedures of CRS followed by HIPEC for peritoneal metastasis of different origin have been performed; of these, 36 procedures were for colorectal PC (31 patients in colonic and 5 in rectal group). Two groups are homogenous after analysis of clinical, operatory, and follow-up data. Median survival (OS) is significantly higher in colonic compared to rectal group (47.83 vs. 22.0 months, p 0.008). 3- and 5-year survival rate is 74 and 50% in colonic group vs. 20 and 0% in rectal group.
Conclusion
Rectal origin PC has a more aggressive behavior compared to colonic origin, reflecting in a worst prognosis of patients affected by rectal origin PC. According to our data and literature, indications of multi-modal treatment including CRS and HIPEC should be more restrictive for rectal cancer PC. Authors should differentiate colonic and rectal origin of PC when reporting cases in the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Purpose
Colorectal cancer (CRC) is the third tumor in Western population with a significant impact on cancer-related mortality. It has been estimated that 10–15% of CR cancers present with peritoneal metastasis or carcinomatosis (PC) and that 20–25% of patients will develop PC at some point of disease evolution, making the peritoneum the second most common site of CRC metastasis after liver [1,2,3].
PC prognosis is poor with a median survival time of 6–9 months if not treated. Modern systemic chemotherapy can increase this survival period, but at the moment cannot offer a curative possibility. In the last 20 years, cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been employed as a curative-intent treatment for CRC peritoneal metastasis [4,5,6,7,8]. A more recent evolution of this concept is involving also poly-metastatic patients, where patient with CRC PC and limited liver involvement could be considered for a curative-intent approach [9, 10].
The majority of papers in the literature consider CRC peritoneal metastasis as a homogeneous disease without differentiating colonic or rectal origin. Some works have reported the difference in biological behavior and survival between colonic and rectal PC [11,12,13]. According to these reports and the experience coming from everyday clinical practice, we reviewed our own series of patients treated with CRS and HIPEC for PC from CRC according to primary tumor origin.
Patients and methods
We retrospectively reviewed data of cytoreductive procedures combined with HIPEC for colorectal peritoneal metastasis in the time period 2004–2015 performed ad MD Anderson Cancer Center–Madrid (Spain). All reported cases have peritoneal disease with biopsy-proven adenocarcinoma of large bowel origin. For the study purpose, colorectal tumors were divided in two groups: tumors originating in the intraperitoneal colon (colonic group) and tumors originating below the peritoneal reflection (rectal group). For each case, we collected patient data (e.g. age, gender, and BSA), cancer-related data (e.g. clinical stage, synchronous or metachronous PC, completeness of cytoreduction (CC score), tumor burden measured by the peritoneal cancer index (PCI)], and surgical procedure data including associated liver resection, number of intestinal anastomosis, duration of surgery, and transfusions. This study included only patients with a complete cytoreduction (CC0 or CC1), treated with HIPEC (mitomycin or oxaliplatin-bidirectional regimen) or early post-operative intraperitoneal chemotherapy (EPIC). Patients with completely resected liver metastasis (R0) at the same time of peritoneal cytoreduction were also included.
The mentioned clinical variables, overall survival (OS), disease-free survival (DFS), and time to death from progression (TTD) of the two groups (colic vs. rectal) have been analyzed using Student T test, Chi-square or Fisher exact test, and log rank (Mantel–Cox) when appropriate, with SPSS software v. 20.0 (SPSS Inc. Chicago IL).
Results
From 114 procedures of CRS followed by HIPEC for peritoneal metastasis of different origins or primary tumor in period 2004–2015, 36 procedures performed for colorectal PC have been selected.
Of these 36 patients, 31 had PC from colonic and 5 from rectal origin. These two groups are homogenous, since there was not any significant difference in collected variables at univariate analysis (see Table 1).
17 patients were males and 19 females (for a detailed gender distribution, see Table 1). 15 (41.9%) patients presented with synchronous PC and 21 (58.1%) developed PC during follow-up after primary tumor treatment, with a global median peritoneal recurrence interval of 19.69 months (range 5.65–73.38) and group-specific recurrence interval of 20.58 months (range 67.53–5.85) for colonic and 14.47 months (range 11.44–17.49) for rectal origin. Out of the 15 patients with synchronous PC, 13 were of colonic and 2 of rectal origin. All patients received systemic chemotherapy before CRS and HIPEC procedure, either as adjuvant treatment after primary tumor resection (23 patients, 21 colonic group, and 2 rectal) or induction treatment before CRS and HIPEC (19 patients, 16 colonic group, and 3 rectal) or both (6 patients, all with metachronous PC in colonic group).
Median global PCI value was 10 (range 2–30), being 10 (range 2–30) in the colonic group and 9 (range 9–26) in the rectal group. 6 (16.7%) patients had a PCI greater than 20, 5 (16.1%) in colonic, and 1 (20%) in rectal group.
