Abstract
Purpose
Aspirin could reduce the risk of cancer metastasis. Circulating tumor cells (CTCs) are a key factor of cancer metastasis, but no evidence has revealed how aspirin affects CTCs and its epithelial–mesenchymal transition (EMT). Here, we conducted a clinical trial to investigate how aspirin affects CTCs in metastatic colorectal cancer (MCC) and breast cancer patients (MBC).
Methods
The trial is retrospective registered at clinicaltrials.gov (NCT02602938). The eligible patients are given 100 mg aspirin q.d. for 8 weeks, and CTCs are evaluated at baseline, 4 and 8 weeks for absolute number, phenotype (epithelial type, E+, mesenchymal type, M+, and biophenotypic type, B+), and vimentin expression.
Results
Data on 21 MCC and 19 MBC patients are analyzed, and it revealed that the CTC numbers decreased with aspirin treatment in MCC (p < 0.001) but not MBC (p = 0.0532); besides, ratio of E+ CTCs increased (p = 0.037) and M+ CTCs decreased at 2 months in MCC (p = 0.013), but neither the ratio of E+ or M+ CTCs changes significantly in MBC; vimentin expression of M+ CTCs is higher than E+ and B+ CTCs either in MBC or MCC patients at baseline (p < 0.01); and aspirin suppresses the vimentin expression in M+ (p = 0.002)and B+ (p = 0.006) CTCs of MCC and M+ CTCs of MBC (p = 0.004); besides it find vimentin expression in B+ (p = 0.004) or M+ (p < 0.001), CTCs are markedly decreased in patients with total CTC numbers declined.
Conclusion
Aspirin could decrease CTCs numbers and block EMT transition in MCC patients and part of MBC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing evidence has confirmed the prognostic value of circulating tumor cells (CTCs) in malignant diseases including colorectal cancer, breast cancer, prostate cancer, and gastric cancer [1,2,3,4]. However, studies have found that most of CTCs are incapable of generating metastatic lesion and epithelial antigen-based approaches often fail to detect the most aggressive CTC subpopulation. Yu et al. [5] revealed that after monitoring the CTCs in advanced breast cancer patients, the fraction of mesenchymal CTCs was much better concordant with the timing of disease progression rather than CTCs’ total number. Since characterizing an epithelial or mesenchymal phenotype in CTCs is challenging, we previously generated an RNA–in situ hybridization (RNA–ISH) method, which can be used to classify the CTCs into epithelial, mesenchymal, and biophenotypic subtypes according to specific markers [6].
It is commonly accepted that the metastatic process of cancer cells involves an epithelial-to-mesenchymal transition (EMT), vascular invasion, protection from the host immune system, and colonization of distant organs [7]. Platelets have been shown to participate in the each of these processes, especially in the induction of EMT in epithelial cells via its activation [8, 9]. Furthermore, it has been reported that an increase in number of platelets negatively correlates with cancer patient outcomes [10]. Growth studies have shown a miraculous effect of single-agent antiplatelet therapies, such as low-dose aspirin, in preventing multiple malignant diseases. These therapies have even improved the prognosis in advanced malignant diseases such as colorectal cancer and breast cancer [11,12,13]. To investigate the effect that aspirin has on CTCs in patients with metastatic colorectal and breast cancer, we conducted a prospective study to test the hypothesis that inhibition of platelets could affect the numbers and EMT phenotype of CTCs.
Patients and methods
Patients and treatment
The trial is registered in clinicaltrial.gov (NCT02602938). Patients with stable metastatic colorectal (MCC) or breast cancer (MBC) were enrolled. The eligibility and exclusion criteria included the following: age from 18 to 80 years; no current intravenous chemotherapy (capecitabine maintenance for more than 1 month before enrolment was allowed); concurrent endocrine therapy, bisphosphonate therapy, and/or monoclonal targeted therapy were permitted (all for at least 2 months before enrollment); estimated survival ≥ 3 months; no platelet inhibitor therapy within 1 month of study, platelet count ≥ 100,000/mm3; no disease of hemorrhagic tendency or history of non-steroid drug allergy; normal kidney and liver function; and no surgery plan within the next 3 months. This project was reviewed and permitted by the ethics committee of Zhejiang Provincial People’s Hospital, and written consent was obtained from all patients before enrollment.
The peripheral blood (7.5 ml) of eligible patients was collected to evaluate CTCs. Those patients with CTCs ≥ 5 were given 100 mg of aspirin per day for the next 2 months, unless they reported severe adverse events (AEs) related to aspirin, an intention to quit the trial, or disease progression.