Completeness of cytoreduction (CC score) was 0 in 31 patients and 1 in 4, being 27 CC0 and 3 CC1 resections in the colonic group and 4 CC0 and 1 CC1 in the rectal group.
Early post-operative chemotherapy (EPIC) was performed in 3 patients, all affected by colonic PC, in the first years of the program; of these, one did only EPIC and two did HIPEC followed by EPIC. 34 patients were treated with HIPEC, 13 with mitomycin regimen, and 21 with oxaliplatin with bidirectional protocol (10 MMC and 19 Oxaliplatin in colonic group, 3 MMC and 2 Oxaliplatin in rectal group).
Median surgical duration was 550 min (range 40–780 min) and there was no difference between the two groups in terms of surgery duration, need for transfusion, associated splenectomy, or numbers of anastomosis done.
10 patients underwent liver resection as part of cytoreductive surgery, 3 of them had liver invasion by peritoneal metastasis, and 7 patients had a pathological confirmed intraparenchymal liver metastasis (tumor covered by intact Glisson capsule at pathological analysis); 6 of them in the colonic group and 1 in the rectal one. In two cases, liver metastasis were more than 3, while median number of liver metastasis was 1 (range 1–6).
Mortality rate was 8.3% (3 deaths out of 36 patients, all in colonic group). Causes of deaths were respiratory failure, aplasia, and abdominal sepsis without evidence of intestinal fistula.
Severe complications (grade III or IV of Clavien–Dindo classification) occurred in seven (19.4%) patients, all patients of colonic group [14]. Complications were two cases of abdominal abscess, one anastomotic leak, one abdominal hematoma, one eventration, one case of wound seroma, and one case of severe neutropenia.
Recurrence rate during follow-up after CRS and HIPEC was 65.5% (19 patients), having peritoneum as the only affected organ in 4 (21%) patients (for a complete list of recurrence site, see Table 1). 11 (52.4%) patients with recurrence had received a second surgical approach: 6 of them with CRS and HIPEC, 3 with liver resection, and 2 with other type of surgery; the majority of re-operated patients were in colonic group (14 out of 15).
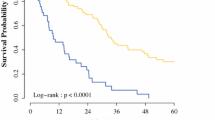
Median overall disease-specific survival (OS) was 40.52 months, with a significant difference between colonic and rectal group (47.83 vs. 22.0 months, respectively, p 0.008). Overall survival rate at 3 and 5 years was 60 and 34%; 3- and 5-year survival rate in colonic group was 74 and 50%, whereas it was 20 and 0% in rectal group (see Fig. 1 and Table 2).
Median disease-free survival (DFS) was 18.05 months without clear difference in the two groups (19.41 months in colonic vs. 15.0 months in rectal, p: ns). Overall disease-free rate at 3 years was 6%; 3-year disease-free rate was 8% in colonic group and 0% in rectal group (see Fig. 1 and Table 2).
Median time to death (TTD) calculated from progression was 23.31 months, with a significant difference (colonic 31.3 vs. rectal 14.0 months, p 0.0001), see Fig. 1 and Table 2.
Considering liver metastasis resection, it was not observed a significant difference in OS and DFS between patients who underwent liver resection as cytoreductive procedure, even though patients with liver metastases had a shorter OS and DFS (median OS 42.6 months for non-liver vs. 24.0 months for liver metastatic; 3-year survival rate 66% non-liver vs. 50% metastatic, p: ns; median DFS 19.26 months for non-liver and 18.0 months for liver metastatic, 3-year disease-free rate 7% for non-liver vs. 0% for liver metastatic, p: ns).
Conclusion
The aim of this paper is not to report a better survival in a selected subgroup of patients affected by PC of CRC origin that can be treated with CRS and HIPEC, as have already been published, but to stress the different behavior of rectal and colonic peritoneal metastasis in terms of survival [15,16,17].
Actually, in the literature, there are a few works that consider rectal and colonic peritoneal metastasis treated with CRS and HIPEC, and in these papers, sample size of patients with PC of rectal origin is small.
In 2003, Verwaal and coll. published a paper in which they analyzed survival in colorectal PC treated with CRS and HIPEC, reporting that rectal origin PC had a shorter median survival compared with colon one (16 vs. 21.6 months) [18].
Another paper published in 2006 by Da Silva and coll., considered 64 patients with colonic and 6 patients with rectal metastasis with optimal cytoreduction. Survival results were similar to what we found in our series, with a clear difference in terms of median survival (17 months for rectal vs. 35 months for colonic origin) [11].