Circulating tumor cells’ evaluation
CTCs were evaluated at baseline, 4 and 8 weeks. Patients’ peripheral blood (7.5 ml) was collected into heparin anticoagulation tubes and subsequently transferred to the lab for CTC measurements within 48 h. The assessment of CTC numbers and phenotype was based on the Canpatrol technique [6]. For detail, Canpatrol CTC filtration system included a filtration tube (8-μm diameter pores calibrated membrane, SurExam, Guangzhou, China), a manifold vacuum plate with valve settings (Millipore, Billerica, USA), an E–Z 96 vacuum manifold (Omega, Norcross, USA), and a vacuum pump (Auto Science, Tianjin, China). Before filtration, erythrocytes were removed with lysis buffer (Sigma, St. Louis, USA). Thereafter, the remaining cells were resuspended with PBS plus 4% formaldehyde (Sigma, St. Louis, USA). The cell suspension was transferred to a filtration tube and pumped with at least 0.08 MPa to obtain CTCs.
The phenotypes of CTCs were classified into three subgroups using the RNA–ISH method: epithelial type (E+ , Cytokeratin 8, 18, 19+, EpCam+); mesenchymal type (M+, Vimentin+, Twist+), and biophenotypic type (B+, express both E+ and M+ markers) (Fig. 1). The sequences of the capture probe and bDNA signal amplification probe are listed in supplementary Tables S1 and S2.
Assessment of vimentin expression in CTCs
The expression of vimentin was also assessed in all MCC and MBC patients by RNA–ISH. The sequences of the capture probe and bDNA signal amplification probe are listed in supplementary Tables S1, S2. The evaluation of vimentin expression level was based on RNA–ISH signal intensity (Table S3, Figs. S2–S4): N or + were considered as low expression (vimentin L), while ++ or +++ were considered as high expression (vimentin H).
Statistical analysis
Data were recorded in Excel (Microsoft Corp, Redmond, WA, USA) and analyzed with SPSS version 17.0.1 (PSSS, Inc, Chicago, IL, USA). Continuous parameters, such as age, were presented as the mean ± standard deviation (SD). Frequencies were calculated for categorical measurements, such as gender and CTC subtype. The time of enrollment was taken as the baseline. The data regarding percent changes were assumed to be normally distributed. The primary efficacy variable is the percent change in CTC numbers at weeks 4 and 8 compared to baseline, and the second efficacy variable is the percent change in M+ CTCs compared to baseline.
The percent change from baseline was determined as
Percent changes from baseline for efficacy variables were analyzed using analysis of covariance (ANCOVA) with the respective baseline value as the covariate. p < 0.05 was considered statistically significant.
Results
Patient characteristics and safety
This was designed as a single-arm study. Thirty-two MCC and thirty-one MBC patients met our criteria between July 2015 and July 2016, as shown by the flow chart in Fig. 2. Twenty-six MCC and twenty-eight MBC patients underwent initial CTC assessment, but four MCC and seven MBC patients were excluded, because their CTC numbers were less than 5/7.5 ml peripheral blood. There were no serious adverse events (SAEs) reported in the whole process, and most patients tolerated the aspirin treatment well. Two MBC patients dropped out, one because of a stomachache thought to be the result of aspirin treatment and the other due to disease progression. Of the MCC patients, one failed to follow up. In total, 21 MCC patients and 19 MBC patients were evaluable for the primary endpoint.
The baseline characteristics of MCC and MBC patients are listed in Table 1. The mean age was 58.2 ± 8.81 years in the MBC group and 62.1 ± 10.56 years in the MCC patient group. All patients had previously received one or more lines of chemotherapy for the control of disease progression. 19 MCC patients and 11 MBC patients had more than two metastatic lesions. According to the inclusion criteria, the baseline CTC numbers were ≥ 5/7.5 ml peripheral blood in all patients. The mean total CTC number was 8.68 ± 3.47 in MBC patients, whereas the E+, M+, and B+ CTC numbers were 4 ± 1.8 (48.2 ± 22.4%), 2.5 ± 1.9 (25.1 ± 14.9%), and 2.2 ± 1.6 (26.7 ± 19.9%), respectively. The mean total CTC number in MCC patients was 10.7 ± 4.76, whereas the E+, M+, and B+ CTC numbers for these patients were 4 ± 1.7 (45.3 ± 27.3%), 2.86 ± 2.8 (22.0 ± 18.5%), and 3.86 ± 3.38 (32.8 ± 18.7%), respectively. We compared the baseline ratio between E+ and M+ CTCs in included patients, and our results revealed that the number of E+ CTCs was significantly higher than that of M+ CTCs both in MBC and MCC patients (MBC, p = 0.0059; MCC, p = 0.0213, Fig. 3). The baseline platelet counts and functional test results were similar between MCC and MBC patients (data not shown).
Aspirin’s effect on circulating tumor cells
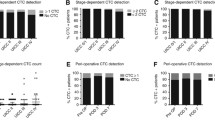
CTC number and phenotype was evaluated at baseline, 4 and 8 weeks. Compared to baseline, CTC numbers decreased significantly in MCC patients at 1 and 2 months (p < 0.001, Fig. 4a). However, although the CTC numbers of MBC patients presented a decreasing trend, a significant change was not observed at either 1 or 2 months (p = 0.053, Fig. 4b). To go into greater detail, 15 MCC and 8 MBC patients observed a reduction of more than 2 CTCs/7.5 ml peripheral blood at 2 months; 5 MCC and 8 MBC patients reached a stable level of CTCs following 2 month aspirin treatment; and one MCC and three MBC patients saw increased numbers of CTCs after 2 months (Table 1).
Aspirin’s effect on CTC numbers and subpopulation. a, b Absolute number of total CTCs in MCC and MBC patients with aspirin treatment; c, d ratio of E+ and M+ CTCs in MCC patients with aspirin treatment; e, f ratio of E+ and M+ CTCs in MCC patients with aspirin treatment. CTC circulating tumor cells, MCC metastatic colorectal cancer, MBC metastatic breast cancer
We then evaluated the subpopulation of CTCs in both MCC and MBC patients. Statistical analysis found that the relative ratio of E+ and M+ CTCs remained fairly stable in MBC and MCC patients at 1 month. Thereafter, the ratio of E+ CTCs markedly increased (p = 0.037, Fig. 4c), and M+ CTCs significantly decreased at 2 months in MCC patients (p = 0.013, Fig. 4d). The ratio of neither E+ nor M+ CTCs changed in MBC patients with aspirin treatment (Fig. 4e, f).
Vimentin expression in circulating tumor cells
Since vimentin was considered as the core factor in epithelial–mesenchymal transition, we assessed vimentin expression in CTCs. Vimentin expression was detected by RNA–ISH method in all MCC and MBC patients (supplementary Table S4). The results revealed that the vimentin expression level was much higher in M+ CTCs than E+ and B+ CTCs either in MBC or MCC patients at baseline: in MCC patients, vimentin H subpopulation accounted for 45.8% (38/83), 67.9% (55/81), and 78.3% (47/60) in E+, B+, and M+ CTCs, respectively, whereas in MBC patients, vimentin H subpopulation accounted for 36.8% (28/76), 80.9% (34/42), and 93.6% (44/47) in E+, B+, and M+ CTCs, respectively.
We then explored whether aspirin could reduce the expression of vimentin in CTCs (Figs. S2–S4). In MCC patients, we found that vimentin expression level had declined significantly following 2 months of aspirin 100 mg q.d. therapy, in B+ (p = 0.006, Fig. 5b) and M+ CTCs (p = 0.002, Fig. 5c), but not in E+ subpopulation (p = 0.418, Fig. 5a); while in MBC patients, its expression level was also decreased in M+ CTCs (p = 0.004, Fig. 5f), but not in E+ (p = 0.734, Fig. 5d) and B+ subpopulations (p = 0.202, Fig. 5e). Since previous publications found that the change of genotype was not exactly in accordance with CTC numbers’ change [5], we further explored if the vimentin expression was accompanied with CTC numbers’ change under aspirin treatment. Since the limited subjects’ number, the data of MBC and MCC patients were combined and grouped by CTC numbers changed or not with 2 month aspirin treatment: our results indicated that the vimentin expression in B+ (p = 0.004, Fig. 6b) or M+ (p < 0.001, Fig. 6c) CTCs was markedly decreased in patients (15 MCC patients and 8 MBC patients) with total CTC numbers also declined, but not in E+ CTCs (p = 0.451, Fig. 6a), while in the rest patients, the vimentin H CTC numbers were similar before and after aspirin therapy in all three subpopulations (E+, p = 0.0701; B+, p = 0.322; M+, p = 0.543, Fig. 6d–f).