Conversely, a more recent work by Votanopoulos and coll. published in 2013, did not reported a marked difference in overall survival and disease-free survival according to PC origin. Sample size of this paper is larger than previous ones (13 patients in the rectal group, 204 in colonic). Authors reported a similar median survival between the two groups of 14.3 months for rectal origin and 17 months for colonic. The lack of difference between rectal and colon PC and the low median survival for colonic origin, can be explained considering that the reported rate of suboptimal cytoreduction, could mask PC origin effect on survival (49% of patients with colonic origin and 46% with rectal origin had a R2 resection) [13].
Some theories have been proposed to explain this difference in terms of survival for PC of rectal origin. One relates rectal worst prognosis to primary tumor resection, since it makes pelvic peritonectomy more difficult and enhances rectal cancer cell entrapment in pelvic wall [19]. Another proposed theory suggests that rectal cancer cells of peritoneal metastasis have to be biologically more aggressive, since they have to gain capability of perforating rectal wall that is thicker than the colon wall. At the moment, there is no defined explanation for this finding [11].
Regarding survival of patients with liver metastasis treated at the same time of PC, they have a shorter median OS and DFS. It has already been reported that poly-metastatic patients have a worst prognosis than single-organ metastatic patients [20, 21]; our data support this finding, but we cannot draw any conclusion because of the small numbers of our series and the fact that the majority of liver-metastatic patients were operated in recent years (2012–2014).
In conclusion, we are aware of these study limitations related to the small number of patients and its retrospective nature, but we underline that our aim is to stress the differential survival between colonic and rectal origin of PC.
According to our findings and literature data, we think that indications of CRS and HIPEC for rectal cancer PC at multidisciplinary discussion should be more restrictive than for colon PC.
We also suggest that authors differentiate colonic and rectal origin of PC when reporting cases in the literature and we call for a large-scale multicentric registry of rectal cancer with peritoneal metastasis treated with CRS and HIPEC as a further contribution to ascertain the role of CRS and HIPEC in rectal PC.
References
Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–50.
Glehen O, Osinsky D, Beaujard AC, Gilly FN. Natural history of peritoneal carcinomatosis from nongynecologic malignancies. Surg Oncol Clin N Am. 2003;12:729–39 xiii.
Mitchard JR, Love SB, Baxter KJ, Shepherd NA. How importantis peritoneal involvement in rectal cancer? A prospective study of 331 cases. Histopathology. 2010;57:671–9.
Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-Year followup of randomized trial: cyto reduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–32.
Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27:681–5.
Elias D, Blot F, El Otmany A, Antoun S, Lasser P, Boige V, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71–6.
Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Ann Surg Oncol. 2007;14:128–33.
Esquivel J, Elias D, Baratti D, Kusamura S, Deraco M. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol. 2008;98:263–7.
Chua TC, Yan TD, Zhao J, Morris DL. Peritoneal carcinomatosis and liver metastases from colorectal cancer treated with cytoreductive surgery perioperative intraperitoneal chemotherapy and liver resection. Eur J Surg Oncol. 2009;35:1299–305.
Kianmanesh R, Scaringi S, Sabate JM, Castel B, Pons-Kerjean N, Coffin B, et al. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg. 2007;245:597–603.
da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. 2006;203:878–86.
Jacquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res. 1996;15:49–58.
Votanopoulos KI, Swett K, Blackham AU, Ihemelandu C, Shen P, Stewart JH, Levine EA. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from rectal cancer. Ann Surg Oncol. 2013;20(4):1088–92.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, Lorimier G, Dubè P, Glehen O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28(1):63–8.
Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ III. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116:3756–62.
Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–32.
Verwaal VJ, van Ruth S, de Bree E, van Slooten GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–43.
Sugarbaker P. Update on the prevention of local recurrence and peritoneal metastases in patients with colorectal cancer. World J Gastroenterol. 2014;20(28):9286–91.
Alzahrani N, Ung L, Valle SJ, Liauw W, Morris DL. Synchronous liver resection with cytoreductive surgery for the treatment of liver and peritoneal metastases from colon cancer: results from an Australian centre. ANZ J Surg. 2017;87(11):E167–72.
De Cuba EMV. Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases: systematic review of all literature and meta-analysis of observational studies. Cancer Treat Rev. 2013;39(4):321–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have conflicts of interest to declare.
Human and animal rights statement
There is no experimental research involving humans or animals.
Informed consent
Informed consent has been collected.
Rights and permissions
About this article
Cite this article
Tonello, M., Ortega-Perez, G., Alonso-Casado, O. et al. Peritoneal carcinomatosis arising from rectal or colonic adenocarcinoma treated with cytoreductive surgery (CRS) hyperthermic intraperitoneal chemotherapy (HIPEC): two different diseases. Clin Transl Oncol 20, 1268–1273 (2018). https://doi.org/10.1007/s12094-018-1857-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1857-9