Aspirin’s effect on Vimentin H CTCs. a–c Absolute number of vimentin H E+/B+/M+CTCs in MCC patients with aspirin treatment; d–f absolute number of Vimentin H E+/B+/M+CTCs in MBC patients with aspirin treatment. CTC circulating tumor cells, MCC metastatic colorectal cancer, MBC metastatic breast cancer
Discussion
Although aspirin has been marketed for over 100 years, it still remains the cornerstone of anti-platelet drugs. Except for its function of cancer prevention, its therapeutic role in cancer received increasing attention. The neoadjuvant use of aspirin was reported to correlate with better tumor down-staging in colorectal cancer [14]; and the most recent comprehensive meta-analysis found that aspirin markedly reduces cancer specific death of colorectal cancer and all-cause mortality [15]. Unlike the consistence result in colorectal cancer, the application of aspirin in breast cancer is still controversial [16, 17]. Here, we first report how aspirin affects CTCs in late-stage colorectal and breast cancer patients, and our result shows that aspirin markedly decreases the CTCs’ numbers and blocks EMT transition in MCC patients, and more than 40% MBC patients also respond to aspirin treatment, although not reach statistically significant.
Weilbaecher et al. [7] first tested whether disruption of platelet function would decrease the number of CTCs in MBC patients, but a negative result was reported. There are several differences with the trial present here: first, patients with detectable CTCs (≥ 1 CTC) were calculated in the previous report, and one quarter of included patients had no CTCs present at baseline, which could underestimate aspirin’s effect, and make it impossible to separate CTCs into further groups, so here, we included patients with more than 5/7.5 ml peripheral blood at baseline; second, the patients only receive 100 mg q.d. aspirin here, while in the previous trial, patients taken both clopidogrel (300 mg loading dose followed by 75 mg orally daily) and aspirin (325 mg orally daily) therapy. In consideration of the high risk of gastrointestinal bleeding, it is important to determine the optimal dosage. An individual patient data meta-analysis demonstrated that aspirin reduces the risk of colorectal adenoma or recurrence of advanced lesions without dose dependent manner, and comparison of higher dose (300 or 325 mg q.d.) versus lower dose (81 or 160 mg q.d.) aspirin showed markedly greater risk reduction with lower dosage [18]. Similarly, another meta-analysis showed that the risk of breast cancer was deceased significantly for only 300 mg aspirin once a week (RR 0.96; 95% CI 0.92–0.99, p = 0.02). However, the optimal dosage of aspirin in adjuvant therapy is still undetermined, several adjuvant trials include newly diagnosed cancers patients, including colorectal, gastroesophageal, breast, and prostate cancer, with various dosages of aspirin have been initiated, and we are looking forward to the final results [19]. In conclusion, we decide to give aspirin to both MCC and MBC patients’ 100 mg q.d., and our result proves its safety and effectiveness.
Aberrant activation of EMT has been implicated play crucial role in tumor metastasis, but it is difficult to identify M+ or E+ type CTCs from hematopoietic cells with the traditional approach. Several approaches have been established to assess EMT-transformed CTCs [20], such as RNA–in situ hybridization (ISH) method [5, 6], cell-surface vimentin-based detection [21], and cytoplasm–vimentin-based detection [22], and it is implicated that EMT-transformed CTCs have greater specificity and sensitivity than total CTC numbers in prognosis prediction and treatment response association. Maheswaran et al. [5] found that the change of M+ CTCs was not accompanied with total CTC numbers in 30% MBC patients (3/10); Li et al. [21] reported high numbers of M+ CTCs in castration-resistant prostate cancer patients but not total CTC numbers detected by CellSearch. We have established RNA–in situ hybridization (ISH) assay to detect several E+ and M+ markers within CTCs previously [6], and here, we find aspirin effectively impede the EMT process of CTCs in most MCC and part of MBC patients; besides, the change of vimentin expression is consistent with total CTCs, and only one MBC patient is found, has a declined vimentin expression, but increased total CTC numbers. Based on all these above, we speculate that aspirin therapy could benefit for those patients, whose total or M+ CTCs numbers increased, which needs to be verified in the near future.
Besides, the leading voice to explain aspirin’s anti-cancer effect could contribute to its anti-platelet function. Platelets play an integral part in cancer metastasis, such as promote the tumor cell EMT transition, degrade the extracellular matrix (ECM) [23, 24], protect CTCs from shear force and immune attack in circulation [25, 26], and activate the TGF-βpathway of CTCs to promote its EMT transformation. Because of the platelets—cancer interaction, anti-platelets therapy could be a rational way to prevent carcinogenesis and metastasis, and we assume that anti-platelet therapy not only affects the CTC number, but also the EMT process, and here, we find that aspirin indeed decreased vimentin expression, which is acknowledged as one of the most important factors in EMT process. Furthermore, it is interesting to see the different responses among various patients with aspirin treatment. Two retrospective studies [27, 28] reveal that the effect of aspirin is associated with reduced recurrence rate in patients with KRAS wild type and PI3KCA mutated tumors; meanwhile; Reimers et al. [29] found that the survival benefit was related to HLA class I antigen-positive tumors. In this trial, we find that most of the responders of MBC patients were HR+ patients (6/8). Since all these HR+ patients have experienced the first- or second-line endocrine therapy failures, PI3K mutation is likely existed in these patients, which was a dominant factor for endocrine therapy resistance [30].
In conclusion, here, we conduct a single-arm, prospective study to evaluate the effect of aspirin on CTCs, and our results indicate that low-dose aspirin could decrease the CTCs number and suppress the EMT transition in MCC patients and part of MBC patients. Our finding provide more evidence to support the hypothesis that the therapeutic effect of aspirin against cancer metastasis, and it is hopefully to help clinicians to find out late-stage cancer patients with specific gene mutation or biomarker, who could indeed benefit from aspirin treatment with well-designed large prospective trials in the future.
Abbreviations
- CTC:
-
Circulating tumor cells
- EMT:
-
Epithelial–mesenchymal transformation
- MCC:
-
Metastatic colorectal cancer
- MBC:
-
Metastatic breast cancer
References
Groot Koerkamp B, Rahbari NN, Buchler MW, Koch M, Weitz J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann Surg Oncol. 2013;20(7):2156–65. https://doi.org/10.1245/s10434-013-2907-8.
Lv Q, Gong L, Zhang T, Ye J, Chai L, Ni C, et al. Prognostic value of circulating tumor cells in metastatic breast cancer: a systemic review and meta-analysis. Clin Transl Oncol Off Publ Fed Spanish Oncol Soc Natl Cancer Inst Mex. 2016;18(3):322–30. https://doi.org/10.1007/s12094-015-1372-1.
Zhou J, Ma X, Bi F, Liu M. Clinical significance of circulating tumor cells in gastric cancer patients. Oncotarget. 2017;. https://doi.org/10.18632/oncotarget.14879.
Zheng Y, Zhang C, Wu J, Cheng G, Yang H, Hua L, et al. Prognostic value of circulating tumor cells in castration resistant prostate cancer: a meta-analysis. Urol J. 2016;13(6):2881–8.
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–4. https://doi.org/10.1126/science.1228522.
Wu S, Liu S, Liu Z, Huang J, Pu X, Li J, et al. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One. 2015;10(4):e0123976. https://doi.org/10.1371/journal.pone.0123976.
Roop RP, Naughton MJ, Van Poznak C, Schneider JG, Lammers PE, Pluard TJ, et al. A randomized phase II trial investigating the effect of platelet function inhibition on circulating tumor cells in patients with metastatic breast cancer. Clin Breast Cancer. 2013;13(6):409–15. https://doi.org/10.1016/j.clbc.2013.08.006.
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res BCR. 2009;11(4):R46. https://doi.org/10.1186/bcr2333.
Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, et al. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307(1):26–36. https://doi.org/10.1016/j.canlet.2011.03.012.
Tsuruo T, Fujita N. Platelet aggregation in the formation of tumor metastasis. Proc Jpn Acad Ser B Phys Biol Sci. 2008;84(6):189–98.
Li P, Wu H, Zhang H, Shi Y, Xu J, Ye Y, et al. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 2015;64(9):1419–25. https://doi.org/10.1136/gutjnl-2014-308260.
Ye XF, Wang J, Shi WT, He J. Relationship between aspirin use after diagnosis of colorectal cancer and patient survival: a meta-analysis of observational studies. Br J Cancer. 2014;111(11):2172–9. https://doi.org/10.1038/bjc.2014.481.
Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2012;23(6):1403–15. https://doi.org/10.1093/annonc/mds113.
Restivo A, Cocco IM, Casula G, Scintu F, Cabras F, Scartozzi M, et al. Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. Br J Cancer. 2015;113(8):1133–9. https://doi.org/10.1038/bjc.2015.336.
Elwood PC, Morgan G, Pickering JE, Galante J, Weightman AL, Morris D, et al. Aspirin in the treatment of cancer: reductions in metastatic spread and in mortality: a systematic review and meta-analyses of published studies. PLoS One. 2016;11(4):e0152402. https://doi.org/10.1371/journal.pone.0152402.
Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(9):1467–72. https://doi.org/10.1200/JCO.2009.22.7918.
Holmes MD, Olsson H, Pawitan Y, Holm J, Lundholm C, Andersson TM, et al. Aspirin intake and breast cancer survival—a nation-wide study using prospectively recorded data in Sweden. BMC Cancer. 2014;14:391. https://doi.org/10.1186/1471-2407-14-391.
Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101(4):256–66. https://doi.org/10.1093/jnci/djn485.
Patrignani P, Patrono C. Aspirin and Cancer. J Am Coll Cardiol. 2016;68(9):967–76. https://doi.org/10.1016/j.jacc.2016.05.083.
Zhang W, Xia W, Lv Z, Ni C, Xin Y, Yang L. Liquid biopsy for cancer: circulating tumor cells, circulating free DNA or exosomes? Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2017;41(2):755–68. https://doi.org/10.1159/000458736.
Satelli A, Batth I, Brownlee Z, Mitra A, Zhou S, Noh H, et al. EMT circulating tumor cells detected by cell-surface vimentin are associated with prostate cancer progression. Oncotarget. 2017;8(30):49329–37. https://doi.org/10.18632/oncotarget.17632.
Lindsay CR, Le Moulec S, Billiot F, Loriot Y, Ngo-Camus M, Vielh P, et al. Vimentin and Ki67 expression in circulating tumour cells derived from castrate-resistant prostate cancer. BMC Cancer. 2016;16:168. https://doi.org/10.1186/s12885-016-2192-6.
Leblanc R, Peyruchaud O. Metastasis: new functional implications of platelets and megakaryocytes. Blood. 2016;128(1):24–31. https://doi.org/10.1182/blood-2016-01-636399.
Orellana R, Kato S, Erices R, Bravo ML, Gonzalez P, Oliva B, et al. Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells. BMC Cancer. 2015;15:290. https://doi.org/10.1186/s12885-015-1304-z.
Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Can Res. 2009;69(19):7775–83. https://doi.org/10.1158/0008-5472.CAN-09-2123.
Placke T, Orgel M, Schaller M, Jung G, Rammensee HG, Kopp HG, et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Can Res. 2012;72(2):440–8. https://doi.org/10.1158/0008-5472.CAN-11-1872.
Domingo E, Church DN, Sieber O, Ramamoorthy R, Yanagisawa Y, Johnstone E, et al. Evaluation of PIK3CA mutation as a predictor of benefit from nonsteroidal anti-inflammatory drug therapy in colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(34):4297–305. https://doi.org/10.1200/JCO.2013.50.0322.
Hua X, Phipps AI, Burnett-Hartman AN, Adams SV, Hardikar S, Cohen SA, et al. Timing of Aspirin and other nonsteroidal anti-inflammatory drug use among patients with colorectal cancer in relation to tumor markers and survival. J Clin Oncol Off J Am Soc Clin Oncol. 2017;. https://doi.org/10.1200/JCO.2017.72.3569.
Reimers MS, Bastiaannet E, Langley RE, van Eijk R, van Vlierberghe RL, Lemmens VE, et al. Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA Intern Med. 2014;174(5):732–9. https://doi.org/10.1001/jamainternmed.2014.511.
De Marchi T, Foekens JA, Umar A, Martens JW. Endocrine therapy resistance in estrogen receptor (ER)-positive breast cancer. Drug Discov Today. 2016;21(7):1181–8. https://doi.org/10.1016/j.drudis.2016.05.012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Ethical approval
The study was approved by the Institutional Ethics Committee of Zhejiang Provincial People’s Hospital.
Informed consent
Written informed consent was obtained from all patients enrolled in this study.
Funding
This project was supported by the Science and Technology Department of Zhejiang Province (Grant Numbers 2015C33097, 2016C33116), the Natural Science Foundation of China (Grant Number 81502463), the key Research Project of Science Technology Department of Zhejiang Province (Grant Number 2015C03030), and the Natural Science Foundation of Zhejiang Province (Grant Numbers Y15H160158, Q15H070012).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, L., Lv, Z., Xia, W. et al. The effect of aspirin on circulating tumor cells in metastatic colorectal and breast cancer patients: a phase II trial study. Clin Transl Oncol 20, 912–921 (2018). https://doi.org/10.1007/s12094-017-1806-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1806-z